Abstract
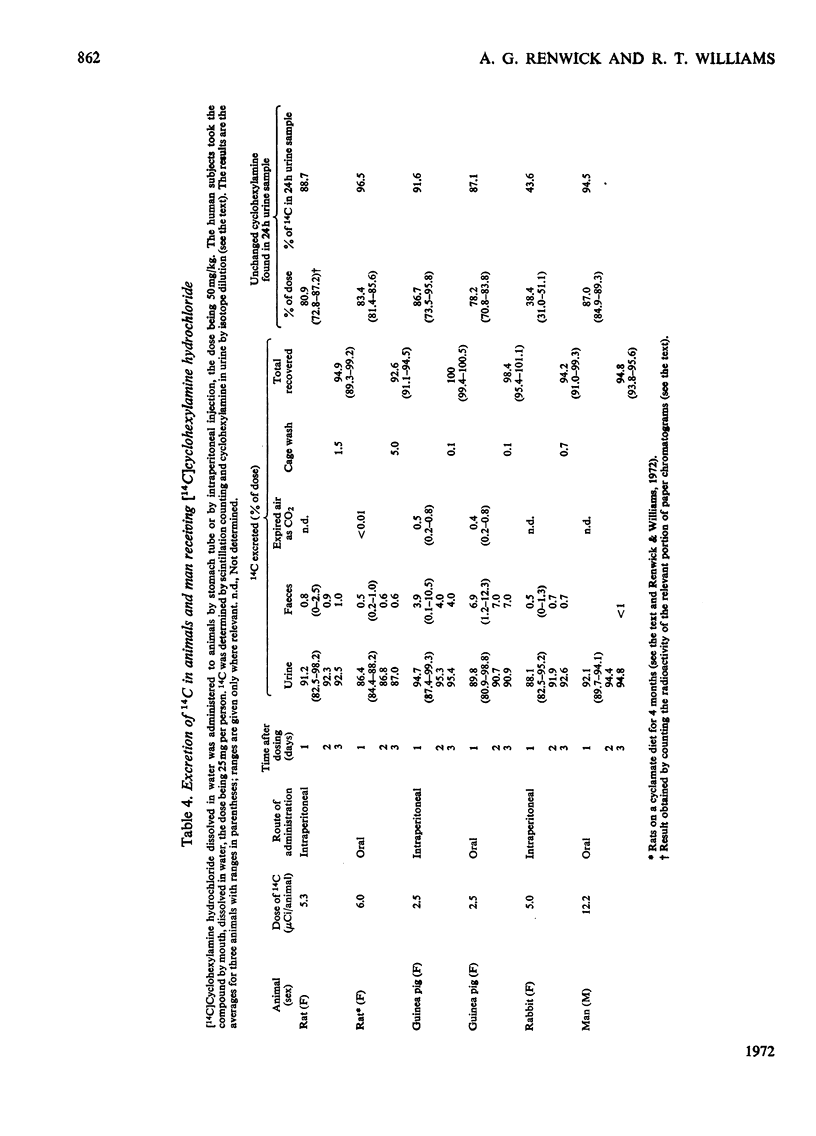
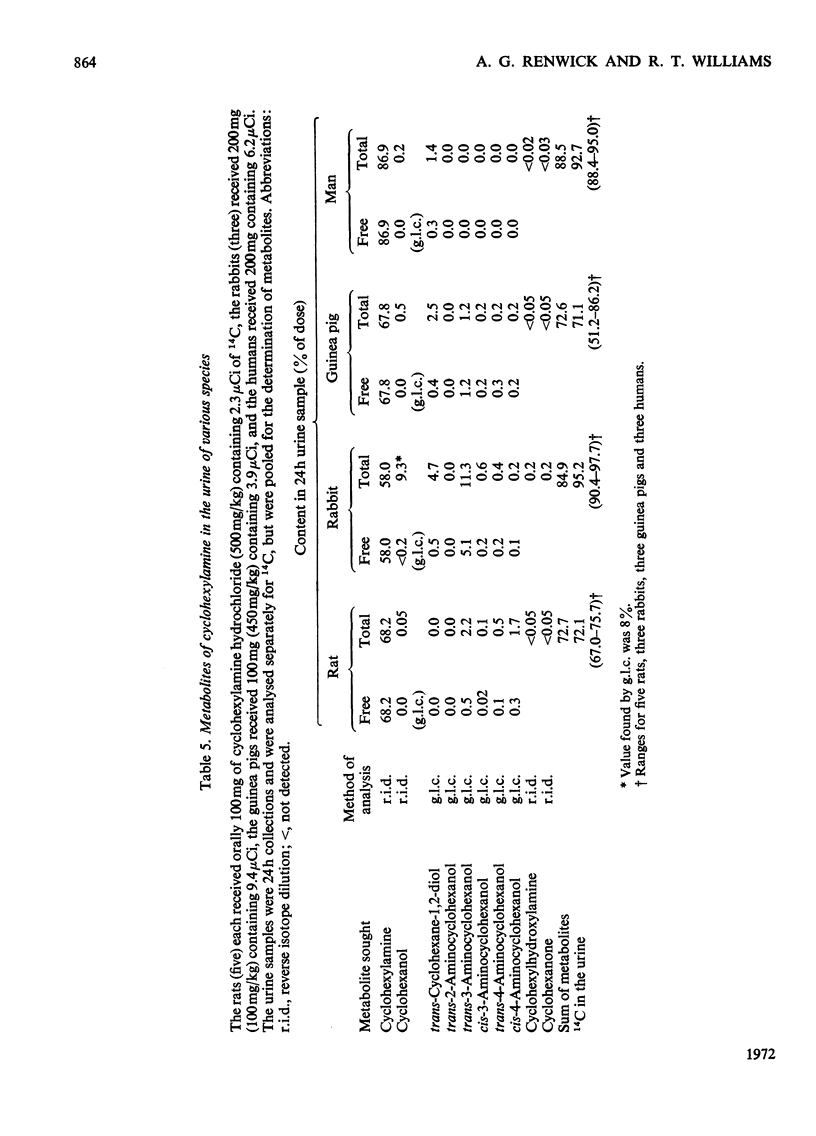
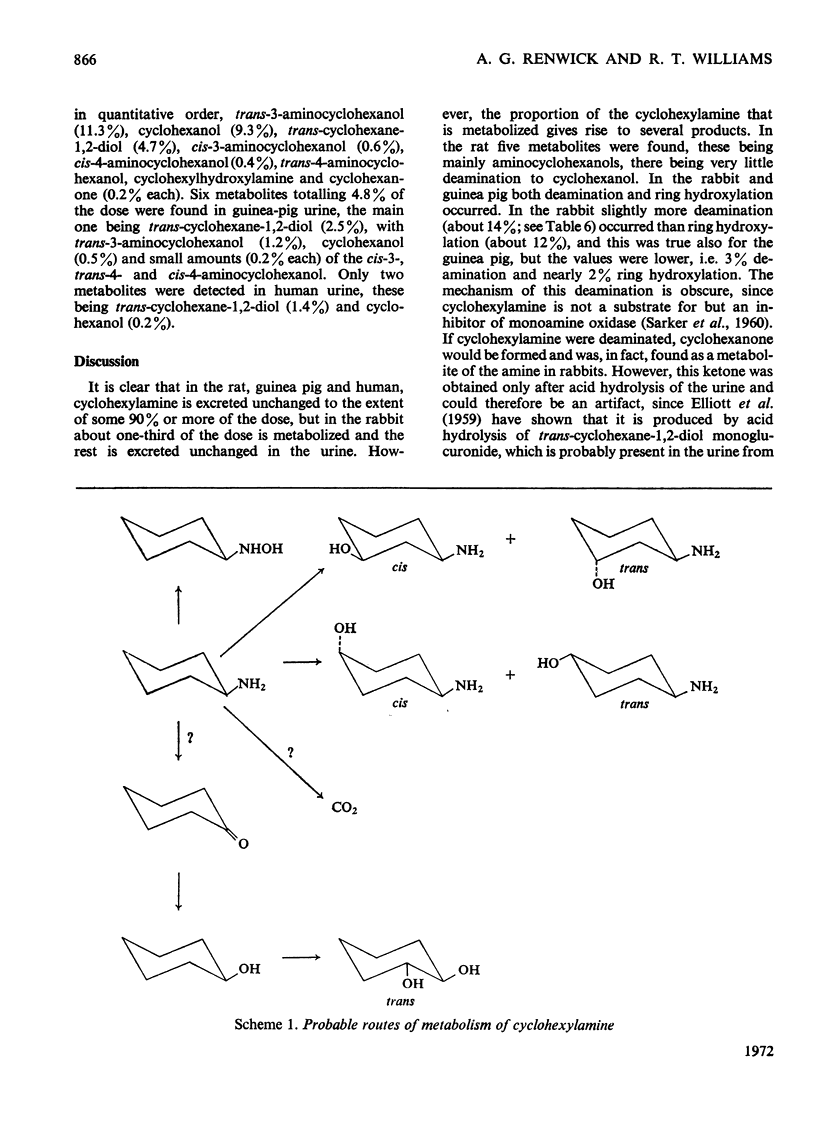
1. [1-14C]Cyclohexylamine hydrochloride was synthesized and given orally or intraperitoneally to rats, rabbits and guinea pigs (dose 50–500mg/kg) and orally to humans (dose 25 or 200mg/person). The 14C is excreted mainly in the urine, most of the excretion occurring in the first day after dosing. Only small amounts (1–7%) are found in the faeces. 2. In the rat, guinea pig and man, the amine is largely excreted unchanged, only 4–5% of the dose being metabolized in 24h in the rat and guinea pig and 1–2% in man. In the rabbit about two-thirds of the dose is excreted unchanged and about 30% is metabolized. 3. In the rat, five minor metabolites were found, namely cyclohexanol (0.05%), trans-3- (2.2%), cis-4- (1.7%), trans-4- (0.5%) and cis-3-aminocyclohexanol (0.1% of the dose in 24h). 4. In the rabbit, eight metabolites were identified, namely cyclohexanol (9.3%), trans-cyclohexane-1,2-diol (4.7%), cyclohexanone (0.2%), cyclohexylhydroxylamine (0.2%) and trans-3- (11.3%), cis-3- (0.6%), trans-4- (0.4%) and cis-4-aminocyclohexanol (0.2%). 