Abstract
Infection of the mouse trigeminal ganglia (TG) is the most commonly used model for the study of herpes simplex virus type 1 (HSV-1) latency. Its popularity is caused, at least in part, by the perception that latent infection can be studied in this system in the absence of spontaneous viral reactivation. However, this perception has never been rigorously tested. To carefully study this issue, the eyes of Swiss–Webster mice were inoculated with HSV-1 (KOS), and 37–47 days later the TG were dissected, serial-sectioned, and probed for HSV-1 ICP4, thymidine kinase, glycoprotein C, and latency-associated transcript RNA by in situ hybridization. Serial sections of additional latently infected TG were probed with HSV-1-specific polyclonal antisera. Analysis of thousands of probed sections revealed abundant expression of viral transcripts, viral protein, and viral DNA replication in about 1 neuron per 10 TG tested. These same neurons were surrounded by a focal white cell infiltrate, indicating the presence of an antigenic stimulus. We conclude that productive cycle viral genes are abundantly expressed in rare neurons of latently infected murine TG and that these events are promptly recognized by an active local immune response. In the absence of detectable infectious virus in these ganglia, we propose the term “spontaneous molecular reactivation” to describe this ongoing process.
Herpes simplex virus type 1 (HSV-1) infections of the skin or eye lead to invasion of the trigeminal ganglia (TG), where the virus follows one of two pathways (1–3). In some infected neurons, the virus replicates and destroys the cell. This lytic cycle path involves regulated viral gene expression in which immediate-early genes are required for the efficient expression of early and late genes. In other neurons, a latent infection is established in which lytic cycle genes are not expressed and infectious virus is not produced. Latent infection of neurons is characterized by the expression of the latency-associated transcripts (LAT), which are encoded in a single region of the HSV-1 genome and which accumulate to high levels in the nucleus of the host cell (4–10). In situ hybridization (ISH) studies of latently infected sensory ganglia have revealed numerous neurons expressing LAT but no other viral RNAs (4, 5, 7, 9–15). Thus, for many years a latent neuron has been defined in molecular terms as a neuron that is positive for LAT and negative for other viral RNAs.
In humans, HSV-1 intermittently reactivates from the latent state, with peripheral shedding of infectious virus. Shedding of infectious virus is similarly observed in rabbit ocular models of HSV infection, both spontaneously and in response to local or systemic stimuli (16, 17). In contrast, latent HSV-1 infection in the mouse seems to be more tightly regulated, and attempts to identify infectious virus or viral antigen in murine sensory ganglia after resolution of primary infection (indicative of a spontaneous reactivation localized to the sensory ganglion) have been unsuccessful (2, 7, 9, 11, 18–26). These findings have led many investigators to use the mouse model to study the latent state and to assume that features of viral gene expression in this model are attributable to latency and not to spontaneous reactivation. However, several observations suggest that low-level expression of productive cycle viral genes may occur in murine TG latently infected with HSV-1. First, cytokine expression and focal inflammatory infiltrates persist in these tissues (27–30). Second, treatment with acyclovir reduces the level of cytokine expression in these tissues (31). Third, through the use of reverse transcriptase–PCR, Kramer and Coen (32) found transcripts of the HSV-1 ICP4 and thymidine kinase (TK) genes in latently infected TG. The ICP4 gene product is required for efficient synthesis of early and late viral RNA, and the viral TK gene is a typical early gene whose transcription is induced by the ICP4 protein. Two divergent models of HSV-1 gene expression may explain these results. Either many latently infected neurons express very low levels of viral RNAs other than LAT, or rare neurons express high levels of lytic cycle RNAs. It is also possible that elements of both models are correct, i.e., that many latently infected neurons express very low levels of lytic cycle genes, but a few rare neurons express high levels of these same genes.
In the present study, we examine the possibility that rare neurons in latently infected sensory ganglia express abundant lytic cycle mRNAs. Using probes for HSV-1 ICP4, TK, glycoprotein C (gC), and LAT RNA, we performed ISH on thousands of sections of latently infected sensory ganglia, looking for the proverbial needle in a haystack. By using alternate sections, we uncovered evidence for rare neurons expressing high levels of ICP4, TK, and gC mRNAs. In rare neurons, we also found evidence of viral DNA replication and protein synthesis. Finally, we observed inflammatory cells surrounding each of the neurons in which we detected expression of lytic cycle genes, indicating a strong immune response to these viral events.
