Abstract
Noradrenaline (NA), a key neurotransmitter of the endogenous pain inhibitory system, acutely inhibits nociceptive transmission (including that mediated by substance P), potentiates opioid analgesia, and underlies part of the antinociceptive effects of the widely prescribed tricyclic antidepressants. Lesions of noradrenergic neurons, however, result in either normal or reduced pain behavior and variable changes in morphine antinociception, undermining the proposed association between noradrenaline (NA) deficiency and chronic pain (hyperalgesia). We used mice lacking the gene coding for dopamine β-hydroxylase, the enzyme responsible for synthesis of NA from dopamine, to reexamine the consequences of a lack of NA on pain behavior. Here, we show that absence of NA in the central nervous system results in a substance P-mediated chronic hyperalgesia (decreased nociceptive threshold) to thermal, but not mechanical, stimuli and decreased efficacy of morphine. Contrary to studies that show substance P-mediated hyperalgesia requires intense stimuli, we found that even a mild stimulus is sufficient to evoke substance P-dependent hyperalgesia in the NA-deficient mice. Restoring central NA normalized both the nociceptive threshold and morphine efficacy, which is consistent with a tonic inhibitory effect of NA on nociceptive transmission. Unexpectedly, however, antagonists to the substance P receptor (the NK1 receptor) could achieve the same effect as NA replacement. We conclude that when unopposed by NA, substance P acting at the NK1 receptor causes chronic thermal hyperalgesia, and that the reduced opioid efficacy associated with a lack of NA is due to increased NK1-receptor stimulation.
Noradrenaline (NA) is an essential neurotransmitter of the endogenous pain inhibitory system (1, 2) that tonically and phasically inhibits spinal nociceptive transmission, including that mediated by substance P (3, 4). By stimulating central nervous system (CNS) α2 adrenoreceptors, NA increases threshold and latency to noxious stimuli without affecting responses to innocuous stimuli, and potentiates the antinociceptive effects of opiates (5–10).
Based on the above understanding, it has been suggested that noradrenergic dysfunction is a key component of certain chronic intractable pain disorders (11). A major caveat is that this hypothesis is based primarily on experimental studies that use acute rather than chronic pain. Although acute lesions or inhibition of noradrenergic spinal afferents produce a state of hyperalgesia and reduced antinociceptive effects of opiates (2, 10, 12), the increased pain behavior abates over time. In fact, long-term effects of noradrenergic denervation have been reported to result in decreased pain behavior, whereas the antinociceptive effect of morphine is either reduced, unchanged, or increased (13–17). These conflicting results can be caused in part by the variability in the extent of the removal of noradrenergic neurons or terminals which, when using a neurotoxin, is both incomplete and nonselective. Moreover, because noradrenergic terminals contain more than one neurotransmitter (18, 19), the effects of their elimination are difficult to interpret. Surgical lesions of noradrenergic nuclei or pathways result in even more difficult interpretative scenarios because of the many nonnoradrenergic neurons and/or projections that are removed simultaneously.
To circumvent the problem of incomplete and variable noradrenergic denervation, we chose to take a genetic approach to studying the role of noradrenergic transmission in nociception and morphine analgesia. Specifically, we studied pain behavior and morphine analgesia in mice missing the gene coding for dopamine β-hydroxylase (DBH). A unique advantage of these knockout mice is that NA can be restored, which allowed us to determine whether the absence of NA, rather than some unrecognized developmental defect, is responsible for the findings. NA is restored by administering a synthetic amino acid precursor of NA, L-threo-3,4-dihydroxyphenylserine (DOPS), which is converted to NA by aromatic amino acid decarboxylase (AADC; ref. 20). When carbidopa, an inhibitor of AADC that does not cross the blood–brain barrier, is coadministered with DOPS, NA is produced essentially only in the CNS.
Materials and Methods
The Dbh−/− mice used in this study were adult males, 20th generation 129/SvEv and C57BL/6J mixed genetic background. Dbh−/− mice were phenotyped by identifying animals with ptosis and delayed growth and were genotyped by PCR. Controls were Dbh+/− littermates with normal levels of NA and adrenaline (21); they are normal for all phenotypes tested (20). Procedures for the maintenance and use of the experimental animals were approved by the Animal Care and Use Advisory Committees at the University of Washington and the University of California San Francisco and were carried out in accordance with National Institutes of Health regulations on animal use.
Behavioral Testing.