5. In the guinea pig, six minor metabolites were found, namely cyclohexanol (0.5%), trans-cyclohexane-1,2-diol (2.5%) and trans-3- (1.2%), cis-3- (0.2%), trans-4- (0.2%) and cis-4-aminocyclohexanol (0.2%). 6. In man only two metabolites were definitely identified, namely cyclohexanol (0.2%) and trans-cyclohexane-1,2-diol (1.4% of the dose), but man had been given a smaller dose (3mg/kg) than the other species (50mg/kg). 7. The hydroxylated metabolites of cyclohexylamine were excreted in the urine in both free and conjugated forms. 8. Although cyclohexylamine is metabolized to only a minor extent, in rats the metabolism was mainly through hydroxylation of the cyclohexane ring, in man by deamination and in guinea pigs and rabbits by ring hydroxylation and deamination.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridges J. W., Davies D. S., Williams R. T. The fate of ethyltin and diethyltin derivatives in the rat. Biochem J. 1967 Dec;105(3):1261–1267. doi: 10.1042/bj1051261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT T. H., PARKE D. V., WILLIAMS R. T. Studies in detoxication. 79. The metabolism of cyclo [14C] hexane and its derivatives. Biochem J. 1959 Jun;72(2):193–200. doi: 10.1042/bj0720193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S., Ichibagase H. Studies on synthetic sweetening agents. 8. Cyclohexylamine, a metabolite of sodium cyclamate. Chem Pharm Bull (Tokyo) 1966 Sep;14(9):971–974. doi: 10.1248/cpb.14.971. [DOI] [PubMed] [Google Scholar]
- Oser B. L., Carson S., Vogin E. E., Sonders R. C. Conversion of cyclamate to cyclohexylamine in rats. Nature. 1968 Oct 12;220(5163):178–179. doi: 10.1038/220178a0. [DOI] [PubMed] [Google Scholar]
- Price J. M., Biava C. G., Oser B. L., Vogin E. E., Steinfeld J., Ley H. L. Bladder tumors in rats fed cyclohexylamine or high doses of a mixture of cyclamate and saccharin. Science. 1970 Feb 20;167(3921):1131–1132. doi: 10.1126/science.167.3921.1131. [DOI] [PubMed] [Google Scholar]
- Renwick A. G., Williams R. T. The fate of cyclamate in man and other species. Biochem J. 1972 Oct;129(4):869–879. doi: 10.1042/bj1290869. [DOI] [PMC free article] [PubMed] [Google Scholar]