Materials and Methods
Preparation of Virus.
HSV1 strain KOS was propagated in rabbit skin cells grown in Eagle's minimal essential media with 5% FCS/100 units/ml penicillin/100 μg/ml streptomycin. Viral titers of 1 × 108 plaque-forming units/ml were typically obtained.
Animals, Inoculations, and Serial Sectioning of Tissue.
Six-week-old female Swiss–Webster mice (Simonsen Laboratories, Gilroy, CA) were anesthetized with 1.5% pentobarbital and inoculated binocularly with 1.5 × 106 plaque-forming units of HSV. The corneas were scratched before inoculation. Thirty-seven to 47 days postinoculation, mice were euthanized by CO2 inhalation and perfused with 0.1 M phosphate buffer with 0.9% NaCl followed by perfusion with either 4% paraformaldehyde (for immunocytochemistry) or 10% buffered Formalin phosphate (for ISH). TG were removed, postfixed, equilibrated in 20% sucrose overnight, batched in groups of 8 or 10, embedded in OCT compound (Miles), and snap-frozen in liquid nitrogen. Serial sections (6 μm) through each frozen block of tissue were collected onto Superfrost Plus slides (Fisher Scientific) and stored at −80°C. This protocol was approved by the University of California, San Francisco committee on animal research.
Preparation of Riboprobes.
Riboprobes were generated from a 282-bp AvaII–SacI fragment of the HSV-1 TK gene, a 225-bp fragment of the HSV-1 ICP4 (IE3) gene (nucleotide positions 131, 108–131, 332), a 233-bp fragment of the HSV-1 gC gene (nucleotide positions 88, 438–88, 670), and a 347-bp fragment of the stable LAT intron (nucleotides 119, 628–119, 974). Briefly, these fragments were cloned into plasmid pBluescript II KS− (Stratagene) and linearized to generate DNA templates. The DNA templates were purified with Wizard DNA clean-up columns (Promega), and the concentrations were determined by spectrophotometry. Radiolabeled riboprobes were generated from a 1-μg template DNA by using the riboprobe combination system (Promega) and 35S-UTP. Riboprobes were purified from unincorporated nucleotides with Sephadex G-50 quick spin columns (Roche Molecular Biochemicals) and stored at −80°C.
ISH.
ISH was carried out as described (33). Briefly, tissue sections were first treated with either DNase or RNase. DNase pretreatment of tissue sections was carried out by using a 20 mM Tris buffer (pH 7.4) containing 10 mM MgCl2/40 units/100 μl RNase free DNase (Promega) for 1 h at 37°C followed by extensive washing with 2× SSC. RNase pretreatment of tissue sections was carried out with a 2× SSC solution containing 5% RNase mixture (Ambion, Austin, TX) for 30 min at 37°C followed by extensive washing with 2× SSC. Tissue sections were subsequently fixed in 10% buffered formalin phosphate for 30 min and treated with 0.1 mg/ml proteinase K (30 min) and 0.25% acetic anhydride in 0.1 M triethanolamine (3 min). Riboprobes were diluted to 1 × 107 cpm/ml in hybridization buffer [62.5% formamide/375 mM NaCl/12.5 mM Tris⋅HCl, pH 8.0/EDTA, pH 8.0/1.25× Denhardt's solution, 12.5% (wt/vol) dextran sulfate], applied to tissue sections, and hybridized for 18–20 h at 60°C. After hybridization, sections were treated with 20 mg/ml RNase A (Sigma) and washed four times through 0.1× solutions of sodium chloride-sodium citrate. After ethanol dehydration, the sections were dipped in NTB3 emulsion (Eastman Kodak) and exposed for 3–9 days at 4°C. Sections were developed in Kodak D-19, washed, fixed in Kodak Rapid Fixer, and counterstained with hematoxylin and eosin.
Immunohistochemistry.
Tissue sections were incubated in 3% normal goat serum in PBS for 30 min followed by a 1-h incubation period in rabbit anti-HSV-1 antisera (Accurate Chemicals) diluted 1:300 in 1% normal goat serum/PBS. Sections were washed with 1% normal goat serum/PBS, incubated for 1 h in biotinylated goat anti-rabbit antisera (Vector Laboratories) diluted 1:200 in 1% normal goat serum/PBS, washed, treated with 3% H2O2 for 30 min, incubated with ABC Elite solution (Vector), and reacted with diaminobenzidine (Pierce). Labeled tissue was subsequently washed and counterstained with hematoxylin.
