Abstract
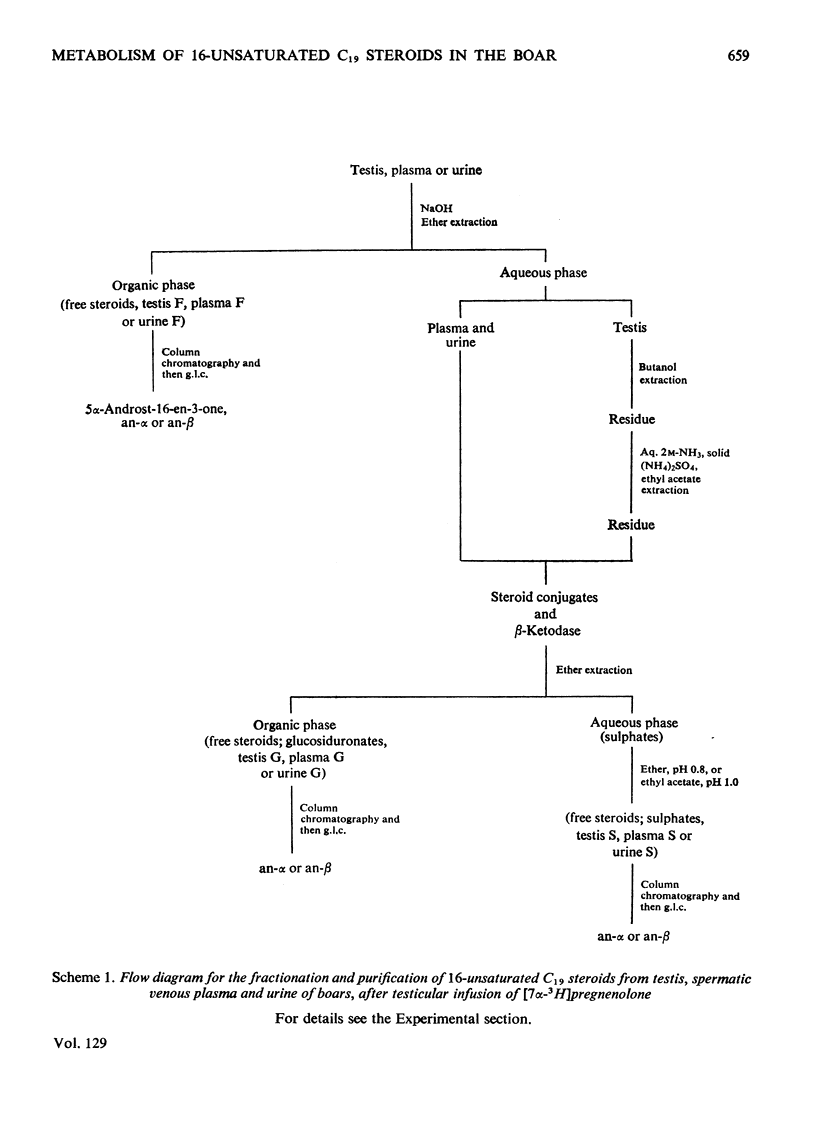
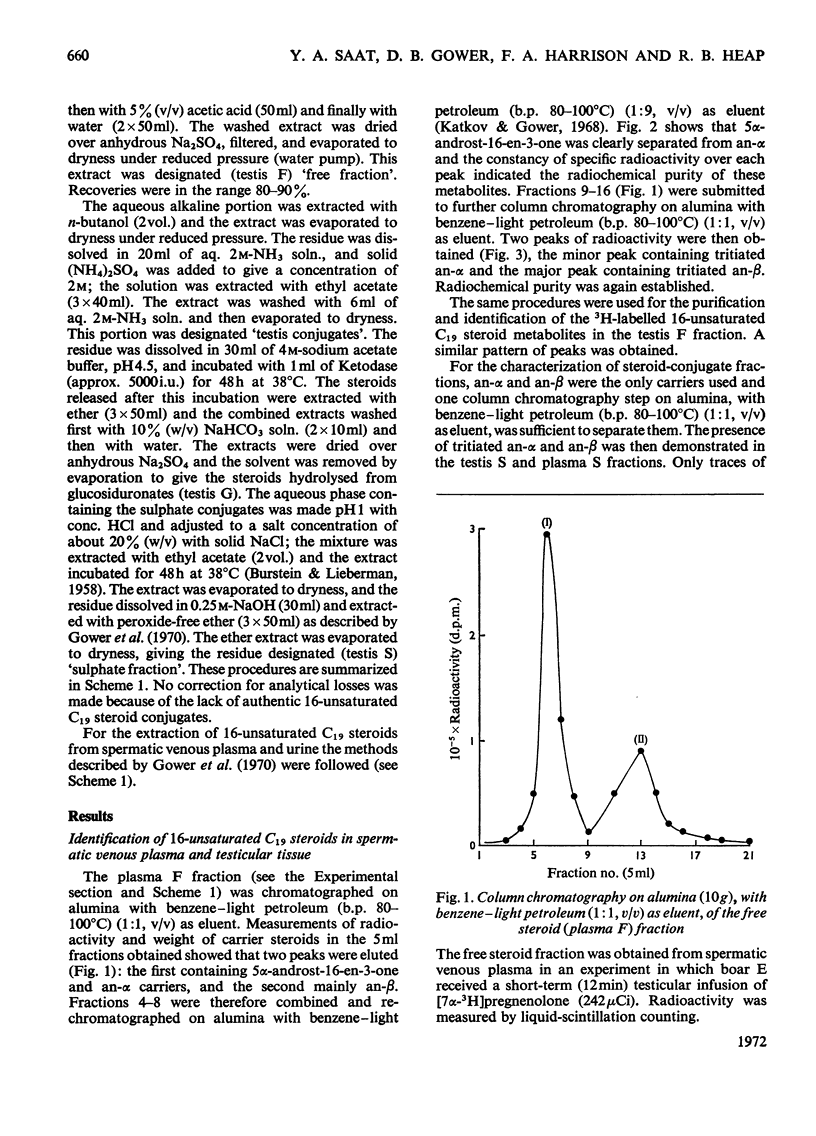
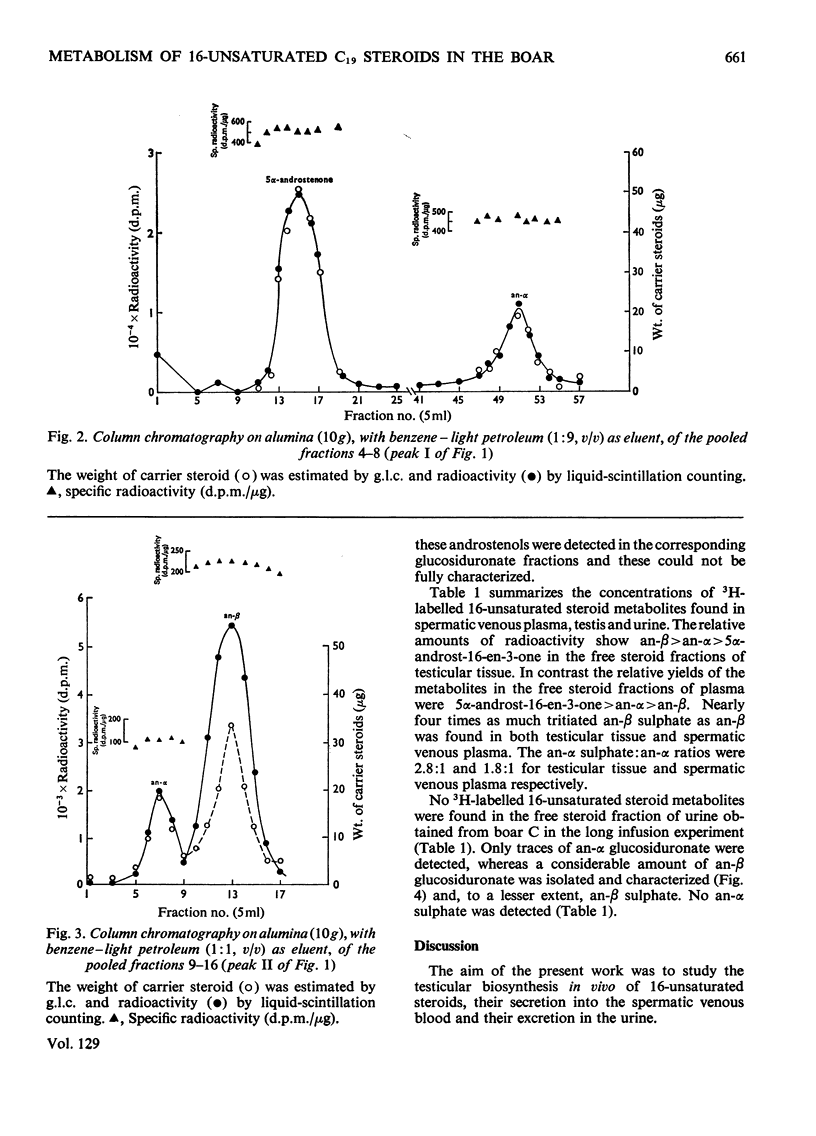
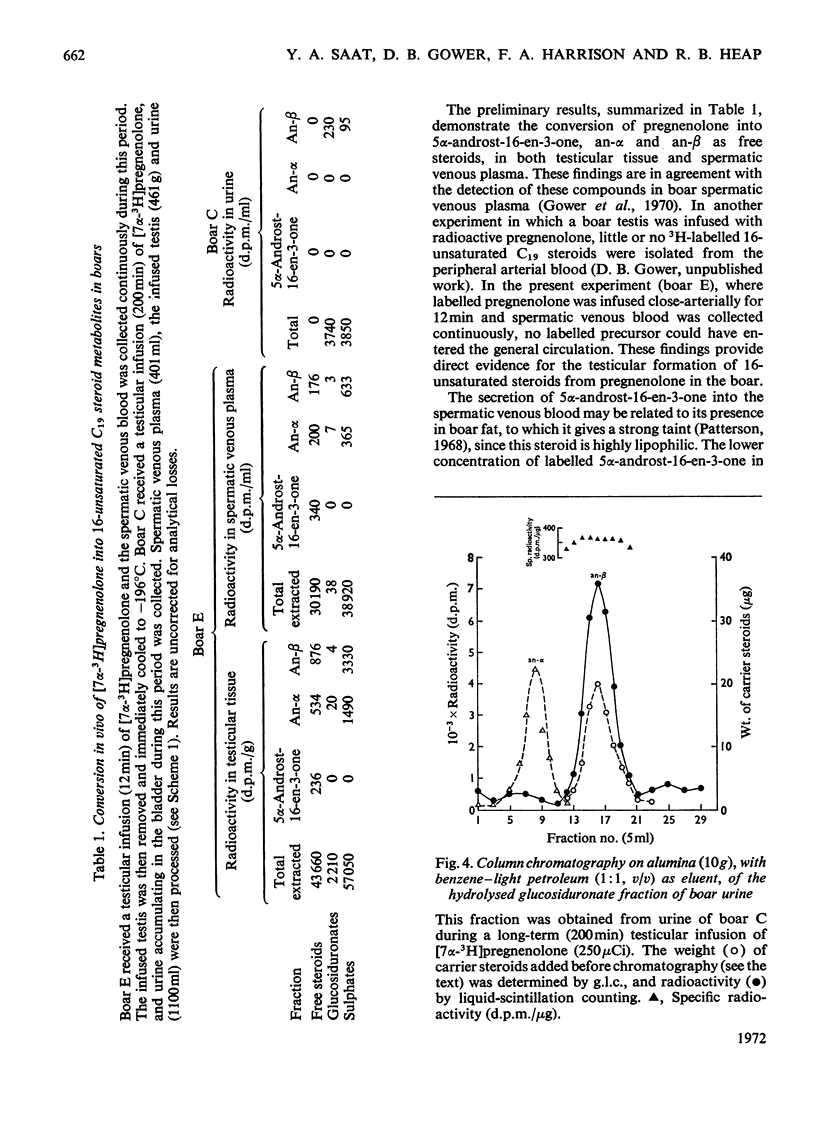
1. In one experiment [7α-3H]pregnenolone was infused continuously for 12min into the left spermatic artery of a sexually mature boar and blood was collected during this period by continuous drainage from the spermatic vein. After infusion, the testis was removed and immediately cooled to −196°C. 2. From both the testicular tissue and the spermatic venous plasma, 3H-labelled 16-unsaturated C19 steroids were isolated and characterized and their radiochemical purity was established. 5α-Androst-16-en-3α- and 3β-ol occurred mainly as sulphate conjugates and to a lesser extent as free steroids. Only traces of these alcohols occurred as glucosiduronate conjugates. 5α-Androst-16-en-3-one was found in the free (ether-extractable) fraction. 3. The isotope concentration of each of the 3H-labelled 16-unsaturated C19 steroids in testicular tissue was different from that in spermatic venous plasma. 4. The ratios of tritiated 5α-androst-16-en-3α- and 3β-ol (free steroids) to their respective sulphate conjugates in the testicular tissue were less than the ratios of the same compounds in the spermatic venous plasma. The possibility that the sulphates are partially hydrolysed by testicular sulphatases before secretion is discussed. 