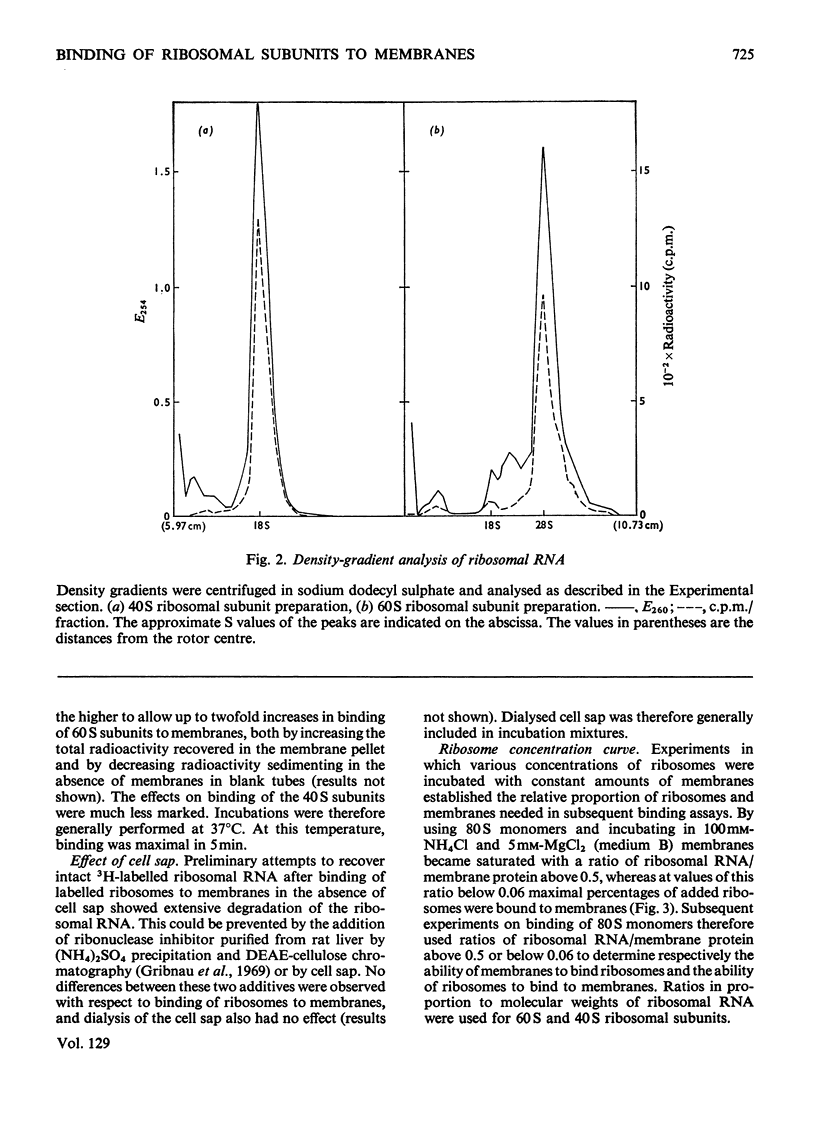
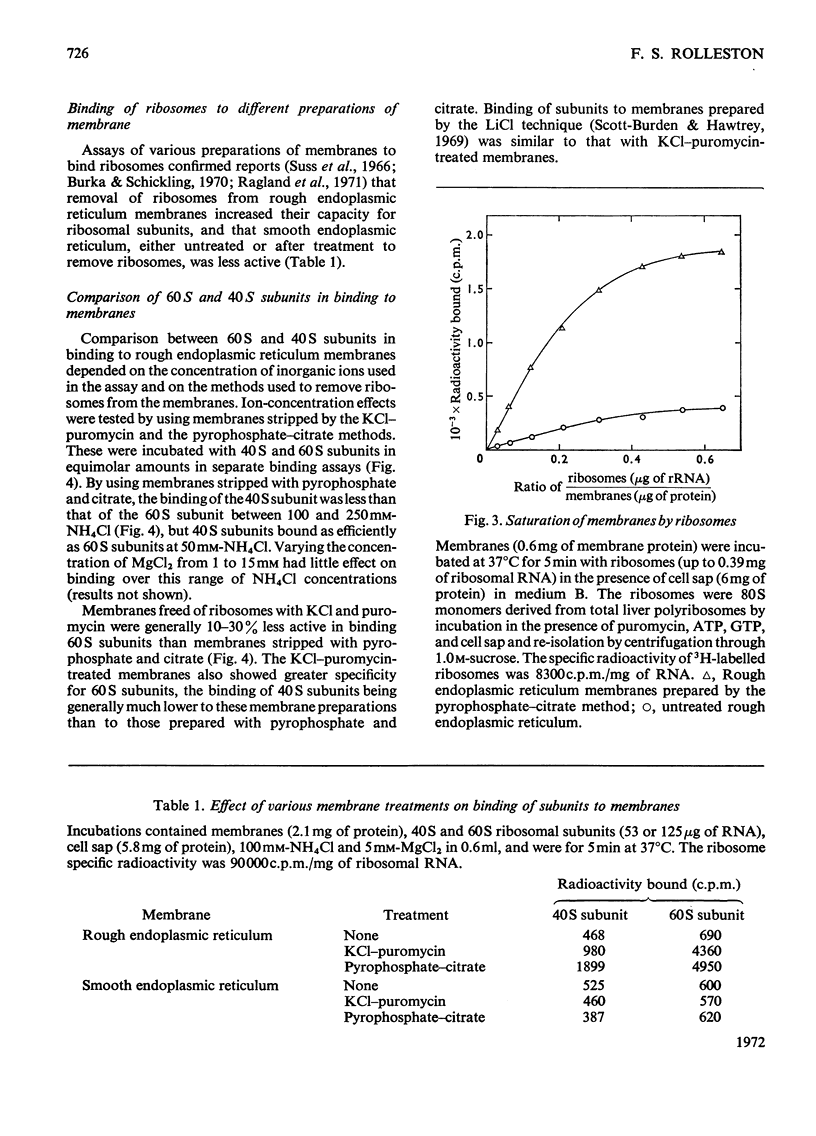
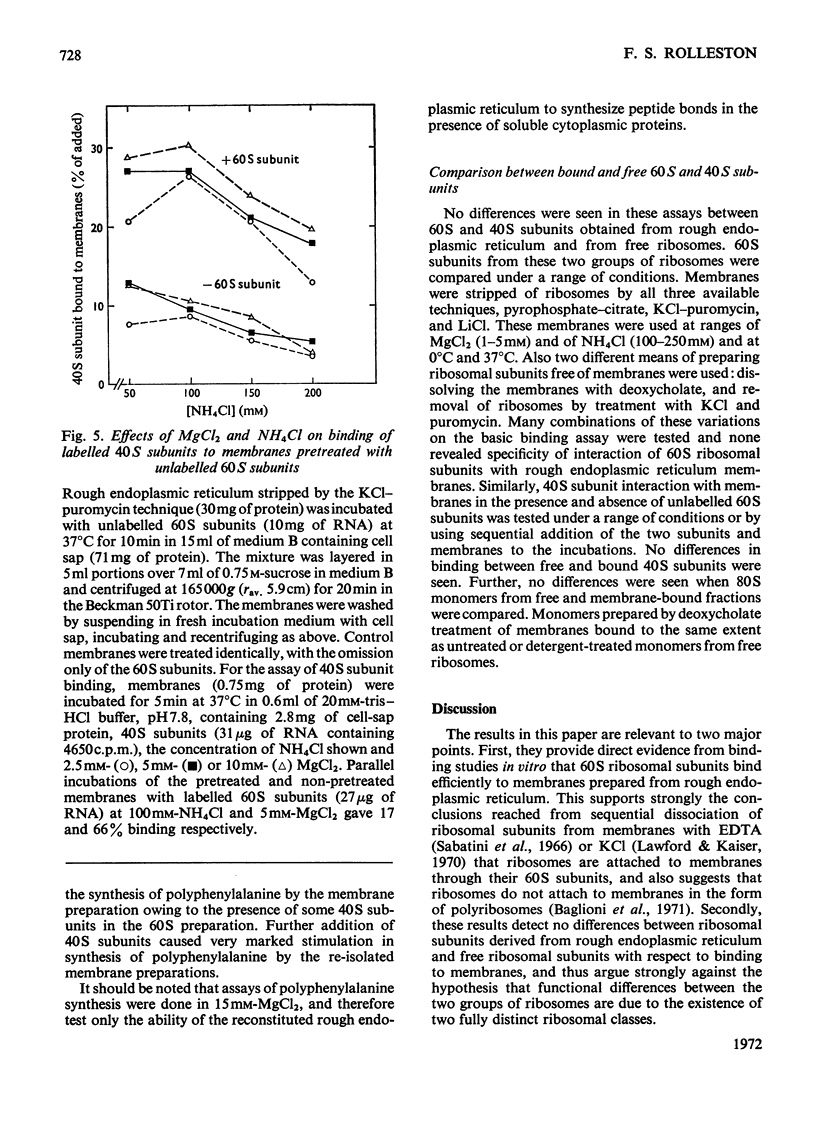
Abstract
The binding of ribosomes and ribosomal subunits to endoplasmic reticulum preparations of mouse liver was studied. (1) Membranes prepared from rough endoplasmic reticulum by preincubation with 0.5m-KCl and puromycin bound 60–80% of added 60S subunits and 10–15% of added 40S subunits. Membranes prepared with pyrophosphate and citrate showed less clear specificity for 60S subunits particularly when assayed at low ionic strengths. (2) Ribosomal 40S subunits bound efficiently to membranes only in the presence of 60S subunits. The reconstituted membrane–60S subunit–40S subunit complex was active in synthesis of peptide bonds. (3) No differences in binding to membranes were seen between subunits derived from free and from membrane-bound ribosomes. (4) It is concluded that the binding of ribosomes to membranes does not require that they be translating a messenger RNA, and that the mechanism whereby bound and free ribosomes synthesize different groups of proteins does not depend on two groups of ribosomes that differ in their ability to bind to endoplasmic reticulum.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. M., Tata J. R. Protein synthesis by membrane-bound and free ribosomes of secretory and non-secretory tissues. Biochem J. 1971 Feb;121(4):683–694. doi: 10.1042/bj1210683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni C., Bleiberg I., Zauderer M. Assembly of membrane-bound polyribosomes. Nat New Biol. 1971 Jul 7;232(27):8–12. doi: 10.1038/newbio232008a0. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Studies on free and membrane-bound ribosomes in rat liver. I. Distribution as related to total cellular RNA. J Mol Biol. 1967 Jun 14;26(2):279–292. doi: 10.1016/0022-2836(67)90297-5. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Studies on free and membrane-bound ribosomes in rat liver. II. Interaction of ribosomes and membranes. J Mol Biol. 1967 Jun 14;26(2):293–301. doi: 10.1016/0022-2836(67)90298-7. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burka E. R., Bulova S. I. Heterogeneity of reticulocyte ribosomes. Biochem Biophys Res Commun. 1971 Mar 5;42(5):801–805. doi: 10.1016/0006-291x(71)90500-6. [DOI] [PubMed] [Google Scholar]
- Burka E. R., Schickling L. F. Attachment of reticulocyte ribosomes to erythroid cell membranes in vitro. Biochemistry. 1970 Feb 3;9(3):459–463. doi: 10.1021/bi00805a002. [DOI] [PubMed] [Google Scholar]
- Dice J. F., Schimke R. T. Turnover and exchange of ribosomal proteins from rat liver. J Biol Chem. 1972 Jan 10;247(1):98–111. [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Falvey A. K., Staehelin T. Structure and function of mammalian ribosomes. I. Isolation and characterization of active liver ribosomal subunits. J Mol Biol. 1970 Oct 14;53(1):1–19. doi: 10.1016/0022-2836(70)90042-2. [DOI] [PubMed] [Google Scholar]
- Fridlender B. R., Wettstein F. O. Differences in the ribosomal protein of free and membrane bound polysomes of chick embryo cells. Biochem Biophys Res Commun. 1970 Apr 24;39(2):247–253. doi: 10.1016/0006-291x(70)90785-0. [DOI] [PubMed] [Google Scholar]
- Ganoza M. C., Williams C. A. In vitro synthesis of different categories of specific protein by membrane-bound and free ribosomes. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1370–1376. doi: 10.1073/pnas.63.4.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribnau A. A., Schoenmakers J. G., Bloemendal H. Purification of rat liver RNase inhibitor and its effect on polyribosome integrity. Arch Biochem Biophys. 1969 Mar;130(1):48–52. doi: 10.1016/0003-9861(69)90007-1. [DOI] [PubMed] [Google Scholar]