For each individual experiment, the same number of animals (between 8 and 12) was used for Dbh−/− and control groups. Thermal nociceptive responses were assessed by using the hot-plate, tail-flick, and cold-plate tests. Individual mice were placed on the hot plate at 50°C (Stoelting), and the delay for the first hindpaw lift was timed. Cut-off was at 30 s, except for experiments with morphine in which 50 s were used. The threshold for heat withdrawal was determined by setting the hot plate at incrementing temperatures (43–47°C) and by counting the number of hindpaw lifts during a period of 4 min for each temperature. A delay of 1 h was allowed before each mouse was exposed to a higher temperature. The tail-flick test was carried out by placing the ventral surface of the middle third of the tail over a radiant heat source (Ugo Basile, Varese, Italy). Withdrawal of the tail stopped the heat source, and the time was recorded automatically. Cut-off was set at 15 s. The cold-plate test (22) was performed by placing each mouse on a 4°C surface for 5 min. The number of hindpaw lifts during 4 min was recorded (22). Mechanical responses were assessed by using the tail-pressure and von Frey filament tests. For the tail-pressure test, mice were placed in a Plexiglas cylinder, and an increasing pressure was applied on the junction of the proximal and middle third of their tails with a Randall-Selitto apparatus (1-mm tip; Stoelting) until the tail was withdrawn. The test was repeated three times every 5 min. The threshold for withdrawal from mechanical stimulation to the plantar aspect of the hindpaws was determined with von Frey monofilaments (Stoelting). Filaments with a bending force from 0.01 g to 2.0 g were applied to the plantar aspect of each hindpaw until a paw withdrawal occurred in more than half of the trials. Progressively finer filaments were applied until the mechanical threshold was found. Motor function was assessed by using a RotaRod (3.2-cm diameter rod, Ugo Basile) which was set at a constant low speed of 50 cm/min. Once all animals were walking, the RotaRod was switched to an accelerating mode, reaching a cut-off speed of 400 cm/min at 5 min. The maximal time in seconds each mouse was able to remain on the RotaRod was recorded. Animals were trained for 1 week before data collections. Exploratory behavior was assessed with the Hole board test (Stoelting). Mice were placed in the middle of a square surface (40 × 40 cm) perforated with 16 equally spaced 2.9-cm holes and allowed to explore freely for 4 min. The number of holes visited during the test period was recorded. Motor function and exploratory behavior were assessed for the highest dose of each drug resulting in an antinociceptive effect to ensure that the increase in withdrawal latency was due to sensory change rather than a psychomotor effect.
Peripheral Vascular Perfusion.
Hindpaw cutaneous blood perfusion was assessed with a 2D Laser Doppler Imager (Moor Instruments, Axminster, U.K.) equipped with a near-infrared (780 nm) laser. This system measures blood flow in deep-lying subcutaneous vessels (23) for both hindpaws without any physical contact. Mice were lightly anesthetized with 1% isoflurane mixed with 100% oxygen, which was delivered by means of a vaporizer connected to a face mask. Scanning was done twice over 60 s at a resolution of 0.2 mm/pixel for each animal.
Immunocytochemistry.
Transverse sections of the aldehyde-fixed brain and spinal cord were cut on a freezing microtome and processed for immunocytochemistry by using antisera against the following antigens: substance P (John Maggio, Univ. of Cincinnati, OH), NK1 (Steve Vigna, Duke Univ., Durham, NC), TH (Abcam, Cambridge, U.K.), DBH and 5-HT (Eugene Tech, Ridgefield, NJ), μ-opioid, α2A, and α2C receptors (Robert Elde, Univ. of Minnesota, Twin Cities, MN), AADC (Biogenesis, Poole, U.K.). Tissue from five Dbh−/− and five control mice was used for each antibody.
Drugs.
The following drugs were used: DOPS (Sumitomo Pharmaceutical, Osaka, Japan), carbidopa, naltrexone, and morphine sulfate (Sigma), RP-67580 and RP-68651 (Rhone-Poulenc Rorer, Vitry-sur-Seine, France), CP-96,345 and CP-96,344 (Pfizer Diagnostics), L-733,060 (Merck), SKF86466 (GlaxoSmithKline), UK14,304, HEAT hydrochloride (Tocris Cookson, Ellisville, MO). The drugs were dissolved in saline except for DOPS, which was dissolved in 0.2 M HCl and neutralized with NaOH immediately before injection. Controls were given the same volume of vehicle. All drugs were given s.c. unless otherwise specified, and a period of 30 min was allowed between drug administration and behavioral testing, except for DOPS/carbidopa, for which 5 h was usually allowed. For each animal, the degree of antinociception was determined by a t test comparing predrug vs. postdrug response value. Some postdrug hot-plate latencies were expressed as a percentage of the maximum possible effect (MPE) [% MPE = (postdrug latency − predrug latency)/(cutoff time − predrug latency) × 100; 100% = when a mouse does not demonstrate any pain behavior at cutoff time]. The use of percentage of MPE takes account of differences in hotplate baseline latencies between Dbh−/− mice and control mice so that these differences do not bias the quantification of the antinociceptive effect of the administered drug.
Statistical Analysis.
By using the outcome variable in each experiment, comparisons of treatment groups were done with the Student's t test or ANOVA. Post hoc analysis of means and SEM using a Scheffé's F test confirmed statistical significance. For the results of von Frey filament tests, nonparametric analysis was done by using an ANOVA and the Wilcoxon test. A value of P < 0.05 indicated statistical difference between the groups compared.
Results
Although NA, adrenaline, and DBH are absent from all tissues of Dbh−/− mice (compare Fig. 1 a and a′), these animals exhibit long-term viability and seem normal except for a mild eyelid ptosis. Dbh−/− mice show no signs of altered somatic sensation or spontaneous pain behavior, and they are comparable to controls in short-term active avoidance learning necessary for nociceptive testing (21). Motor performance with a RotaRod and exploratory behavior with the hole-board test were normal (see Fig. 5 a and b, which is published as supporting information on the PNAS web site, www.pnas.org).
