Abstract
Synaptic depression is thought to underlie the loss of cortical responsiveness to an eye deprived of vision. Here, we establish a fundamental role for type 2 metabotropic glutamate receptors (mGluR2) in long-term depression (LTD) of synaptic transmission within primary visual cortex. Direct mGluR2 activation by (2S,2′R,3′R-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV) persistently depressed layer 2/3 field potentials in slices of mouse binocular zone when stimulated concomitantly. Chemical LTD was independent of N-methyl-d-aspartate (NMDA) receptors but occluded conventional LTD by low-frequency stimulation, indicating shared downstream events. Antagonists or targeted disruption of mGluR2 conversely prevented LTD induction by electrical low-frequency stimulation to layer 4. In contrast, Schaeffer collateral synapses did not exhibit chemical LTD, revealing hippocampal area CA1, naturally devoid of mGluR2, to be an inappropriate model for neocortical plasticity. Moreover, monocular deprivation remained effective in mice lacking mGluR2, and receptor expression levels were unchanged during the critical period in wild-type mice, indicating that experience-dependent plasticity is independent of LTD induction in visual cortex. Short-term depression that was unaffected by mGluR2 deletion may better reflect circuit refinement in vivo.
Early experience shapes neuronal circuits in the developing brain. Both short-term depression (STD; refs. 1–3) and long-term synaptic depression (LTD; refs. 4–7) within neocortex have been proposed to signal susceptibility to sensory deprivation. Homosynaptic weakening of inputs is an attractive model for the loss of responsiveness in visual cortex after monocular occlusion (8). Saturating LTD requires residual activity from the closed eye to have maximal effects, and evidence has been offered in favor of this view (9). Yet, open eye inputs are also readily lost in the presence of d-amino-5-phosphonovalerate (D-APV) (10), an N-methyl-d-aspartate (NMDA) receptor antagonist that prevents LTD induction (5). Detailed understanding of the mechanisms that trigger homosynaptic LTD within visual cortex is required to determine whether it can be induced by monocular deprivation (MD) and contribute to the subsequent loss of responsiveness in vivo.
Direct demonstration of LTD dissociation from MD effects has come from gene-targeted animals (11, 12), as well as infusion of the metabotropic glutamate receptor (mGluR) antagonist α-methyl-4-carboxyphenylglycine (MCPG) into kitten visual cortex (13, 14). The ability to block visual cortical LTD with MCPG has been contested, however, on the basis of analogy with area CA1 of the hippocampus (15, 16). Indeed, MCPG is a broad-spectrum antagonist (17), and it is essential to identify which of the eight individual mGluR subtypes, if any, is involved in visual cortical LTD (18). It has further been reported that synergistic action of multiple mGluRs may potently activate cAMP production in correlation with the peak of the critical period for MD effects in kittens (19). Moreover, activation of protein synthesis (20) or mitogen-activated protein kinases (21) by synaptic mGluRs may link rapid physiological events with structural changes known to accompany sensory deprivation (22, 23).
To specifically address a role for mGluRs in visual cortical plasticity, we have focused on the type 2 isoform for several reasons. Group II mGluR laminar expression and sensitivity to agonists is correlated with the critical period in kittens, and all are delayed by dark-rearing from birth (24, 25). In addition, mGluR2 is a well known presynaptic autoreceptor (17, 26). Accumulating glutamate during brief trains of neuronal activity may transiently depress synaptic transmission by binding to these sites (27). Finally, highly selective pharmacological reagents (28–30) as well as mice lacking mGluR2 are now available (31). These tools offer an ideal opportunity to probe the link between synaptic depression and ocular dominance plasticity.
Our findings confirm a fundamental role for mGluRs in LTD of visual cortex that is distinct from hippocampal area CA1. The results suggest a unified framework for understanding previously conflicting experimental paradigms for inducing neocortical LTD by either high-frequency stimulation (HFS; refs. 4, 32, and 33) or low-frequency stimulation (LFS; refs. 5 and 6). Moreover, direct comparison to MD effects in mGluR2 knockout (KO) mice verifies mechanistic dissociation from homosynaptic LTD and mGluR2 function (13, 14). Appropriate synaptic gain control by STD is instead strengthened as an important indicator of experience-dependent plasticity during cortical development (2, 3).
