Abstract
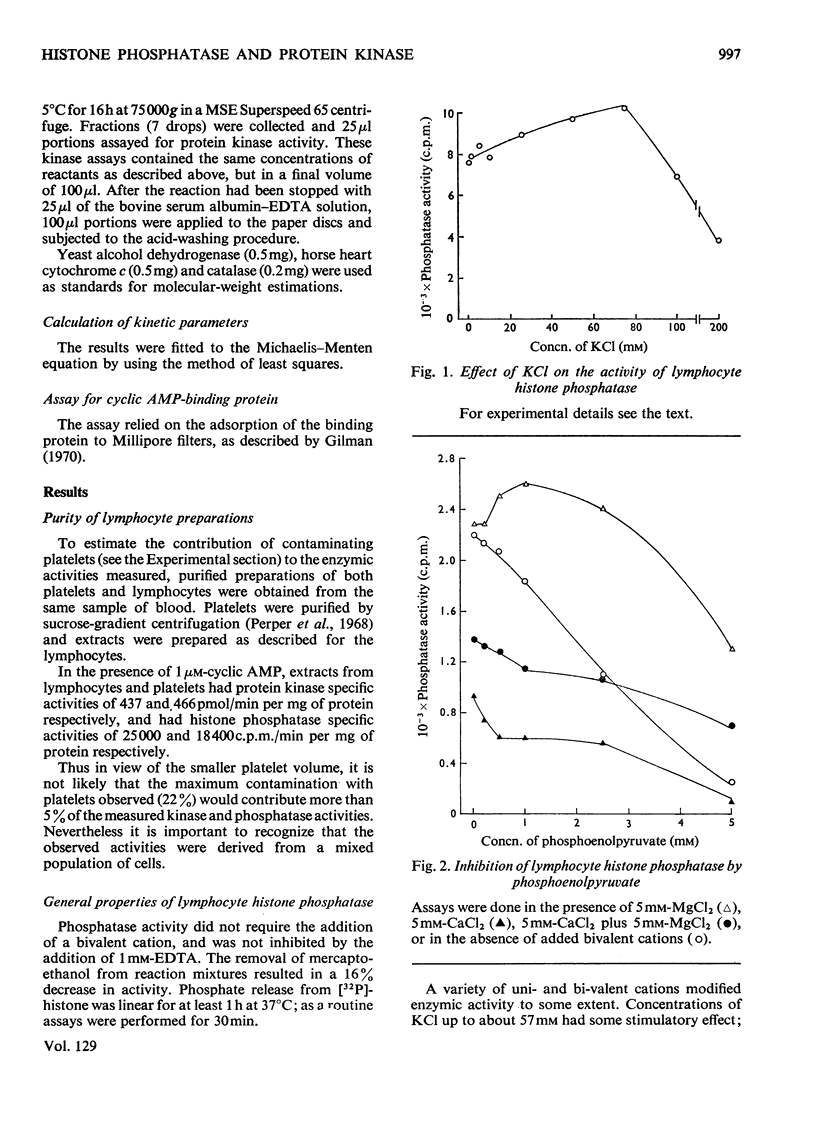
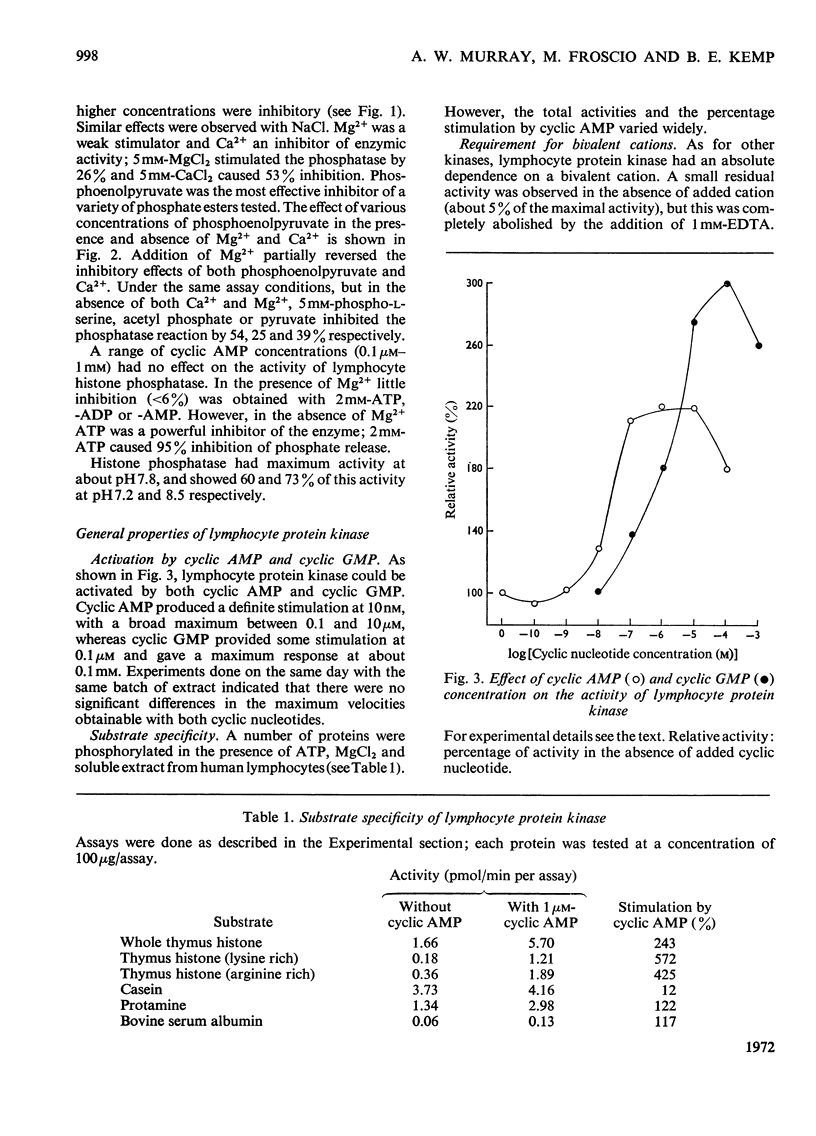
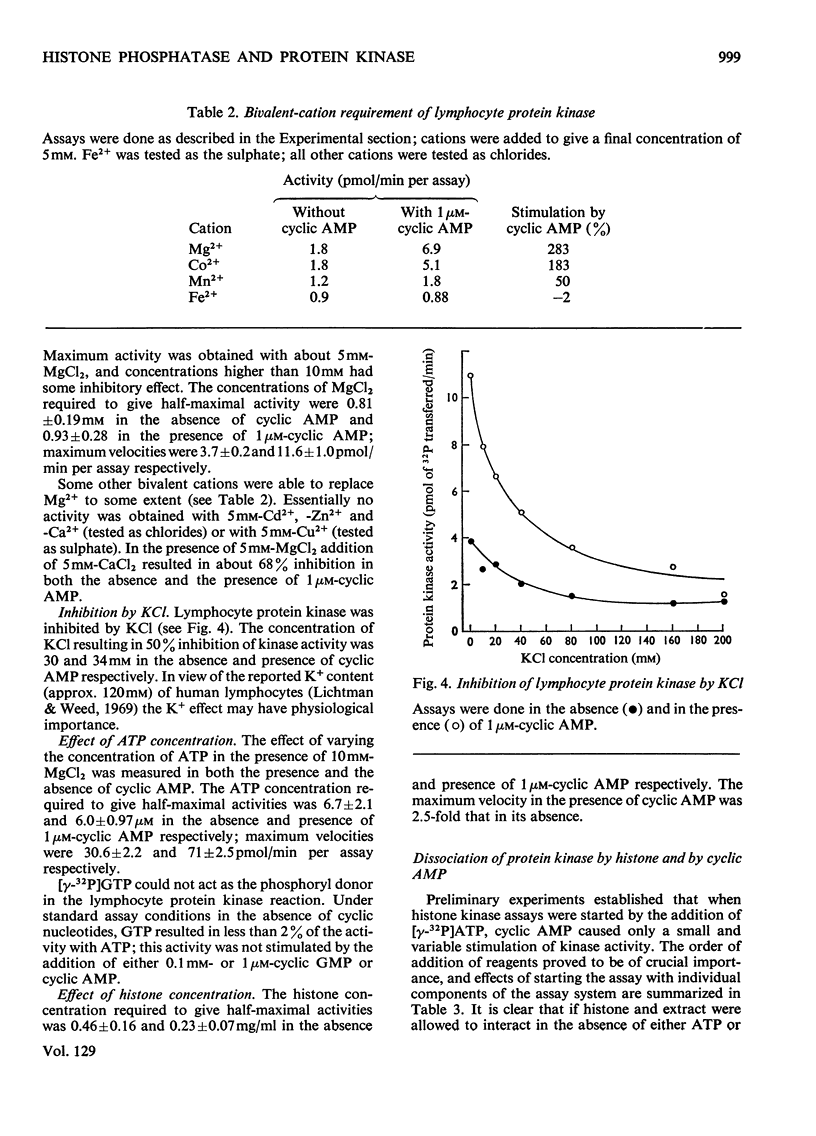
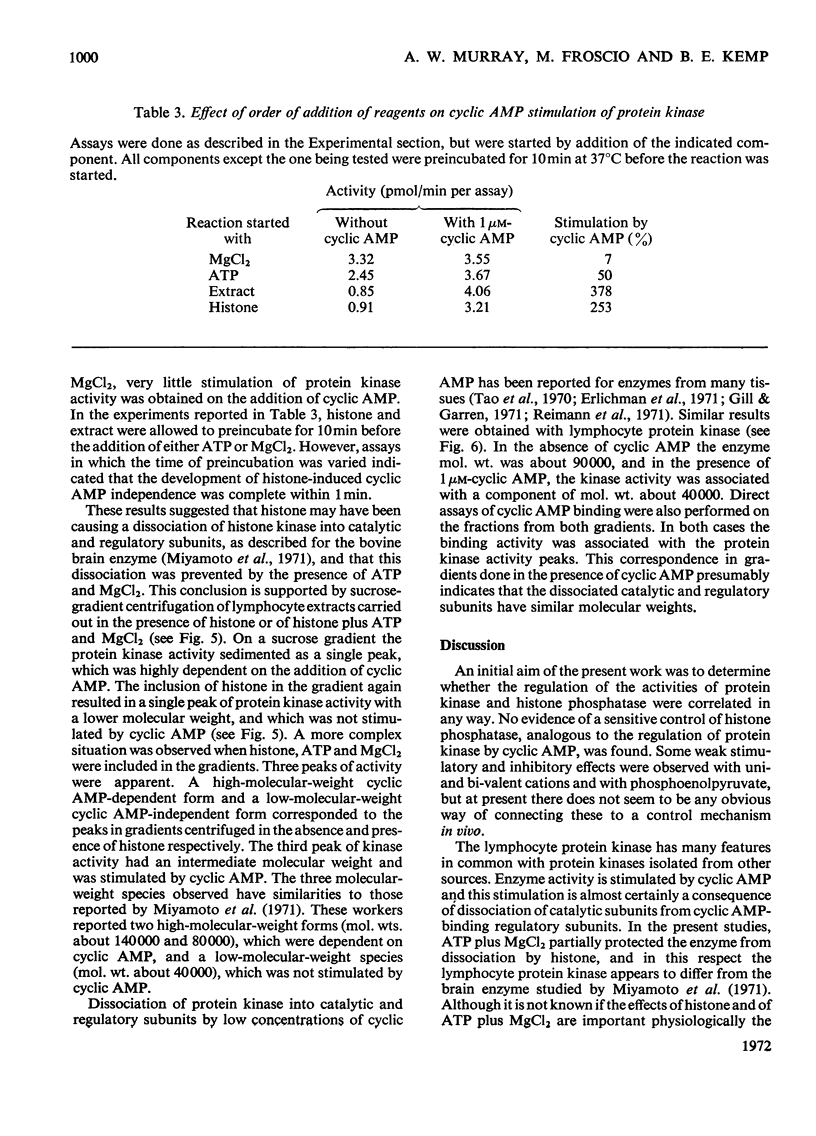
1. Extracts of human peripheral blood lymphocytes contained a histone phosphatase that catalysed the release of Pi from phosphorylated whole thymus histone. 2. Stimulation of the phosphatase was obtained by concentrations of KCl and NaCl of up to 75mm, and by MgCl2; CaCl2 inhibited the enzymic activity. 3. In the absence of MgCl2, phosphoenol-pyruvate inhibited histone phosphatase activity; this inhibition could be partially reversed by adding MgCl2 to assays. 4. Lymphocyte extracts contained a protein kinase activity which was maximally stimulated by 1μm-cyclic AMP (adenosine 3′:5′-cyclic monophosphate) or by 0.1mm-cyclic GMP (guanosine 3′:5′-cyclic monophosphate). 5. Incubation of the enzyme with histone in the absence of ATP or MgCl2 resulted in the dissociation of the enzyme into a lower-molecular-weight species that was not stimulated by cyclic AMP. This effect could be prevented if ATP and MgCl2 were present in reaction mixtures before histone and enzyme were allowed to interact. 