Abstract
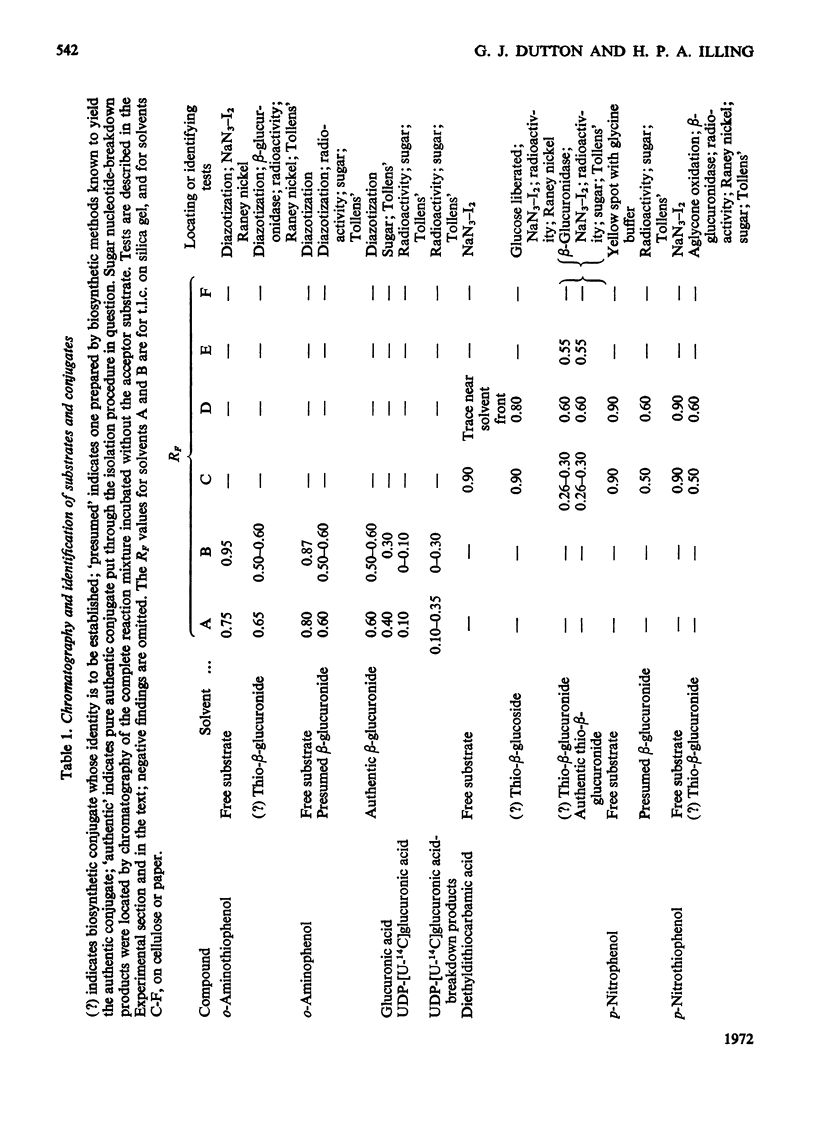
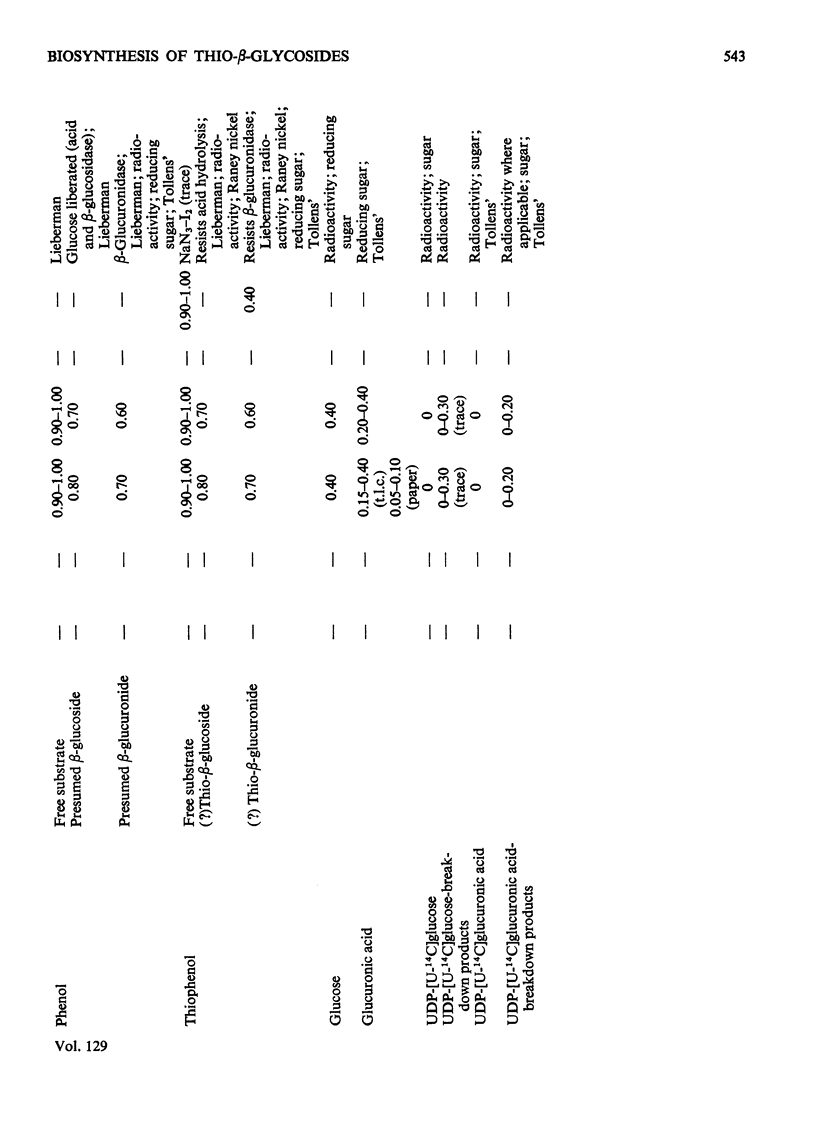
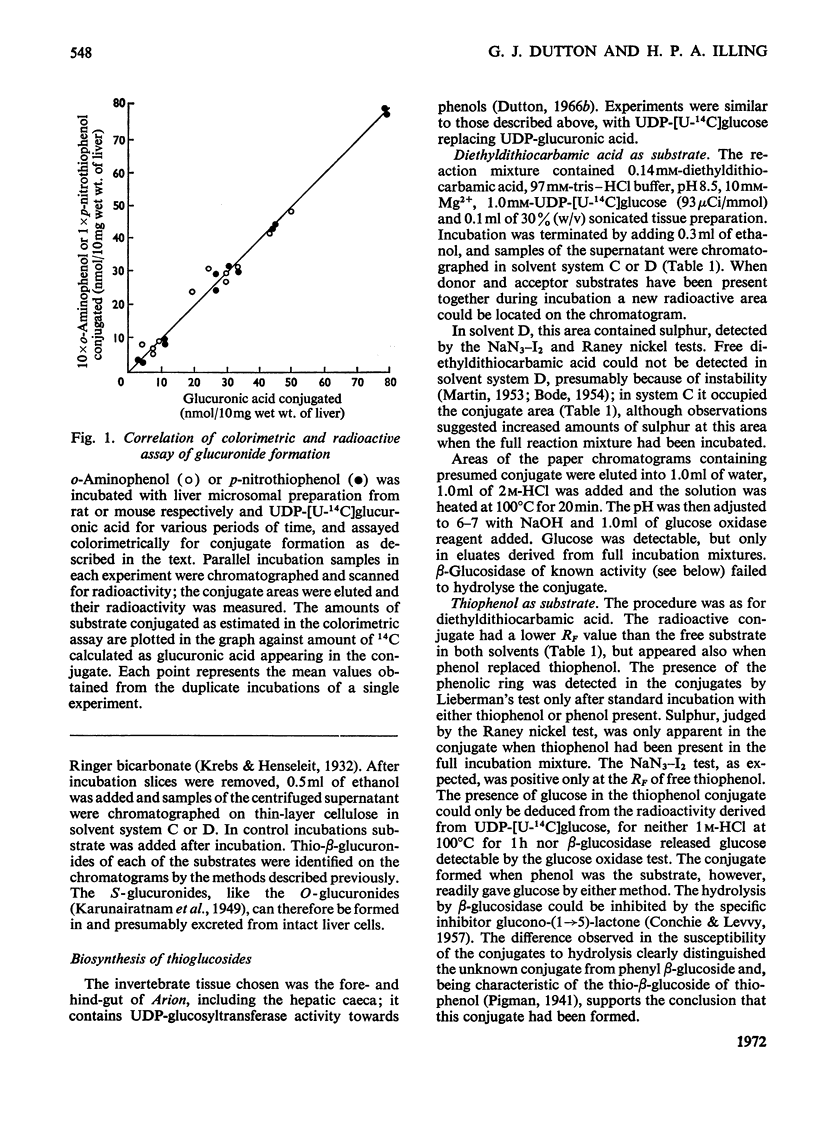
1. The thio-β-d-glucosiduronic acids (thio-β-glucuronides) of o-aminothiophenol, diethyldithiocarbamic acid, p-nitrothiophenol and thiophenol are formed biosynthetically in broken- and intact-cell preparations of mouse liver. 2. For this biosynthesis to occur in homogenates or microsomal fractions, UDP-glucuronic acid was required during incubation; glucose, glucuronic acid or UDP could not replace it. UDP was a product of the reaction. 3. The biosynthetic mechanism linking glucuronic acid to thiol and carbodithioic groups therefore requires UDP-glucuronyltransferase activity and resembles that forming the various types of O-glucuronides. 4. An analogous enzymic mechanism employing UDP-glucose synthesizes the thio-β-d-glucosides of diethyldithiocarbamic acid and thiophenol in gut preparations of the mollusc Arion ater; this mechanism resembles that forming the O-glucosides. The thio-β-d-glucosides are formed also in intact cells. 5. As expected from the distribution of O-glycosides, S-glucuronides of these aglycones were not detectable with the invertebrate, nor were the S-glucosides with the vertebrate. 6. Despite their similar biosyntheses, S- and O-β-glycosides differ in susceptibility to hydrolysis by β-glycosidases. Rat preputial-gland β-glucuronidase hydrolysed thioglucuronides of o-aminothiophenol, diethyldithiocarbamic acid and p-nitrothiophenol, hydrolysis being inhibited by glucarolactone; the thioglucuronide of thiophenol was not hydrolysed by preputial-gland or liver β-glucuronidase. The two S-glucosides resisted hydrolysis by β-glucosidase from almond emulsin.