5. In a second experiment, a continuous close-arterial infusion of [7α-3H]pregnenolone into the left testis was performed over a 200min period and all the urine that accumulated during the infusion was collected for analysis. 6. No 3H-labelled 16-unsaturated C19 steroids were detected in the urine as free steroids. Only a trace of 5α-androst-16-en-3α-ol was detected conjugated as glucosiduronate, whereas the corresponding 3β-alcohol occurred mainly as glucosiduronate and to a lesser extent as sulphate. 7. The absence of 5α-androst-16-en-3β-ol glucosiduronate in the spermatic venous blood and its presence in considerable amount in the urine may be attributed to hepatic glucuronyl transferase activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURSTEIN S., DORFMAN R. I. Determination of mammalian steroid sulfatase with 7 alpha-H3-3beta-hydroxyandrost-5-en-17-one sulfate. J Biol Chem. 1963 May;238:1656–1660. [PubMed] [Google Scholar]
- BURSTEIN S., LIEBERMAN S. Hydrolysis of ketosteroid hydrogen sulfates by solvolysis procedures. J Biol Chem. 1958 Aug;233(2):331–335. [PubMed] [Google Scholar]
- Baulieu E. E., Fabre-Jung I., Huis in't Veld L. G. Dehydroepinadrosterone sulfate: a secretory product of the boar testis. Endocrinology. 1967 Jul;81(1):34–38. doi: 10.1210/endo-81-1-34. [DOI] [PubMed] [Google Scholar]