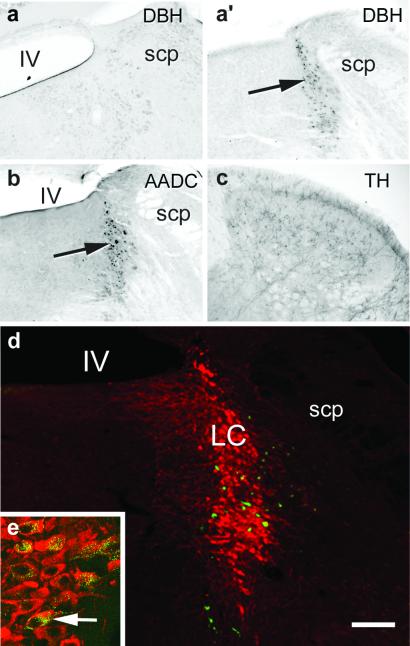
Figure 1.
Brainstem noradrenergic cell groups and their projections. (a, a′) Immunolabeling for DBH was absent from Dbh−/− brainstem noradrenergic cell groups such as the locus coeruleus compared with normal labeling in control mice (arrow). (b) Neurons in noradrenergic cells groups contain equivalent amounts of AADC in Dbh−/− (arrow pointing to locus coeruleus) and control mice (see arrow, Fig. 6, which is published as supporting information on the PNAS web site). (c, d) Tyrosine hydroxylase (TH)-labeled fibers present in normal amounts in the lumbar spinal dorsal horn of Dbh−/− mice and cell bodies in the brainstem suggests that the bulbospinal catecholaminergic projections are intact in Dbh−/− mice. An injection of 0.3 μl of green fluorescent beads into the dorsolateral quadrant of the spinal cord resulted in many retrogradely labeled cells (green) in the locus coeruleus of Dbh−/−mice (d) that were also double-immunolabeled with TH (red). (e) A high-magnification confocal image of double-labeled cells (one indicated by white arrow; 2 μm confocal slice). IV, fourth ventricle; LC, locus coeruleus; scp, superior cerebellar peduncle. (Bar: a, a′ = 250 μm; c = 175 μm; d = 100 μm; e = 30 μm.)
Behavioral Studies.
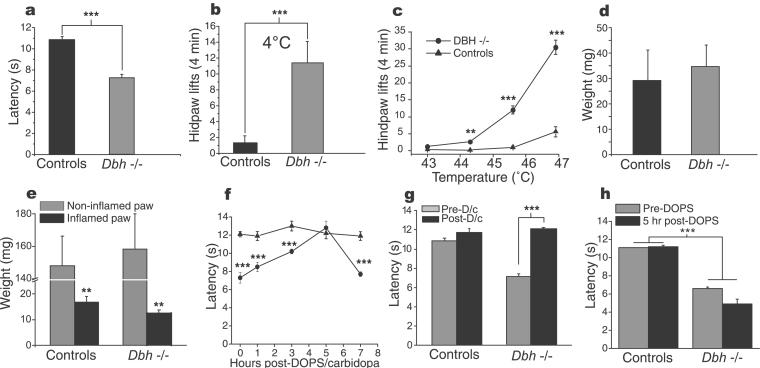
Results of nociceptive tests determined that Dbh−/− mice exhibit increased responses (paw lifts) to nociceptive thermal stimuli (heat and cold hyperalgesia; Fig. 2 a–c). Laser Doppler imaging showed that Dbh−/− mice had increased peripheral blood flow (Dbh−/−, 95.15 flux; controls, 22.8 flux; P < 0.001) leading us to conclude that the thermal hyperalgesia present in the Dbh−/− mice did not result from a loss of thermal buffering capacity caused by low blood perfusion in the vicinity of nociceptors. The thermal hyperalgesia was not accompanied by a mechanical hyperalgesia, as the Dbh−/− mice exhibited normal responses to von Frey filament (Fig. 2d) and tail-pinch tests (see Fig. 5c, which is published as supporting information on the PNAS web site). However, mechanical hyperalgesia could be produced after unilateral paw inflammation was induced by injecting complete Freund's adjuvant (CFA, 25 μl, Sigma) s.c. into the footpad. Unexpectedly, both Dbh−/− and control mice presented an equivalent decrease in mechanical threshold (Fig. 2e). The inflammatory response in the two groups also was similar (pre-CFA mid-paw thickness for Dbh−/− vs. controls, 0.14 ± 0.004 cm vs. 0.14 ± 0.003 cm; 1 day post-CFA for Dbh−/− vs. controls, 0.37 ± 0.004 cm vs. 0.33 ± 0.02 cm; 30 days post-CFA for Dbh−/− vs. controls, 0.21 ± 0.008 cm vs. 0.21 ± 0.007 cm; P > 0.05 for all times).
Figure 2.
Nociceptive testing before and after noradrenergic replacement. (a) Thermal hyperalgesia in Dbh−/− mice is demonstrated by their shorter withdrawal latency on the hot-plate test (50°C) and by (b) an increased number of paw lifts when freely walking on 4°C surface. (c) The lowered heat threshold for paw withdrawal on the hot-plate test is shown when mice are tested for 4 min at temperatures that are near nociceptive threshold (≈45°C). (d) In contrast to the increased response to thermal stimuli, no signs of hyperalgesia were observed when mechanical stimuli were used (von Frey filaments). (e) However, mechanical hyperalgesia (von Frey filaments test) was present after sensitization of one hindpaw with CFA, indicating that mechanical transduction is similar in Dbh−/− and control mice. Note that whereas the hyperalgesia is significant in the inflamed paw, the contralateral paw seems to be hypoalgesic compared with the pre-CFA values (P < 0.01) in both groups. The hypoalgesia results from a protective behavior the mice use to avoid standing on the inflamed hindpaw. (f) Reversal of heat hyperalgesia in Dbh−/− mice is maximal 5 h after receiving a single dose of DOPS/carbidopa (1.0 g/kg, 125 mg/kg), at which time the withdrawal latency in Dbh−/− mice becomes identical to that of controls (g, 50°C). (h) When the same dose of DOPS (1.0 g/kg) was given without carbidopa (mainly, to increase peripheral NA), no significant effect on nociceptive responses was observed. **, P < 0.01; ***, P < 0.001; D/c = DOPS/carbidopa.