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLAPP J. W. A new metabolic pathway for a sulfonamide group. J Biol Chem. 1956 Nov;223(1):207–214. [PubMed] [Google Scholar]
- COLUCCI D. F., BUYSKE D. A. THE BIOTRANSFORMATION OF A SULFONAMIDE TO A MERCAPTAN AND TO MERCAPTURIC ACID AND GLUCURONIDE CONJUGATES. Biochem Pharmacol. 1965 Apr;14:457–466. doi: 10.1016/0006-2952(65)90218-2. [DOI] [PubMed] [Google Scholar]
- CONCHIE J., LEVVY G. A. Inhibition of glycosidases by aldonolactones of corresponding configuration. Biochem J. 1957 Feb;65(2):389–395. doi: 10.1042/bj0650389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton G. J. Uridine diphosphate glucose and the synthesis of phenolic glucosides by mollusks. Arch Biochem Biophys. 1966 Sep 26;116(1):399–405. doi: 10.1016/0003-9861(66)90046-4. [DOI] [PubMed] [Google Scholar]
- GOODMAN I., FOUTS J. R., BRESNICK E., MENEGAS R., HITCHINGS G. H. A mammalian thioglycosidase. Science. 1959 Aug 21;130(3373):450–451. doi: 10.1126/science.130.3373.450. [DOI] [PubMed] [Google Scholar]
- Gessner T., Jakubowski M. Diethyldithiocarbamic acid methyl ester. A metabolite of disulfiram. Biochem Pharmacol. 1972 Jan 15;21(2):219–230. doi: 10.1016/0006-2952(72)90272-9. [DOI] [PubMed] [Google Scholar]