- Booth W. D. The occurrence of some C19 steroids and vitamin A in boar testis. J Reprod Fertil. 1970 Dec;23(3):533–534. doi: 10.1530/jrf.0.0230533. [DOI] [PubMed] [Google Scholar]
- Brooksbank B. W., Gower D. B. The estimation of 3 alpha-hydroxy-5 alpha-androst-16-ene and other C19-delta 16-steroids in urine by gas-liquid chromatography. Acta Endocrinol (Copenh) 1970 Jan;63(1):79–90. doi: 10.1530/acta.0.0630079. [DOI] [PubMed] [Google Scholar]
- Brophy P. J., Gower B. D. 16-unsaturated C 19 3-oxo steroids as metabolic intermediates in boar testis. Biochem J. 1972 Jul;128(4):945–952. doi: 10.1042/bj1280945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTENSEN A. K., MASON N. R. COMPARATIVE ABILITY OF SEMINIFEROUS TUBULES AND INTERSTITIAL TISSUE OF RAT TESTES TO SYNTHESIZE ANDROGENS FROM PROGESTERONE-4-14C IN VITRO. Endocrinology. 1965 Apr;76:646–656. doi: 10.1210/endo-76-4-646. [DOI] [PubMed] [Google Scholar]
- Gower D. B. 16-Unsaturated C 19 steroids. A review of their chemistry, biochemistry and possible physiological role. J Steroid Biochem. 1972 Jan;3(1):45–103. doi: 10.1016/0022-4731(72)90011-8. [DOI] [PubMed] [Google Scholar]
- Gower D. B., Harrison F. A., Heap R. B. The identification of C19-16-unsaturated steroids and estimation of 17-oxosteroids in boar spermativ vein plasma and urine. J Endocrinol. 1970 Jul;47(3):357–365. [PubMed] [Google Scholar]