Central α2 Receptor Involvement.
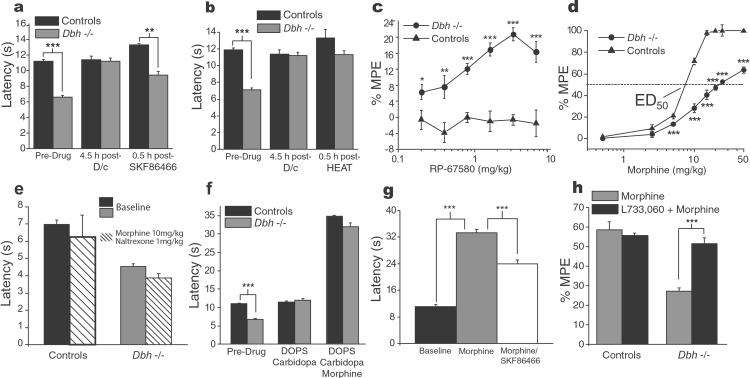
Restoring NA in the CNS by administering DOPS (1.0 g/kg) in combination with carbidopa (125 mg/kg) abolished the hyperalgesia by 5 h in the Dbh−/− mice but was without effect in the controls (Fig. 2 f and g). The 5-h delay in the reversal of hyperalgesia corresponds to the time necessary for NA to reach its highest level in the CNS (20). When DOPS (0.5 g/kg) was given without carbidopa, to elevate peripheral NA but to elevate central NA minimally, no effect on nociceptive responses was observed (Fig. 2h), despite a prolonged (18 h) remission of their eyelid ptosis, thus confirming the presence of peripheral NA. Carbidopa alone was without any effect on the hyperalgesia of Dbh−/− (data not shown). Administration of the α2 adrenoreceptor agonist UK14,304 (0.0025 to 0.1 mg/kg) produced analgesia in the Dbh−/− mice for heat paw withdrawal (predrug 6.8 ± 0.2 s vs. postdrug 13.3 ± 0.3 s; P < 0.001) and in the controls (predrug 10.9 ± 0.1 vs. postdrug 12.4 ± 0.2 s; P < 0.01) at the highest dose tested (0.1 mg/kg). The antinociceptive effect of DOPS/carbidopa was reversed by the α2 adrenergic-receptor antagonist SKF86466 (0.6 mg/kg; Fig. 3a) but not by the α1 antagonist HEAT (3.4 mg/kg, i.p.; Fig. 3b). SKF86466 produced hyperalgesia in the controls but not in the Dbh−/− mice when higher doses were given alone (2.4 mg/kg, P < 0.01 and 4.8 mg/kg, P < 0.001; Fig. 5d, which is published as supporting information on the PNAS web site).
Figure 3.
Nociceptive testing after noradrenergic replacement, morphine, and NK1-receptor antagonist. (a) The antinociceptive effect of DOPS/carbidopa was reversed by the selective α2 adrenergic-receptor antagonist SKF86466 given 5 h after DOPS/carbidopa administration. (b) The same protocol was used for the selective α1 antagonist HEAT but no effect was seen. (c) The hyperalgesia in Dbh−/− mice was found to depend upon NK1-receptor activation, as latency for hot-plate (50°C) paw withdrawal was normalized by the NK1-receptor antagonist RP-67580 in the Dbh−/− mice, although it had no effect on control mice. (d) The antinociceptive effect of morphine on hot-plate behavior (50°C) was decreased in Dbh−/− mice. Control mice, having reached 100% of the maximal possible effect (% MPE) at 15 mg/kg, displayed no pain behavior at the cutoff time (50 s). Regression analysis showed the curve of Dbh−/− mice had a decreased slope and ED50 value, indicating a decreased efficacy of morphine. When 50 mg/kg of morphine were given, motor effects appeared in Dbh−/− mice. (e) Administration of naltrexone (1 mg/kg) blocks morphine analgesia (10 mg/kg) in controls and Dbh−/− mice on the hot-plate test at 50°C. (f) Five hours after DOPS/carbidopa (2 g/kg,125 mg/kg) administration, morphine efficacy was restored on the hot-plate test. (g) In control mice, the α2 antagonist SKF86466 (4.8 mg/kg) produces a state of resistance to morphine (10 mg/kg) on the 50°C hot-plate test, comparable to that found in Dbh−/− mice. (h) Morphine efficacy also was restored in Dbh−/− mice by administration of the NK1 antagonist L-733,060 (15 mg/kg) 30 min before administering 10 mg/kg morphine (ED70 of controls). (c and d) x axis is log scale. *, P < 0.05; **, P < 0.01; ***, P < 0.001; D/c = DOPS/carbidopa.