- HANSEN H. J., GILES W. G., NADLER S. B. Metabolism of 9-ethyl-6-MP-S35 and 9-butyl-6-MP-S35 in humans. Proc Soc Exp Biol Med. 1963 May;113:163–165. doi: 10.3181/00379727-113-28307. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Hétu C., Gianetto R. Synthetic 1-thio- -D-glucosiduronic acids as substrates for rat-liver -glucuronidase. Can J Biochem. 1970 Jul;48(7):799–804. doi: 10.1139/o70-124. [DOI] [PubMed] [Google Scholar]
- KARUNAIRATNAM M. C., KERR L. M. H., LEVVY G. A. The glucuronide-synthesizing system in the mouse and its relationship to beta-glucuronidase. Biochem J. 1949;45(4):496–499. doi: 10.1042/bj0450496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASLANDER J. Formation of an S-glucuronide from tetraethylthiuram disulfide (Antabuse) in man. Biochim Biophys Acta. 1963 Jun 4;71:730–731. doi: 10.1016/0006-3002(63)91149-1. [DOI] [PubMed] [Google Scholar]
- KASLANDER J., KAARS SIJPESTEIJN A., van der KERK G. On the transformation of dimethyldithiocarbamate into its beta-glucoside by plant tissues. Biochim Biophys Acta. 1961 Sep 16;52:396–397. doi: 10.1016/0006-3002(61)90695-3. [DOI] [PubMed] [Google Scholar]
- LEVVY G. A., McALLAN A., MARSH C. A. Purification of beta-glucuronidase from the preputial gland of the female rat. Biochem J. 1958 May;69(1):22–27. doi: 10.1042/bj0690022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVVY G. A. The preparation and properties of beta-glucuronidase. IV. Inhibition by sugar acids and their lactones. Biochem J. 1952 Nov;52(3):464–472. doi: 10.1042/bj0520464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- RANDERATH K. A comparison between thin-layer chromatography and paper chromatography of nucleic acid derivatives. Biochem Biophys Res Commun. 1962 Jan 24;6:452–457. doi: 10.1016/0006-291x(62)90374-1. [DOI] [PubMed] [Google Scholar]