6. Cyclic AMP also dissociated the kinase into a lower-molecular-weight species. 7. In the presence of 1μm-AMP, half-maximal activities were obtained with 0.92mm-MgCl2, 6.0μm-ATP and 0.23mg of whole thymus histone/ml.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cross M. E., Ord M. G. Changes in histone phosphorylation and associated early metabolic events in pig lymphocyte cultures transformed by phytohaemagglutinin or 6-N,2'-O-dibutyryladenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Aug;124(1):241–248. doi: 10.1042/bj1240241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlichman J., Hirsch A. H., Rosen O. M. Interconversion of cyclic nucleotide-activated and cyclic nucleotide-independent forms of a protein kinase from beef heart. Proc Natl Acad Sci U S A. 1971 Apr;68(4):731–735. doi: 10.1073/pnas.68.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G. N., Garren L. D. Role of the receptor in the mechanism of action of adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Apr;68(4):786–790. doi: 10.1073/pnas.68.4.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G., Mirsky A. E. Phosphoprotein metabolism in isolated lymphocyte nuclei. Proc Natl Acad Sci U S A. 1966 May;55(5):1182–1189. doi: 10.1073/pnas.55.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. The monovalent cation content and adenosine triphosphatase activity of human normal and leukemic granulocytes and lymphocytes: relationship to cell volume and morphologic age. Blood. 1969 Nov;34(5):645–660. [PubMed] [Google Scholar]
- Meisler M. H., Langan T. A. Characterization of a phosphatase specific for phosphorylated histones and protamine. J Biol Chem. 1969 Sep 25;244(18):4961–4968. [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto E., Petzold G. L., Harris J. S., Greengard P. Dissociation and concomitant activation of adenosine 3',5'-monophosphate-dependent protein kinase by histone. Biochem Biophys Res Commun. 1971 Jul 16;44(2):305–312. doi: 10.1016/0006-291x(71)90600-0. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Froscio M. Cyclic adenosine 3':5'-monophosphate and microtubule function: specific interaction of the phosphorylated protein subunits with a soluble brain component. Biochem Biophys Res Commun. 1971 Sep;44(5):1089–1095. doi: 10.1016/s0006-291x(71)80197-3. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Perper R. J., Zee T. W., Mickelson M. M. Purification of lymphocytes and platelets by gradient centrifugation. J Lab Clin Med. 1968 Nov;72(5):842–848. [PubMed] [Google Scholar]
- Reimann E. M., Brostrom C. O., Corbin J. D., King C. A., Krebs E. G. Separation of regulatory and catalytic subunits of the cyclic 3',5'-adenosine monophosphate-dependent protein kinase(s) of rabbit skeletal muscle. Biochem Biophys Res Commun. 1971 Jan 22;42(2):187–194. doi: 10.1016/0006-291x(71)90086-6. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Newberry W. M., Jr, Parker C. W. Cyclic adenosine 3',5'-monophosphate in human lymphocytes. Alterations after phytohemagglutinin stimulation. J Clin Invest. 1971 Feb;50(2):432–441. doi: 10.1172/JCI106510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao M., Salas M. L., Lipmann F. Mechanism of activation by adenosine 3':5'-cyclic monophosphate of a protein phosphokinase from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1970 Sep;67(1):408–414. doi: 10.1073/pnas.67.1.408. [DOI] [PMC free article] [PubMed] [Google Scholar]