Anatomical Studies.
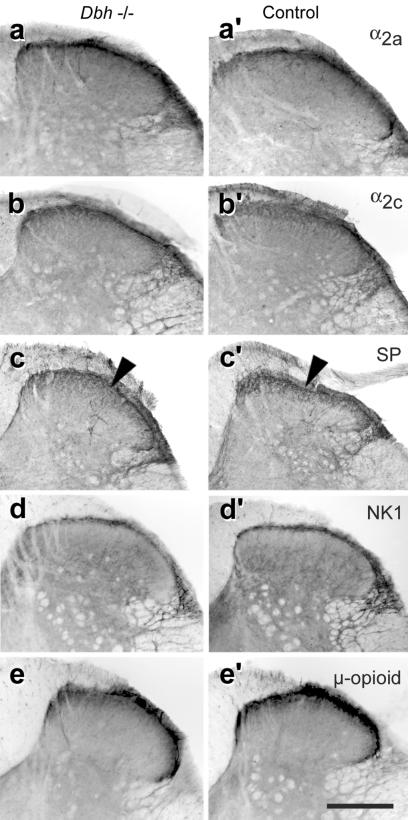
Immunocytochemistry showed a normal distribution of AADC (Fig. 1b and see Fig. 6, which is published as supporting information on the PNAS web site) and TH-containing (Fig. 1d) neuronal profiles in the brainstem noradrenergic nuclei. TH fibers were normally distributed in the spinal cord of Dbh−/− mice (Fig. 1c). Injection of a retrograde tracer into the spinal cord resulted in comparable neuronal labeling in the locus coeruleus and other brainstem catecholaminergic cell groups in Dbh−/− (Fig. 1 d and e) and control mice. The α2A and α2C adrenoreceptors on which NA acts to produce antinociception (24, 25) had a normal distribution in the Dbh−/− mice (Fig. 4 a, a′, b, and b′). We conclude that noradrenergic neurons and their projections are preserved in Dbh−/− mice and that these neurons are likely to be responsible for the restoration of normal nociceptive thresholds after administration of DOPS/carbidopa.
Figure 4.
Immunocytochemistry of the spinal dorsal horn from Dbh−/− and control mice. (a, a′) Immunostaining for α2A receptor. (b, b′) α2C receptor. NK1 receptor (d, d′) and μ-opioid receptor (e, e′) showed no difference between Dbh−/− and control mice. (c, c′) Substance P (SP) labeling differed between Dbh−/− and control mice. There was decreased staining in the Dbh−/− mice, which was confirmed for the superficial layers of the dorsal horn (black arrowhead) by densitometry (see text). For all antigens, the Dbh−/− and control tissues were processed at the same time in adjacent wells under the same conditions. (Bar = 300 μm.)
Effect of NK1 Antagonists on Nociceptive Behavior.
Because part of the antinociceptive effect of noradrenergic-receptor stimulation is thought to be caused by the inhibition of substance P release (3, 26, 27), we examined the effect of three selective substance P-receptor (NK1) antagonists, RP-67580 (0.2 to 6.4 mg/kg; Fig. 3c), CP-96,345 (0.5 to 8.0 mg/kg), and L-733,060 (3.0 to 15.0 mg/kg), on the heat hyperalgesia in Dbh−/− mice. Antagonists to other tachykinin receptors were not tested because the NK1 receptor is likely to be preponderant in the maintenance of a decreased nociceptive threshold (28, 29). All three compounds, but not the inactive enantiomers RP-68651 (6.4 mg/kg) and CP-96,344 (8.0 mg/kg), normalized the hot-plate pain behavior in Dbh−/− mice whereas it had no effect in controls. At the maximal dose of each drug, motor and exploratory behaviors were unaffected. No antinociceptive response to RP-67580 (6.4 mg/kg) was found in Dbh−/− mice in which the nociceptive threshold to hot-plate stimulation had been reestablished to normal values by prior administration of DOPS/carbidopa (1.0 g/kg, 125 mg/kg, given 4.5 h before the NK1 antagonist, data not shown). Densitometric analysis revealed significantly decreased substance P immunoreactivity (Dbh−/−, 3.8 ± 0.12 pixel/103 μm2; controls, 5.35 ± 0.18 pixel/103 μm2; P < 0.01) in the spinal dorsal horn of Dbh−/− mice (Fig. 4 c and c′), whereas NK1-receptor immunoreactivity (Fig. 4 d and d′) was unchanged (Dbh−/−, 13.13 ± 0.36 pixel/103 μm2; controls, 11.7 ± 0.29 pixel/103 μm2; P > 0.05). The antinociceptive effect of the NK1 antagonists in Dbh−/− mice suggests a greater release of substance P in these animals, which is consistent with the decrease in substance P immunoreactivity seen in their dorsal horn compared with controls (Fig. 4 c and c′).
Decreased Antinociceptive Effect of Morphine.