- STEVENSON I. H., DUTTON G. J. Glucuronide synthesis in kidney and gastrointestinal tract. Biochem J. 1962 Feb;82:330–340. doi: 10.1042/bj0820330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOEBER F. Sur la biosynthèse induite de la beta-glucuronidase chez Escherichia coli. C R Hebd Seances Acad Sci. 1957 Feb 11;244(7):950–952. [PubMed] [Google Scholar]
- STRUME J. H. METABOLISM OF DISULFIRAM AND DIETHYLDITHIOCARBAMATE IN RATS WITH DEMONSTRATION OF AN IN VIVO ETHANOL-INDUCED INHIBITION OF THE GLUCURONIC ACID CONJUGATION OF THE THIOL. Biochem Pharmacol. 1965 Apr;14:393–410. doi: 10.1016/0006-2952(65)90213-3. [DOI] [PubMed] [Google Scholar]
- Stromme J. H., Eldjarn L. Distribution and chemical forms of diethyldithiocarbamate and tetraethylthiuram disulphide (disculfiram) in mice in relation to radioprotection. Biochem Pharmacol. 1966 Mar;15(3):287–297. doi: 10.1016/0006-2952(66)90300-5. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- TRIVELLONI J. C. Biosynthesis of glucosides and glycogen in the locust. Arch Biochem Biophys. 1960 Jul;89:149–150. doi: 10.1016/0003-9861(60)90027-8. [DOI] [PubMed] [Google Scholar]
- YAMAHA T., CARDINI C. E. The biosynthesis of plant glycosides. I. Monoglucosides. Arch Biochem Biophys. 1960 Jan;86:127–132. doi: 10.1016/0003-9861(60)90379-9. [DOI] [PubMed] [Google Scholar]