Results
Thousands of Mouse Neurons in Latently Infected Tissue Express Only LAT RNA.
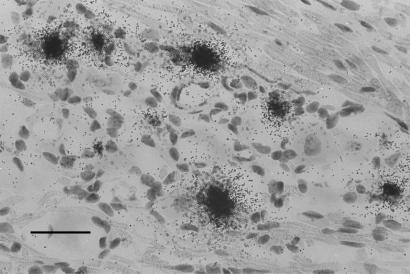
To begin the investigation of viral gene expression in latent neurons, TG of five mice were infected by ocular inoculation with HSV-1 strain KOS. At 37 days postinfection, well into the latent state, ganglia were removed, sectioned in a serial fashion, and assayed by ISH for LAT (every fourth section) and ICP4 transcripts (all remaining sections). Casual review of the latently infected tissue revealed numerous neurons that expressed LAT (Fig. 1) but none that expressed ICP4. This result was expected based on the previous work of others (4–10). A more careful review of the sections probed for LAT revealed 5,276 positive neurons, or about 500 LAT-positive neurons per latently infected ganglion. This finding may be a minimal estimate of the total number of latent sites, as there is evidence that there are TG neurons that harbor latent HSV but express LAT below the limits of in situ detection (34).
Figure 1.
ISH for LAT in the murine TG 37 days after ocular inoculation with KOS strain HSV-1. Note the presence of numerous LAT-positive neurons. (Bar = 40 μm.)
Rare Neurons in Latently Infected Tissue Express ICP4 mRNA.
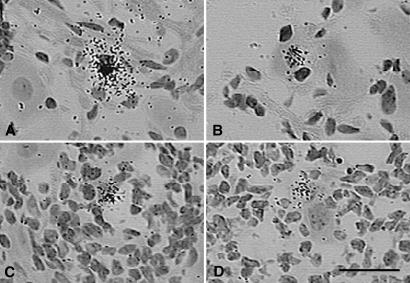
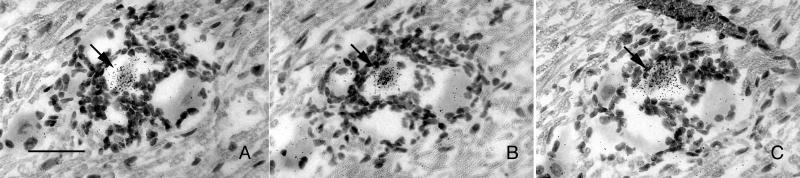
A comprehensive review of all of the tissue probed by ISH in the above experiment revealed evidence of expression of the ICP4 gene in rare neuronal and non-neuronal cells of latently infected TG (≈10 positives per block of 10 ganglia). Examples of these ICP4-positive cells are presented in Fig. 2. Note that although silver grains were tightly clustered over these cells, for most of them (≈90%) we were unable to verify ICP4 expression in a consecutive tissue section, primarily because of technical difficulties in identifying the same cell in serial frozen sections. However, we were able to identify about one neuron in each tissue block of 10 ganglia (≈440 sections) in which ICP4 transcripts were found in consecutive tissue sections (Fig. 3). As judged by the intensity of the ISH signal, the level of ICP4 transcription in these neurons was similar to that in control tissue sections of TG productively infected with HSV-1. Further evidence for the expression of productive cycle genes in these rare neurons was the presence of a focal infiltrate of inflammatory cells.
Figure 2.
ISH for HSV-1 ICP4 mRNA in the murine TG 37 days after ocular inoculation with KOS strain HSV-1. Examples of neuronal cell bodies with overlying tightly clustered silver grains where we were unable to verify ICP4 expression in consecutive tissue sections are shown. Note the infiltrating white blood cells in C and D. (Bar = 50 μm.)
Figure 3.
ISH for HSV-1 ICP4 mRNA in three consecutive frozen tissue sections of the murine TG 37 days after ocular infection with KOS strain HSV-1. Labeling for ICP4 is noted in the same poorly preserved neuronal cell body (arrows) in three consecutive sections. Note the focal infiltrate of white cells. (Bar = 40 μm.)
Rare Neurons in Latently Infected Tissue Express TK mRNA.