As NA is known to increase opioid antinociception (9, 25), we determined the antinociceptive effect of morphine on hot-plate behavior. As determined by regression analysis, results showed a decrease in the slope of the dose–response curve in Dbh−/− mice (Fig. 3d), indicating a decreased efficacy of this drug. At 50 mg/kg, morphine caused the Dbh−/− mice to be cataleptic and unable to stay on the RotaRod for more than 3 s compared with the cutoff time of 300 s for controls, suggesting that the withdrawal latency on the hot-plate test at this dose was increased because of motor impairment. Evidence that the effect of morphine was mediated through opioid receptors in both groups was obtained by blocking the antinociception by administration of naltrexone (1.0 mg/kg, Fig. 3e). The lack of NA was responsible for the resistance to morphine, as DOPS/carbidopa pretreatment restored the antinociceptive efficacy of morphine in Dbh−/− mice (Fig. 3f). This effect of DOPS/carbidopa was reversed by systemic administration of the α2 antagonist SKF86466 (0.6 mg/kg, data not shown). As expected, administration of SKF86466 (4.8 mg/kg) to control mice reduced the antinociceptive effect of morphine (Fig. 3g). Notably, the μ-opioid receptor on which morphine acts to produce antinociception (30) was equally distributed in the CNS of both Dbh−/− and control mice (Fig. 4 e and e′).
Antagonizing the NK1 Receptor Restores Morphine Efficacy.
Because substance P can block the antinociceptive effect of morphine (31), we tested the possibility that the reduced morphine antinociception seen in Dbh−/− involved NK1-receptor stimulation. Administration of the NK1 antagonist L-733,060 (15 mg/kg) 30 min before administering morphine (10 mg/kg; corresponding to the ED70 of control mice) restored morphine analgesia in Dbh−/− mice (Fig. 3h), indicating a role for substance P in reduced morphine antinociception.
Discussion
Our results suggest that a chronic dysfunction of the endogenous pain inhibitory system, of which NA is a major neurotransmitter, is sufficient to produce a state of chronic hyperalgesia. An ongoing (tonic) stimulation of α2 adrenergic receptors is necessary for mice to maintain a normal nociceptive threshold in accord with early suggestions that NA exerts a tonic inhibition on nociceptive transmission (1, 2). The subtype of α2 adrenoreceptor (A, B, or C) responsible for the tonic activity of NA remains to be determined. Although the α2A receptor is the most likely candidate because it primarily mediates the antinociceptive effect of noradrenergic agonists (32), mice with a point mutation of the α2A receptor have no signs of hyperalgesia (33), which suggests that at least one other subtype, such as the α2C receptor (25), is implicated in the tonic inhibitory effect of NA on nociceptive threshold. Interestingly, mice with a point mutation in the α2A gene show a decreased analgesic effect of morphine (33), indicating that altered response to morphine in the presence of noradrenergic dysfunction is not dependent on the occurrence of hyperalgesia.
The present data also support the notion that substance P plays a critical role in hyperalgesia associated with a lack of NA (3, 4), even in the response to mild noxious stimuli. Although an increased stimulation of the NK1 receptor is an essential feature of the hyperalgesia in the Dbh−/− mice, it must be associated with glutamatergic cotransmission (34). This conclusion is based on observations that release of substance P alone does not result in spontaneous pain behavior (35, 36); rather, it acts as a modulator of nociceptive neurotransmission by contributing to central sensitization (34, 37). Here, the increased release of substance P would be a consequence of chronic decreased NA (4), leading to a reduced nociceptive threshold to thermal stimuli by increasing the gain of nociceptive neurons (38). Finally, in view of the previous reports that NK1 mediates hyperalgesia to highly noxious stimuli (28, 39, 40) or contributes to the pain behavior associated with prolonged or repeated stimuli (41), the present findings that substance P is involved in the withdrawal reflexes to light stimuli was unanticipated. We conclude that NK1-mediated hyperalgesia can be evoked by stimuli resembling those encountered by chronic pain patients. The response of Dbh−/− mice to mild noxious stimuli is analogous to the hyperalgesia reported by individuals suffering from fibromyalgia (42), which is often associated with lower levels of the principal metabolite of NA (3-methoxy-4-hydroxyphenethylene) in the cerebrospinal fluid and increased substance P levels (43–46). Significantly, lack of NA prevents tachyphylaxis to the nociceptive effects of substance P (47, 48) resulting in a sustained decrease in thermal nociceptive threshold (38).
We suggest that the present findings help resolve the contradictory results of previous chronic lesion studies of noradrenergic nuclei (13–17) by showing that altered noradrenergic transmission, whether transitory or chronic, results in a sustained decreased antinociceptive effect of morphine. Because morphine inhibits substance P release in the spinal cord (49), and because NK1-receptor stimulation is essential for the manifestation of the hyperalgesia in Dbh−/− mice, the decreased morphine antinociception in these animals was unexpected. Reestablishment of morphine efficacy by antagonizing the NK1 receptor indicates that substance P is released despite the presence of morphine. This finding concurs with the report of increased morphine antinociception in preprotachykinin A gene knockout mice (40) and the finding that morphine reduces only minimally substance P-induced NK1-receptor internalization (50).
We propose that substance P and opioids have opposite effects on pain behavior, the nociceptive effect of the former being balanced by the antinociceptive effect of the latter. When hyperalgesia is associated with the reduced antinociceptive effect of opioids, this balance is likely to be restored by NK1 antagonists. Whereas most clinical trials have been unable to demonstrate a significant antinociceptive effect of NK1 antagonists (51), these findings suggest that these antagonists might be considered as an adjunct therapy in chronic pain patients receiving morphine.