- Hicks S. J., Drysdale J. W., Munro H. N. Preferential synthesis of ferritin and albumin by different populations of liver polysomes. Science. 1969 May 2;164(3879):584–585. doi: 10.1126/science.164.3879.584. [DOI] [PubMed] [Google Scholar]
- Khawaja J. A. Interaction of ribosomes and ribosomal subparticles with endoplasmic reticulum membranes in vitro: effect of spermine and magnesium. Biochim Biophys Acta. 1971 Nov 29;254(1):117–128. doi: 10.1016/0005-2787(71)90118-3. [DOI] [PubMed] [Google Scholar]
- Khawaja J. A., Raina A. Effect of spermine and magnesium on the attachment of free ribosomes to endoplasmic reticulum membranes. Biochem Biophys Res Commun. 1970 Oct 23;41(2):512–518. doi: 10.1016/0006-291x(70)90536-x. [DOI] [PubMed] [Google Scholar]
- Martin T. E., Rolleston F. S., Low R. B., Wool I. G. Dissociation and reassociation of skeletal muscle ribosomes. J Mol Biol. 1969 Jul 14;43(1):135–149. doi: 10.1016/0022-2836(69)90084-9. [DOI] [PubMed] [Google Scholar]
- Puro D. G., Richter G. W. Ferritin synthesis by free and membrane-bound (poly)ribosomes of rat liver. Proc Soc Exp Biol Med. 1971 Nov;138(2):399–403. doi: 10.3181/00379727-138-35906. [DOI] [PubMed] [Google Scholar]
- Ragland W. L., Shires T. K., Pitot H. C. Polyribosomal attachment to rat liver and hepatoma endoplasmic reticulum in vitro. A method for its study. Biochem J. 1971 Jan;121(2):271–278. doi: 10.1042/bj1210271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnotti G., Lawford G. R., Campbell P. N. Biosynthesis of microsomal nicotinamide-adenine dinucleotide phosphate-cytochrome c reductase by membrane-bound and free polysomes from rat liver. Biochem J. 1969 Apr;112(2):139–147. doi: 10.1042/bj1120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader R. W., Stanners C. P. On the significance of ribosome dimers in extracts of animal cells. J Mol Biol. 1967 Sep 14;28(2):211–223. doi: 10.1016/s0022-2836(67)80004-4. [DOI] [PubMed] [Google Scholar]