To determine whether mRNAs other than ICP4 were produced during HSV latent infection, a second group of mice was infected, and latently infected ganglia were pooled, serial sectioned, and hybridized to probes for TK and LAT. As in the first experiment, the majority of neurons that were positive by ISH expressed only LAT and not TK RNA (data not shown). However, a comprehensive review of all probed tissue sections revealed evidence of expression of the TK gene in rare neuronal and non-neuronal cells (≈10 positives per tissue block), although difficulty in identifying the same cell in adjacent tissue sections prevented us from verifying TK expression in most (≈90%) of these cells. However, we were able to identify a single neuron in each tissue block of 10 ganglia that was positive for HSV TK expression in consecutive tissue sections (Fig. 4). This neuron was also positive for LAT and was surrounded by an inflammatory infiltrate. These results demonstrate that not only are transcripts from an HSV immediate early gene, ICP4, expressed in a few rare neurons of latently infected ganglia, but that transcripts from TK, an HSV early gene, are expressed as well. The presence of inflammatory cells further suggests that these transcripts were translated into viral proteins. Finally, the abundant LAT in TK-positive neurons strongly suggests that they were or had been latent sites of infection (1, 3).
Figure 4.
ISH for TK mRNA (A and C) and LAT (B) in three consecutive frozen tissue sections of the murine TG 37 days after ocular infection with KOS strain HSV-1. Note that the same neuron is positive for TK mRNA and LAT in consecutive tissue sections (arrows). Note the focal infiltrate of white cells. (Bar = 50 μm.)
Rare Neurons in Latently Infected Tissue Express Both ICP4 and TK mRNA.
Because TK viral gene expression is induced by action of the ICP4 protein, it is reasonable to assume that a cell that expresses TK mRNA also expresses the ICP4 mRNA. Furthermore, the presence of both ICP4 mRNA and TK mRNA within the same neuron would lend credence to the biological significance of the transcripts. In the next experiment, we tried to confirm the findings of the previous two experiments to show that a neuron that is expressing TK transcripts is also expressing ICP4 transcripts. In this study, five mice were infected for 46 days. TG were removed and sectioned. Alternate sections were then hybridized with probes for ICP4 and TK. Once again, by careful review of the probed tissue, we were able to identify a single neuron in this block of 10 latently infected ganglia in which lytic cycle genes were expressed, this time with evidence of both ICP4 and TK transcripts in the same neuron (Fig. 5).
Figure 5.
ISH for TK (A and C) and ICP4 (B) mRNA in three consecutive frozen tissue sections of the murine TG 46 days after ocular infection with KOS strain HSV-1. Note that the same neuron labels for both TK and ICP4 mRNA (arrows). (Bar = 50 μm.)
Rare Neurons in Latently Infected Tissue Express gC mRNA.
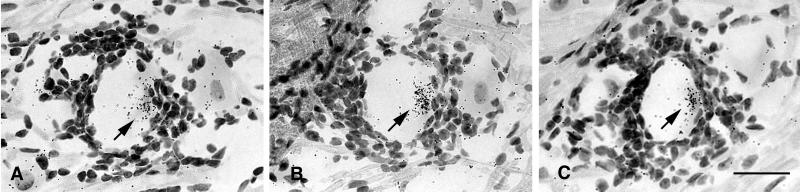
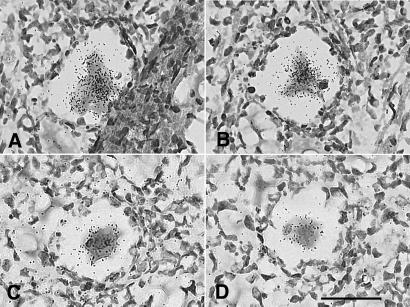
We next sought evidence of HSV-1 late gene expression in sensory ganglia latently infected with HSV-1. To accomplish this, serial sections of a block of eight latently infected TG were hybridized with probes for gC mRNA. In this block of tissue, we identified two neurons in which the HSV-1 late gene gC was expressed. The presence of gC transcripts in these two neurons was confirmed by ISH of adjacent tissue sections (Fig. 6). As judged by the intensity of the ISH signal, the level of gC transcription in these neurons was similar to that in control tissue sections of TG productively infected with HSV-1. A focal inflammatory infiltrate was associated with each of the neurons expressing gC mRNA.
Figure 6.