Supplementary Material
Acknowledgments
We thank J. D. Levine, G. Wilcox, and A. I. Basbaum for their critical comments on the manuscript, J. P. Hieble and GlaxoSmithKline for providing SKF86466, Pfizer (New York) for providing CP-96,345 and CP-96,344, J. E. Maggio and P. W. Mantyh for suppling RP-67580, and Sumitomo Pharmaceutical (Osaka, Japan) for providing DOPS. This work was supported by the American Fibromyalgia Syndrome Association and by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Abbreviations
- NA
noradrenaline
- CNS
central nervous system
- DBH
dopamine β-hydroxylase
- DOPS
L-threo-3,4-dihydroxyphenylserine
- AADC
aromatic amino acid decarboxylase
- MPE
maximum possible effect
- CFA
complete Freund's adjuvant
Footnotes
See commentary on page 549.
Present address: Department of History, Stanford University, Stanford, CA 94305.
References
- 1.Jones S L. Prog Brain Res. 1991;88:381–394. doi: 10.1016/s0079-6123(08)63824-8. [DOI] [PubMed] [Google Scholar]
- 2.Sagen J, Proudfit H K. Brain Res. 1984;310:295–301. doi: 10.1016/0006-8993(84)90152-5. [DOI] [PubMed] [Google Scholar]
- 3.Bourgoin S, Pohl M, Mauborgne A, Benoliel J J, Collin E, Hamon M, Cesselin F. Neuropharmacology. 1993;32:633–640. doi: 10.1016/0028-3908(93)90076-f. [DOI] [PubMed] [Google Scholar]
- 4.Kuraishi Y, Hirota N, Sato Y, Kaneko S, Satoh M, Takagi H. Brain Res. 1985;359:177–182. doi: 10.1016/0006-8993(85)91426-x. [DOI] [PubMed] [Google Scholar]
- 5.Izenwasser S, Kornetsky C. Pain. 1988;33:363–368. doi: 10.1016/0304-3959(88)90297-7. [DOI] [PubMed] [Google Scholar]
- 6.Kellstein D E, Malseed R T, Ossipov M H, Goldstein F J. Neuropharmacology. 1988;27:1–14. doi: 10.1016/0028-3908(88)90194-3. [DOI] [PubMed] [Google Scholar]
- 7.Monasky M S, Zinsmeister A R, Stevens C W, Yaksh T L. J Pharmacol Exp Ther. 1990;254:383–392. [PubMed] [Google Scholar]
- 8.Ossipov M H, Suarez L J, Spaulding T C. Anesth Analg. 1989;68:194–200. [PubMed] [Google Scholar]
- 9.Bohn L M, Xu F, Gainetdinov R R, Caron M G. J Neurosci. 2000;20:9040–9045. doi: 10.1523/JNEUROSCI.20-24-09040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hylden J L, Thomas D A, Iadarola M J, Nahin R L, Dubner R. Eur J Pharmacol. 1991;194:135–143. doi: 10.1016/0014-2999(91)90097-a. [DOI] [PubMed] [Google Scholar]
- 11.Russell I J, Vaeroy H, Javors M, Nyberg F. Arthritis Rheum. 1992;35:550–556. doi: 10.1002/art.1780350509. [DOI] [PubMed] [Google Scholar]
- 12.Basbaum A I, Fields H L. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 13.Berge O G, Ogren S O. Neuropharmacology. 1984;23:1179–1185. doi: 10.1016/0028-3908(84)90236-3. [DOI] [PubMed] [Google Scholar]
- 14.Sawynok J, Reid A. Brain Res. 1987;419:156–165. doi: 10.1016/0006-8993(87)90579-8. [DOI] [PubMed] [Google Scholar]
- 15.Zhong F X, Ji X Q, Tsou K. Eur J Pharmacol. 1985;116:327–330. doi: 10.1016/0014-2999(85)90171-2. [DOI] [PubMed] [Google Scholar]
- 16.Martin W J, Gupta N K, Loo C M, Rohde D S, Basbaum A I. Pain. 1999;80:57–65. doi: 10.1016/s0304-3959(98)00194-8. [DOI] [PubMed] [Google Scholar]
- 17.Fasmer O B, Berge O G, Tveiten L, Hole K. Pharmacol Biochem Behav. 1986;24:1441–1444. doi: 10.1016/0091-3057(86)90207-8. [DOI] [PubMed] [Google Scholar]
- 18.Stjernquist M, Owman C, Sjoberg N O, Sundler F. Biol Reprod. 1987;36:149–155. doi: 10.1095/biolreprod36.1.149. [DOI] [PubMed] [Google Scholar]
- 19.Iravani M M, Zar M A. Br J Pharmacol. 1994;113:877–882. doi: 10.1111/j.1476-5381.1994.tb17074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas S A, Marck B T, Palmiter R D, Matsumoto A M. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- 21.Thomas S A, Palmiter R D. Behav Neurosci. 1997;111:579–589. doi: 10.1037//0735-7044.111.3.579. [DOI] [PubMed] [Google Scholar]
- 22.Jasmin L, Kohan L, Franssen M, Janni G, Goff J R. Pain. 1998;75:367–382. doi: 10.1016/s0304-3959(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 23.Lockhart J C, Ferrell W R, Angerson W J. Int J Microcirc Clin Exp. 1997;17:130–137. doi: 10.1159/000179220. [DOI] [PubMed] [Google Scholar]
