Abstract
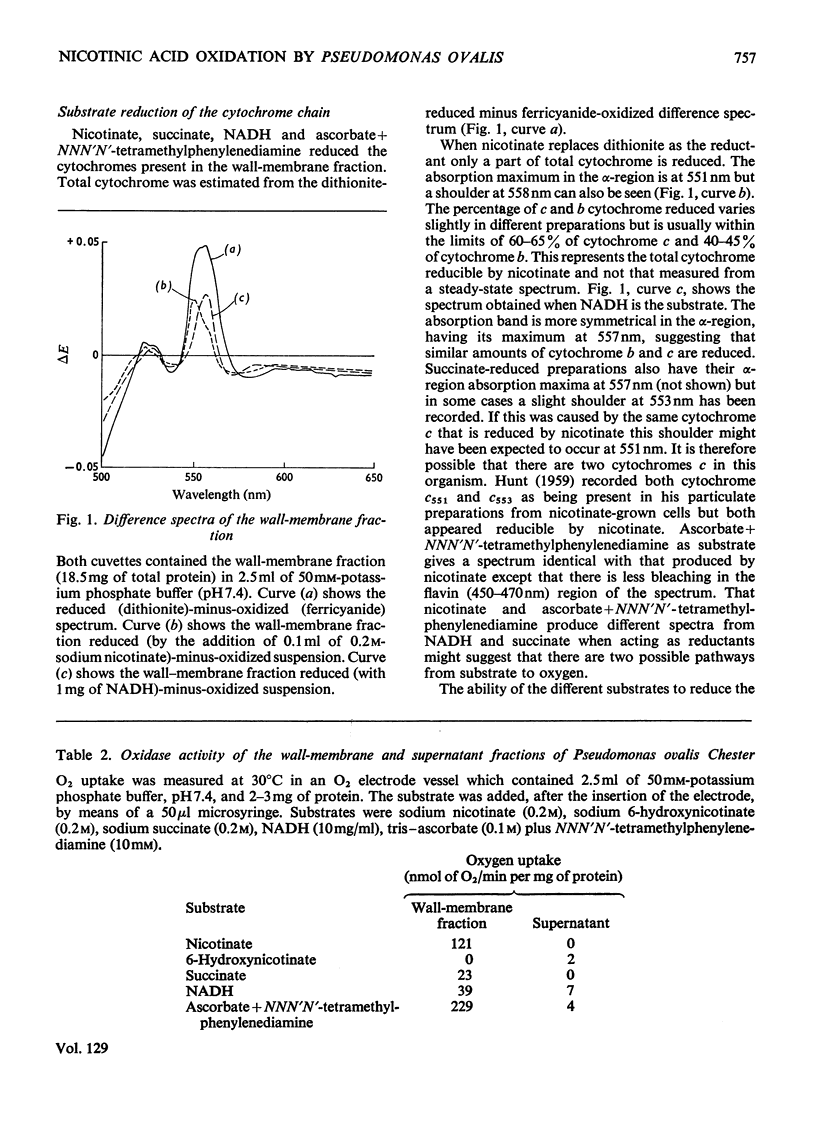
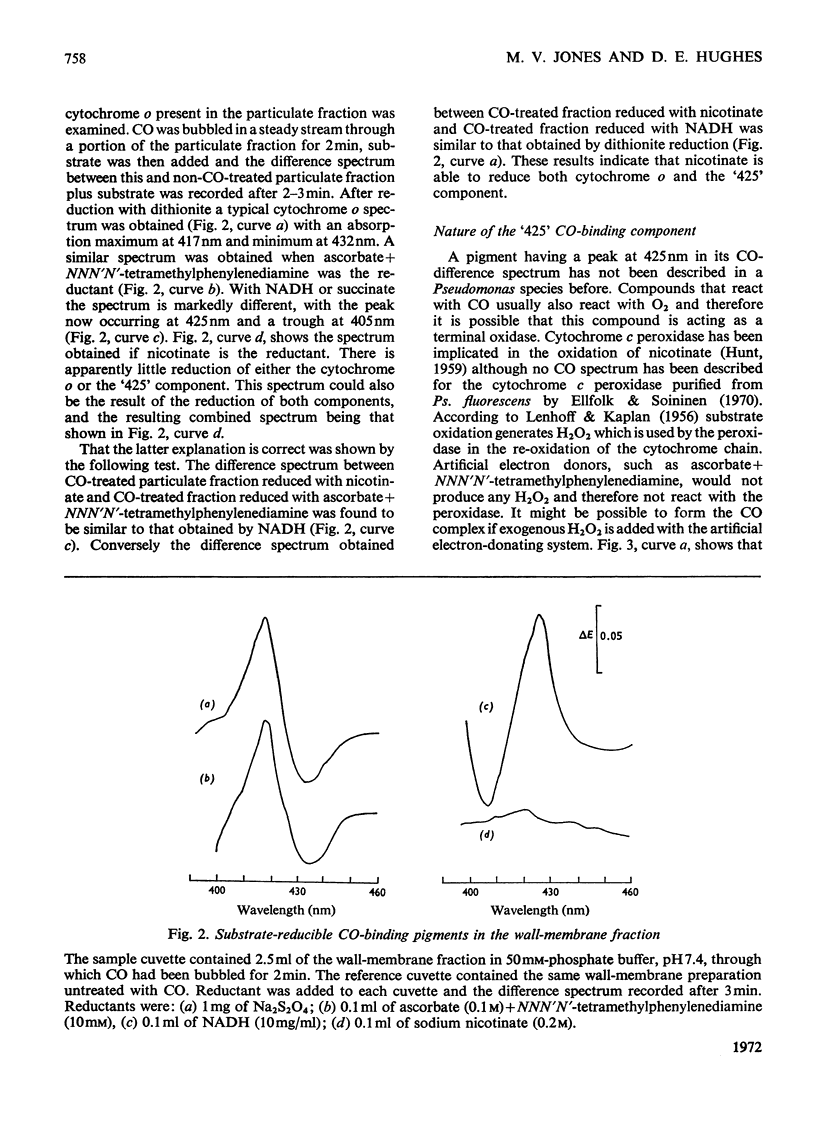
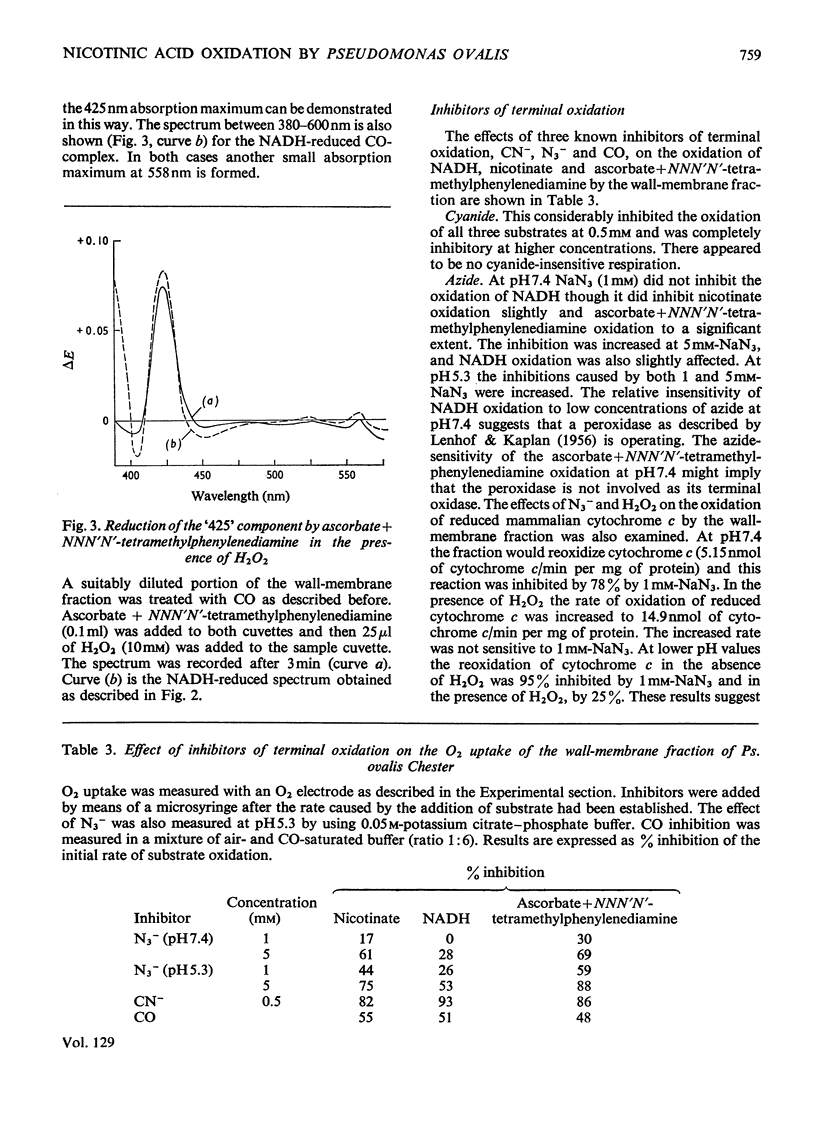
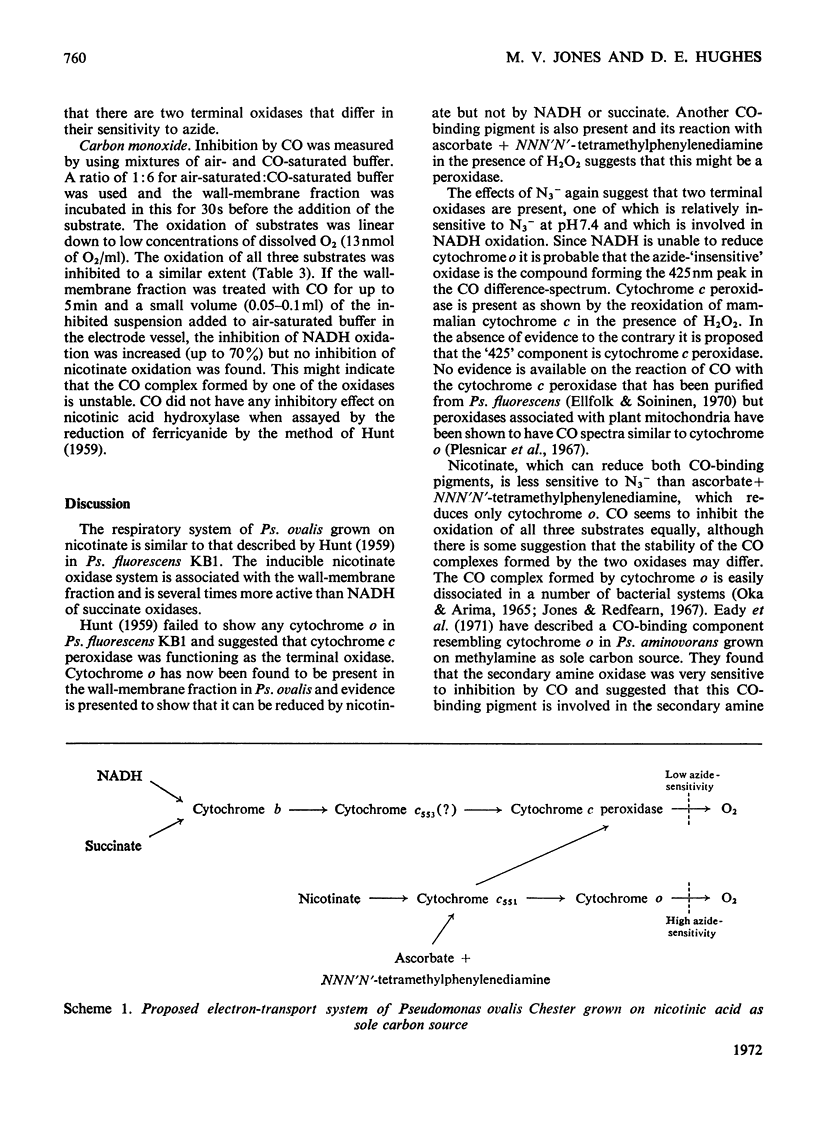
In cell-free extracts of Pseudomonas ovalis nicotinic acid oxidase is confined to the wallmembrane fraction. It is associated with an electron-transport chain comprising b- and c-type cytochromes only, differing proportions of which are reduced by nicotinate and NADH. CO difference-spectra show two CO-binding pigments, cytochrome o (absorption maximum at 417nm) and another component absorbing maximally at 425nm. Cytochrome o is not reduced by NADH or by succinate but is by nicotinate, which can also reduce the `425' CO-binding pigment. The effects of inhibitors of terminal oxidation support the idea of two terminal oxidases and a scheme involving the `425' CO-binding pigment and the other components of the electron-transport chain is proposed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEHRMAN E. J., STANIER R. Y. The bacterial oxidation of nicotinic acid. J Biol Chem. 1957 Oct;228(2):923–945. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- Daniel R. M. The electron transport system of Acetobacter suboxydans with particular reference to cytochrome. Biochim Biophys Acta. 1970 Sep 1;216(2):328–341. doi: 10.1016/0005-2728(70)90224-0. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Jarman T. R., Large P. J. Microbial oxidation of amines. Partial purification of a mixed-function secondary-amine oxidase system from Pseudomonas aminovorans that contains an enzymically active cytochrome-P-420-type haemoprotein. Biochem J. 1971 Nov;125(2):449–459. doi: 10.1042/bj1250449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellfolk N., Soininen R. Pseudomonas cytochrome c peroxidase. I. Purification procedure. Acta Chem Scand. 1970;24(6):2126–2136. doi: 10.3891/acta.chem.scand.24-2126. [DOI] [PubMed] [Google Scholar]
- FRANCIS M. J., HUGHES D. E., KORNBERG H. L., PHIZACKERLEY P. J. THE OXIDATION OF L-MALATE BY PSEUDOMONAS SP. Biochem J. 1963 Dec;89:430–438. doi: 10.1042/bj0890430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES D. E. 6-Hydroxynicotinic acid as an intermediate in the oxidation of nicotinic acid by Pseudomonas fluorescens. Biochem J. 1955 Jun;60(2):303–310. doi: 10.1042/bj0600303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- HUNT A. L., HUGHES D. E., LOWENSTEIN J. M. The hydroxylation of nicotinic acid by Pseudomonas fluorescens. Biochem J. 1958 Jun;69(2):170–173. doi: 10.1042/bj0690170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT A. L. Purification of the nicotinic acid hydroxylase system of Pseudomonas fluorescens KB1. Biochem J. 1959 May;72(1):1–7. doi: 10.1042/bj0720001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. W., Redfearn E. R. The cytochrome system of Azotobacter vinelandii. Biochim Biophys Acta. 1967 Sep 6;143(2):340–353. doi: 10.1016/0005-2728(67)90088-6. [DOI] [PubMed] [Google Scholar]
- Katagiri M., Ganguli B. N., Gunsalus I. C. A soluble cytochrome P-450 functional in methylene hydroxylation. J Biol Chem. 1968 Jun 25;243(12):3543–3546. [PubMed] [Google Scholar]
- LENHOFF H. M., KAPLAN N. O. A cytochrome peroxidase from Pseudomonas fluorescens. J Biol Chem. 1956 Jun;220(2):967–982. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Oka T., Arima K. Cyanide Resistance in Achromobacter II. Mechanism of Cyanide Resistance. J Bacteriol. 1965 Sep;90(3):744–747. doi: 10.1128/jb.90.3.744-747.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N. Regulation of catabolic pathways in Pseudomonas. Bacteriol Rev. 1971 Jun;35(2):87–116. doi: 10.1128/br.35.2.87-116.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. A. Cytochrome content of two pseudomonads containing mixed-function oxidase systems. J Bacteriol. 1970 Sep;103(3):714–721. doi: 10.1128/jb.103.3.714-721.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesnicar M., Bonner W. D., Jr, Storey B. T. Peroxidase associated with higher plant mitochondria. Plant Physiol. 1967 Mar;42(3):366–370. doi: 10.1104/pp.42.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]