ISH for gC mRNA in tissue sections of murine TG 47 days after ocular infection with KOS strain HSV-1. Adjacent tissue sections of a gC-positive neuron are presented in A and B, and adjacent sections of a second positive neuron are presented in C and D. (Bar = 40 μm.)
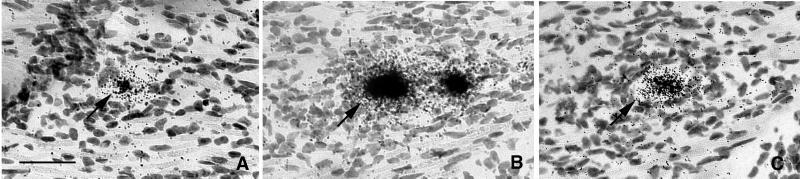
Rare Neurons in Latently Infected Tissue Exhibit High Levels of Viral DNA.
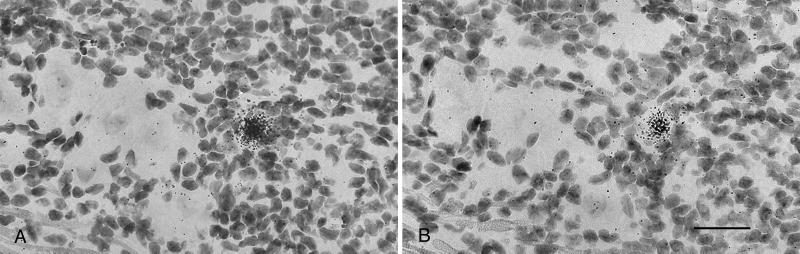
In view of the abundant expression of ICP4 and TK mRNA in rare neurons of latently infected TG, we next sought evidence of viral DNA replication in rare neurons of latently infected TG. Once again, the TG from latently infected mice were removed, sectioned, and hybridized with riboprobes for ICP4 and TK. However, unlike the studies described above in which all tissue sections were treated with DNase before hybridization, in this experiment all of the tissue sections were pretreated with RNase. Careful review of the probed tissue revealed a single neuron in the block of 10 latently infected ganglia that hybridized strongly with both the ICP4 and TK riboprobes in adjacent sections (Fig. 7), indicating the presence of abundant viral DNA in this neuron. This pattern of neuronal labeling was very similar to that in RNase-treated tissue sections of TG productively infected with HSV-1. Because ISH has not previously been shown to be effective at detecting latent HSV DNA, we interpret these results as indicating the presence of replicating viral DNA in rare neurons of latently infected mouse TG. Additional support for this conclusion comes from the observation that the labeled neuron in this experiment was surrounded by an inflammatory infiltrate and that in experiments described above we found late viral gene expression (gC) in rare neurons of latently infected TG. However, an alternative interpretation is that this cell represents a latently infected neuron with very high genome copy number.
Figure 7.
ISH for HSV-1 DNA in murine TG latently infected with KOS strain HSV-1. Tissue sections were treated with RNase, and then consecutive sections were probed with TK-specific (A) or ICP4-specific (B) riboprobes. (Bar = 40 μm.)
Rare Neurons in Latently Infected Tissue Express Viral Antigens.
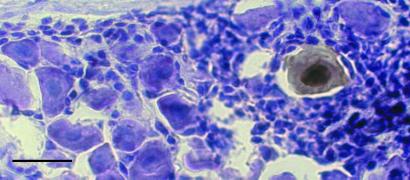
To obtain direct evidence for the synthesis of viral proteins in rare neurons of latently infected murine TG, we next assayed sections of latently infected tissue by immunohistochemistry. By using HSV-1-specific polyclonal antisera, we found one antigen-positive neuron, confirmed in adjacent sections, in each block of 10 latently infected TG that we studied (Fig. 8). Furthermore, a focal inflammatory infiltrate was associated with each antigen-positive neuron. To confirm that this result was not mouse strain specific, we repeated this experiment with BALB/c mice. As expected, we also found rare neurons that stained for HSV-1 antigens in latently infected TG of BALB/c mice.
Figure 8.
Immunohistochemistry of HSV-1 antigen-positive neuron in a murine TG latently infected with KOS strain HSV-1. Note the presence of reaction product throughout the nucleus and cytoplasm. The expression of antigen in this neuron was confirmed in adjacent tissue sections. (Bar = 40 μm.)