- 24.Stone L S, Broberger C, Vulchanova L, Wilcox G L, Hokfelt T, Riedl M S, Elde R. J Neurosci. 1998;18:5928–5937. doi: 10.1523/JNEUROSCI.18-15-05928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fairbanks, C., Posthumus, I., Nguyen, H., Kitto, K., Stone, L. & Wilcox, G. (2002) J. Pharmacol. Exp. Ther., in press. [DOI] [PubMed]
- 26.Collin E, Frechilla D, Pohl M, Bourgoin S, Mauborgne A, Hamon M, Cesselin F. Naunyn–Schmiedeberg's Arch Pharmacol. 1994;349:387–393. doi: 10.1007/BF00170885. [DOI] [PubMed] [Google Scholar]
- 27.Takano M, Takano Y, Yaksh T L. Peptides. 1993;14:371–378. doi: 10.1016/0196-9781(93)90055-l. [DOI] [PubMed] [Google Scholar]
- 28.De Felipe C, Herrero J F, O'Brien J A, Palmer J A, Doyle C A, Smith A J, Laird J M, Belmonte C, Cervero F, Hunt S P. Nature (London) 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- 29.Dougherty P M, Palecek J, Paleckova V, Willis W D. J Neurophysiol. 1994;72:1464–1475. doi: 10.1152/jn.1994.72.4.1464. [DOI] [PubMed] [Google Scholar]
- 30.Loh H H, Liu H C, Cavalli A, Yang W, Chen Y F, Wei L N. Brain Res Mol Brain Res. 1998;54:321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- 31.Sawynok J, Moochhala S M, Pillay D J. Neuropharmacology. 1984;23:741–747. doi: 10.1016/0028-3908(84)90106-0. [DOI] [PubMed] [Google Scholar]
- 32.Hunter J C, Fontana D J, Hedley L R, Jasper J R, Lewis R, Link R E, Secchi R, Sutton J, Eglen R M. Br J Pharmacol. 1997;122:1339–1344. doi: 10.1038/sj.bjp.0701520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone L S, MacMillan L B, Kitto K F, Limbird L E, Wilcox G L. J Neurosci. 1997;17:7157–7165. doi: 10.1523/JNEUROSCI.17-18-07157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrero J F, Laird J M, Lopez-Garcia J A. Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 35.Malcangio M, Ramer M S, Boucher T J, McMahon S B. Eur J Neurosci. 2000;12:139–144. doi: 10.1046/j.1460-9568.2000.00890.x. [DOI] [PubMed] [Google Scholar]
- 36.Sweeney M I, Sawynok J. Can J Physiol Pharmacol. 1986;64:1324–1327. doi: 10.1139/y86-224. [DOI] [PubMed] [Google Scholar]
- 37.Holland L N, Goldstein B D. Pain. 1994;56:339–346. doi: 10.1016/0304-3959(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 38.Dirig D M, Yaksh T L. Neurosci Lett. 1996;220:93–96. doi: 10.1016/s0304-3940(96)13230-4. [DOI] [PubMed] [Google Scholar]
- 39.Mantyh P W, Rogers S D, Honore P, Allen B J, Ghilardi J R, Li J, Daughters R S, Lappi D A, Wiley R G, Simone D A. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 40.Cao Y Q, Mantyh P W, Carlson E J, Gillespie A M, Epstein C J, Basbaum A I. Nature (London) 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- 41.Duggan A W, Riley R C, Mark M A, MacMillan S J, Schaible H G. Neuroscience. 1995;65:849–858. doi: 10.1016/0306-4522(94)00541-c. [DOI] [PubMed] [Google Scholar]
- 42.Staud R, Vierck C J, Cannon R L, Mauderli A P, Price D D. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 43.Russell I J, Orr M D, Littman B, Vipraio G A, Alboukrek D, Michalek J E, Lopez Y, MacKillip F. Arthritis Rheum. 1994;37:1593–1601. doi: 10.1002/art.1780371106. [DOI] [PubMed] [Google Scholar]
- 44.Vaeroy H, Helle R, Forre O, Kass E, Terenius L. Pain. 1988;32:21–26. doi: 10.1016/0304-3959(88)90019-X. [DOI] [PubMed] [Google Scholar]
- 45.Welin M, Bragee B, Nyberg F, Kristiansson M. J Musc Pain. 1995;3:4. [Google Scholar]
- 46.White K P, Harth M. Curr Pain Headache Rep. 2001;5:320–329. doi: 10.1007/s11916-001-0021-2. [DOI] [PubMed] [Google Scholar]
- 47.Larson A A. Pain. 1988;32:367–374. doi: 10.1016/0304-3959(88)90049-8. [DOI] [PubMed] [Google Scholar]
- 48.Holland L N, Goldstein B D, Aronstam R S. Brain Res. 1993;600:89–96. doi: 10.1016/0006-8993(93)90405-c. [DOI] [PubMed] [Google Scholar]
- 49.Aimone L D, Yaksh T L. Peptides. 1989;10:1127–1131. doi: 10.1016/0196-9781(89)90003-x. [DOI] [PubMed] [Google Scholar]
- 50.Trafton J A, Abbadie C, Marchand S, Mantyh P W, Basbaum A I. J Neurosci. 1999;19:9642–9653. doi: 10.1523/JNEUROSCI.19-21-09642.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rupniak N M, Hill R G. In: Novel Aspects of Pain Management: Opioids and Beyond. Sawynok J, Cowan A, editors. New York: Wiley–Liss; 1999. pp. 135–155. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.