- Gower D. B., Loke K. H. Studies on the subcellular location and stability of the enzyme system involved in the biosynthesis of 5,16-androstadien-3 -ol from 3 -hydroxy-5-pregnen-20-one (pregnenolone). Biochim Biophys Acta. 1971 Dec 15;250(3):614–616. doi: 10.1016/0005-2744(71)90267-1. [DOI] [PubMed] [Google Scholar]
- HERD J. A., BARGER A. C. SIMPLIFIED TECHNIQUE FOR CHRONIC CATHETERIZATION OF BLOOD VESSELS. J Appl Physiol. 1964 Jul;19:791–792. doi: 10.1152/jappl.1964.19.4.791. [DOI] [PubMed] [Google Scholar]
- Katkov T., Gower D. B. The biosynthesis of 5 alpha-androst-16-en-3-one from progesterone by boar testis homogenate. Biochim Biophys Acta. 1968 Sep 2;164(1):134–136. doi: 10.1016/0005-2760(68)90082-9. [DOI] [PubMed] [Google Scholar]
- Melrose D. R., Reed H. C., Patterson R. L. Androgen steroids associated with boar odour as an aid to the detection of oestrus in pig artificial insemination. Br Vet J. 1971 Oct;127(10):497–502. doi: 10.1016/s0007-1935(17)37337-2. [DOI] [PubMed] [Google Scholar]
- van der Molen H. J., Eik-Nes K. B. Biosynthesis and secretion of steroids by the canine testis. Biochim Biophys Acta. 1971 Nov 5;248(2):343–362. doi: 10.1016/0005-2760(71)90023-3. [DOI] [PubMed] [Google Scholar]