These results confirm and extend our ISH studies, making it highly unlikely that either set of observations (ISH or immunohistochemistry) is an artifact. Even the frequency with which we found HSV antigen-positive neurons in latently infected TG was similar to that observed for neurons expressing lytic cycle viral mRNAs.
Discussion
Most primary sensory neurons that harbor latent HSV-1 DNA are in a relatively quiescent state. For many years investigators have observed that these neurons express only LAT RNA and no viral antigens. Our findings demonstrate that HSV-1 latency in mice is not so simple. Through the use of ISH and immunohistochemistry, we have demonstrated that there are rare neurons in latently infected murine sensory ganglia that express a high level of HSV-1 lytic cycle transcripts, viral DNA, and protein. Furthermore, these neurons are surrounded by an inflammatory cell infiltrate, implying recognition of these events by the murine immune system. These results suggest that the low-level expression of ICP4, ICP27, and TK transcripts detected in latently infected ganglia by reverse transcriptase–PCR (32, 35, 36) can be accounted for, at least in part, by rare neurons expressing high levels of productive cycle mRNAs. However, this finding does not rule out the possibility that additional neurons in latently infected ganglia express viral IE and E genes below the sensitivity of our in situ assays.
In the course of our work, we consistently observed about one neuron expressing high levels of viral mRNA, DNA, or protein per 5,000 latent sites. We find this event to be surprisingly common, as it represents about one neuron in every group of five mice (10 TG) studied, or about one neuron expressing high-level productive cycle viral genes in each ganglion every 10 days. The combination of productive cycle gene expression and the presence of inflammatory cells raises important questions about the fate of these neurons. If these neurons do not survive, then the reservoir of latently infected neurons should decrease over time. However, all evidence to date demonstrates that this decrease does not occur (37, 38). This finding suggests that either the latent reserve is replenished by means of subsequent latent infection of neurons adjacent to the rare neurons that express viral transcripts or changes in viral load are so small that it is unlikely that they would be detected by the methods that have been used. Assuming loss of one reactivating neuron per 5,000 latent sites per day, the latent viral load would only decrease by about 15% over a 2-year period.
Although we have no direct evidence that the neurons observed in our study represent rare sites of spontaneous viral reactivation, we believe this explanation of our data to be the most likely. First, these neurons were LAT positive, suggesting that they had been latently infected. Second, these neurons expressed immediate early, early, and late viral transcripts at levels equivalent to that observed in control sections of productively infected ganglionic neurons. Third, these neurons contained viral DNA detectable by ISH, whereas latently infected neurons do not contain viral DNA in a state or amount that is detectable by this technique. Fourth, these neurons expressed enough viral protein to be detected by both immunocytochemistry and the host immune system. However, other possible explanations are worth considering. For instance, these neurons might represent abortive attempts at viral reactivation, attempts that were suppressed by late checkpoints or the immune system before infectious virus was produced. Alternatively, these neurons might represent limited foci of persistent productive infection, rather than true latent infection. However, it seems unlikely that persistently infected neurons expressing high levels of viral protein could survive for months, especially while facing an aggressive immune response.
Persistent cytokine expression and inflammatory infiltrates have been repeatedly described in murine TG latently infected with HSV-1, and it has been hypothesized that this may be a consequence of ongoing low-level spontaneous viral reactivation (27–31). However, recent studies of latent infection with a reactivation-deficient TK deletion virus (dlstpk) suggest that cytokine expression can be maintained in sensory ganglia in the absence of propagation of infectious virus, albeit at significantly lower levels than KOS-infected controls (38). In the current study, we observed mostly focal, rather than diffuse, inflammatory infiltrates in latently infected ganglia. Furthermore, all neurons with in situ evidence of productive cycle viral gene expression were surrounded by these infiltrates. In contrast, most LAT-positive neurons were free of associated inflammatory cells (see Fig. 1). Thus, whereas low-level expression of IE and E viral genes (detectable by reverse transcriptase–PCR but not ISH) may be responsible for some element of the persistent immune response in latently infected ganglia, our data suggest that at least a portion of the chronic immune response in latently infected ganglia is generated by rare neurons expressing high levels of productive cycle viral genes.