- Redman C. M. Biosynthesis of serum proteins and ferritin by free and attached ribosomes of rat liver. J Biol Chem. 1969 Aug 25;244(16):4308–4315. [PubMed] [Google Scholar]
- Redman C. M., Cherian M. G. The secretory pathways of rat serum glycoproteins and albumin. Localization of newly formed proteins within the endoplasmic reticulum. J Cell Biol. 1972 Feb;52(2):231–245. doi: 10.1083/jcb.52.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Tashiro Y., Palade G. E. On the attachment of ribosomes to microsomal membranes. J Mol Biol. 1966 Aug;19(2):503–524. doi: 10.1016/s0022-2836(66)80019-0. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Hawtrey A. O. Preparation of ribosome-free membranes from rat liver microsomes by means of lithium chloride. Biochem J. 1969 Dec;115(5):1063–1069. doi: 10.1042/bj1151063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Burden T., Hawtrey A. O. Studies on the reattachment of ribosomes and ribosomal subunits to ribosome-free membranes prepared from rat liver microsomes by means of lithium chloride. Hoppe Seylers Z Physiol Chem. 1971 Apr;352(4):575–582. doi: 10.1515/bchm2.1971.352.1.575. [DOI] [PubMed] [Google Scholar]
- Shires T. K., Narurkar L. M., Pitot H. C. Polysome interaction in vitro with microsomal membranes from rat liver. Biochem Biophys Res Commun. 1971 Dec 3;45(5):1212–1218. doi: 10.1016/0006-291x(71)90147-1. [DOI] [PubMed] [Google Scholar]
- Shires T. K., Narurkar L., Pitot H. C. The association in vitro of polyribosomes with ribonuclease-treated derivatives of hepatic rough endoplasmic reticulum. Characteristics of the membrane binding sites and factors influencing association. Biochem J. 1971 Nov;125(1):67–79. doi: 10.1042/bj1250067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine G. H., Williams D. J., Rabin B. R. Role for steroid hormones in the interaction of ribosomes with the endoplasmic membranes of rat liver. Nat New Biol. 1971 Mar 31;230(13):133–136. doi: 10.1038/newbio230133a0. [DOI] [PubMed] [Google Scholar]