The results of our work with HSV-1 may have important implications for the study of latent infection with other infectious agents such as cytomegalovirus, Epstein–Barr virus, human herpesvirus 8, and even HIV. Latent tissue burden with these viruses is often estimated by using quantitative PCR, an approach that assumes that none of the infected cells in the tissue being assayed harbors reactivating virus. A similar problem exists in using highly sensitive techniques such as reverse transcriptase–PCR and oligonucleotide arrays to analyze latent viral gene expression in a heterogeneous tissue with thousands of infected cells. Under these circumstances, moderate expression of a single viral gene in just a handful of cells is easily misinterpreted as low-level expression in all infected cells. Our discovery of rare neurons that express HSV-1 lytic cycle transcripts in latently infected murine ganglia illustrates our relatively poor understanding of the biology of latent HSV infection and highlights the importance of using caution in interpreting the results of PCR-based studies of latent viral infection.
It is interesting that productive cycle viral genes are also present, in the absence of infectious virus, in sensory ganglia latently infected with Varicella Zoster virus (39–42). This finding implies that HSV and Varicella Zoster virus latency may be more similar than previously appreciated and that like Varicella Zoster virus latent infection, late checkpoints also may play a role in the transcription of HSV lytic cycle genes that prevent spontaneous reactivations of HSV from becoming symptomatic.
Acknowledgments
This work was supported by National Institutes of Health Grants EY10008 and EY02162 (to T.P.M) and AI45679 (to L.T.F.). T.P.M. is also supported by a Research to Prevent Blindness (New York) Lew Wasserman Award.
Abbreviations
- TG
trigeminal ganglia
- HSV-1
herpes simplex virus type 1
- LAT
latency-associated transcript
- TK
thymidine kinase
- gC
glycoprotein C
- ISH
in situ hybridization
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Margolis T P, Sedarati F, Dobson A T, Feldman L T, Stevens J G. Virology. 1992;189:150–160. doi: 10.1016/0042-6822(92)90690-q. [DOI] [PubMed] [Google Scholar]
- 2.Speck P G, Simmons A. J Virol. 1991;65:4001–4005. doi: 10.1128/jvi.65.8.4001-4005.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speck P G, Simmons A. J Gen Virol. 1992;73:1281–1285. doi: 10.1099/0022-1317-73-5-1281. [DOI] [PubMed] [Google Scholar]
- 4.Deatly A M, Spivack J G, Lavi E, Fraser N W. Proc Natl Acad Sci USA. 1987;84:3204–3208. doi: 10.1073/pnas.84.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens J G, Wagner E K, Devi-Rao G B, Cook M L, Feldman L T. Science. 1987;235:1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- 6.Croen K D, Ostrove J M, Dragovic L J, Smialek J E, Straus S E. N Engl J Med. 1987;317:1427–1431. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- 7.Deatly A M, Spivack J G, Lavi E, O'Boyle D R, Fraser N W. J Virol. 1988;62:749–756. doi: 10.1128/jvi.62.3.749-756.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rock D L, Nesburn A B, Ghiasi H, Ong J, Lewis T L, Lokensgard J R, Wechsler S L. J Virol. 1987;61:3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spivack J G, Fraser N W. J Virol. 1987;61:3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner E K, Devi-Rao G B, Feldman L T, Dobson A T, Zhang Y F, Flanagan W M, Stevens J G. J Virol. 1988;62:1194–1202. doi: 10.1128/jvi.62.4.1194-1202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devi-Rao G, Bloom D C, Stevens J G, Wagner E K. J Virol. 1994;68:1271–1282. doi: 10.1128/jvi.68.3.1271-1282.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ecob-Prince M S, Rixon F J, Preston C M, Hassan K, Kennedy P G E. J Gen Virol. 1993;74:995–1002. doi: 10.1099/0022-1317-74-6-995. [DOI] [PubMed] [Google Scholar]
- 13.Puga A, Notkins A L. J Virol. 1987;61:1700–1703. doi: 10.1128/jvi.61.5.1700-1703.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puga A, Rosenthal J D, Openshaw H, Notkins A L. Virology. 1987;89:102–111. doi: 10.1016/0042-6822(78)90044-2. [DOI] [PubMed] [Google Scholar]