- Süss R., Blobel G., Pitot H. C. Rat liver and hepatoma polysome-membrane interaction in vitro. Biochem Biophys Res Commun. 1966 May 3;23(3):299–304. doi: 10.1016/0006-291x(66)90545-6. [DOI] [PubMed] [Google Scholar]
- Takagi M., Ogata K. Direct evidence for albumin biosynthesis by membrane bound polysomes in rat liver. Biochem Biophys Res Commun. 1968 Oct 10;33(1):55–60. doi: 10.1016/0006-291x(68)90254-4. [DOI] [PubMed] [Google Scholar]
- Takagi M., Tanaka T., Ogata K. Functional differences in protein synthesis between free and bound polysomes of rat liver. Biochim Biophys Acta. 1970 Sep 17;217(1):148–158. doi: 10.1016/0005-2787(70)90131-0. [DOI] [PubMed] [Google Scholar]
- WILLIAMS C. A., GANOZA M. C., LIPMANN F. EFFECT OF BACTERIAL INFECTION ON THE SYNTHESIS OF SERUM PROTEINS BY A MOUSE LIVER CELL-FREE SYSTEM. Proc Natl Acad Sci U S A. 1965 Mar;53:622–626. doi: 10.1073/pnas.53.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. J., Gurari D., Rabin B. R. The effects of ribosomes on the activity of a membrane bound enzyme catalysing thiol-disulphide interchange. FEBS Lett. 1968 Dec;2(2):133–135. doi: 10.1016/0014-5793(68)80123-1. [DOI] [PubMed] [Google Scholar]
- Williams D. J., Rabin B. R. Distruption by carcinogens of the hormone dependent association of membranes with polysomes. Nature. 1971 Jul 9;232(5306):102–105. doi: 10.1038/232102a0. [DOI] [PubMed] [Google Scholar]
- Williams D. J., Rabin B. R. The effects of aflatoxin B(1) and steroid hormones on polysome binding to microsomal membranes as measured by the activity of an enzyme catalysing disulphide interchange. FEBS Lett. 1969 Jul;4(2):103–107. doi: 10.1016/0014-5793(69)80207-3. [DOI] [PubMed] [Google Scholar]