- 15.Vali-Nagy T, Deshmane S, Dillner A, Fraser N W. J Virol. 1991;65:4142–4152. doi: 10.1128/jvi.65.8.4142-4152.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laibson P R, Kibrick S. Invest Ophthalmol. 1969;8:346–350. [PubMed] [Google Scholar]
- 17.Hill J M, Dudley J B, Shimomura Y, Kaufman H. Curr Eye Res. 1986;5:241–246. doi: 10.3109/02713688609020049. [DOI] [PubMed] [Google Scholar]
- 18.Cook M L, Bastone V B, Stevens J G. Infect Immun. 1974;9:946–951. doi: 10.1128/iai.9.5.946-951.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knotts F B, Cook M L, Stevens J G. J Infect Dis. 1974;130:16–27. doi: 10.1093/infdis/130.1.16. [DOI] [PubMed] [Google Scholar]
- 20.Laycock K A, Lee S F, Brady R H, Pepose J S. Invest Ophthalmol Visual Sci. 1991;32:2741–2746. [PubMed] [Google Scholar]
- 21.Leib D A, Coen D M, Bogard C L, Hicks K A, Yager D R, Knipe D M, Tyler K L, Schaffer P A. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell W J, Gressens P, Martin J P, DeSanto R. J Gen Virol. 1994;75:1201–1210. doi: 10.1099/0022-1317-75-6-1201. [DOI] [PubMed] [Google Scholar]
- 23.Pepose J S, Foos R Y, Stevens J G. Graefe's Arch Clin Exp Ophthalmol. 1986;224:341–345. doi: 10.1007/BF02150027. [DOI] [PubMed] [Google Scholar]
- 24.Sawtell N M, Thompson R L. J Virol. 1992;66:2150–2156. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steiner I, Mador N, Reibstein I, Spivack J G, Fraser N W. Neuropathol Appl Neurobiol. 1994;20:253–260. doi: 10.1111/j.1365-2990.1994.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 26.Stevens J G, Cook M L. Science. 1971;173:843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- 27.Cantin E M, Hinton D R, Chen J, Openshaw H. J Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimeld C, Whiteland J L, McNicholls S M, Grinfeld E, Easty D L, Gao H, Hill T J. J Neuroimmunol. 1995;61:7–16. doi: 10.1016/0165-5728(95)00068-d. [DOI] [PubMed] [Google Scholar]
- 29.Liu T, Tang Q, Hendricks R L. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halford W P, Gebhardt B M, Carr D J. J Immunol. 1996;157:3542–3549. [PubMed] [Google Scholar]
- 31.Halford W P, Gephardt B M, Carr D J. Virology. 1997;238:53–63. doi: 10.1006/viro.1997.8806. [DOI] [PubMed] [Google Scholar]
- 32.Kramer M F, Coen D M. J Virol. 1995;69:1389–1399. doi: 10.1128/jvi.69.3.1389-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunningham E T, Jr, De Souza E B. In: Methods in Neuroscience: Neurobiology of Cytokines, Part A. De Souza E B, editor. New York: CRC; 1994. pp. 112–127. [Google Scholar]
- 34.Ramakrishnan R, Levine M, Glorioso J G. J Virol. 1994;68:7083–7091. doi: 10.1128/jvi.68.11.7083-7091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tal-Singer R, Lasner T M, Podrzucki W, Skokotas A, Leary J J, Berger S L, Fraser N W. J Virol. 1997;71:5268–5276. doi: 10.1128/jvi.71.7.5268-5276.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen S, Kramer M F, Schaffer P A, Coen D M. J Virol. 1997;71:5878–5884. doi: 10.1128/jvi.71.8.5878-5884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sedarati F, Izumi K M, Wagner E K, Stevens J G. J Virol. 1989;63:4455–4458. doi: 10.1128/jvi.63.10.4455-4458.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen S-H, Garber D, Schaffer P A, Knipe D M, Coen D M. Virology. 2000;278:207–216. doi: 10.1006/viro.2000.0643. [DOI] [PubMed] [Google Scholar]
- 39.Mahalngham R, Wellish M, Cohrs R, Debrus S, Piette J, Rentier B, Gilden D H. Proc Natl Acad Sci USA. 1996;93:2122–2124. doi: 10.1073/pnas.93.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lungu O, Panagiotidis C A, Annunziato P W, Gershon A A, Silverstein S J. Proc Natl Acad Sci USA. 1998;95:7080–7085. doi: 10.1073/pnas.95.12.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meier J L, Holman R P, Croen K D, Smialek J E, Straus S E. Virology. 1993;193:193–200. doi: 10.1006/viro.1993.1115. [DOI] [PubMed] [Google Scholar]
- 42.Cohrs R J, Barbour M, Gilden D H. J Virol. 1996;70:2789–2796. doi: 10.1128/jvi.70.5.2789-2796.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]