Methods
Mice carrying a functional disruption of mGluR2 were generated by insertion of a neomycin-resistance cassette into exon 2 of the gene, as reported (31). Electro-physiological recordings in vitro were obtained at the peak of the critical period [postnatal days 24–35 (P24–35)] by using standard methods, as described (11). Coronal slices (350 μm) of mouse visual cortex were cut and incubated (>1 h, 33°C) in equilibrated (95% O2/5% CO2) artificial cerebrospinal fluid (ACSF). The ACSF contained: 119 mM NaCl, 2.5 mM KCl, 1.3 mM MgSO4, 10 mM NaH2PO4, 26.2 mM NaHCO3, 2.5 mM CaCl2, and 11 mM glucose. Slices were transferred to a recording chamber superfused with the same ACSF (33°C). Half-maximal amplitudes of field potentials evoked from layer 4 by a bipolar glass stimulating electrode filled with ACSF were recorded through a glass electrode filled with 1 M NaCl (1–3 MΩ) in layer 2/3 of the binocular zone. Input–output curves, latency, and half-widths did not differ between mGluR2 wild-type (WT) and KO recorded blind to genotype.
LTD Experiments.
Stable, baseline responses were recorded (>15 min, 0.1 Hz) before applying drugs or LFS (900 pulses, 1 Hz). Subtype-selective mGluR reagents (Tocris Neuramin, Bristol, U.K.) were diluted from stock solution (0.1 M NaOH or H2O) into bath ACSF at final concentrations indicated in text: (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV; refs. 28 and 29), (2S,3S,4S)-2-methyl-2-(carboxycyclopropyl)glycine (MCCG; ref. 30), and (R,S)-1-aminoindan-1,5-dicarboxylic acid (AIDA; ref. 34). Ionotropic glutamate receptor antagonists were applied during conditioning or to confirm the synaptic component of field responses after each recording: 6,7-cyanonitroquinoxaline dione (CNQX, Tocris Neuramin); d-APV (Sigma). In some experiments, NMDA receptor function was enhanced by increasing extracellular CaCl2 (5 mM), decreasing MgSO4 (0.1 mM), and including a partial agonist, d-cycloserine (35) (Sigma).
STD Experiments.
Stable baseline responses (>5 min, 0.2 Hz) were followed by trains (30 pulses) of various frequencies at >1-min rest interval between each. Six frequencies were randomly tested during an experiment, and each train was repeated five times. Synaptic responses were carefully monitored to ensure no long-term changes in efficacy occurred and were confirmed pharmacologically. Maximum field potential amplitude was measured from the synaptic component of the raw response. Five responses before, all responses during, and five responses immediately after each stimulus train were normalized to baseline. Steady-state STD was taken as the mean final response/train.
Ocular Dominance Experiments.
Mice were prepared blind to genotype for electro-physiological recording in vivo under Nembutal (50 mg/kg, Abbott) and chlorprothixene (0.2 mg, Sigma) anesthesia by using standard techniques (11, 12, 36). For each animal, 5–8 cells (>75 μm apart) were recorded in each of 4–6 vertical penetrations spaced evenly (>200-μm intervals) across the mediolateral extent of primary visual cortex to map the monocular and binocular zones and to avoid sampling bias. Receptive fields of isolated single units were plotted on a tangent screen with a hand-held projection lamp. Cells were assigned ocular dominance scores according to the seven-point classification scheme of Wiesel and Hubel (37). A weighted average of the bias toward one eye or the other, the contralateral bias index, was calculated for each binocular zone according to the formula: contralateral bias index = [(n1 − n7) + (2/3)(n2 − n6) + (1/3)(n3 − n5) + N]/2N, where N = total number of cells and nx = number of cells of ocular dominance score equal to x. For MD experiments, eyelid margins were trimmed and sutured shut under halothane anesthesia for 4 days. Recordings were obtained contralateral to deprived eye. Daily intracerebroventricular injections of AIDA (50 mM) were performed throughout MD to block group I mGluRs in vivo. Effective concentration was estimated to be 100-fold less within visual cortex on the basis of previous diffusion measurements for molecules of similar size (e.g., diazepam, d-APV, and MCPG) (3, 10, 12, 13).
mGluR2 Immunohistochemistry.
Mice before (<P23), during (P24–35), or after (>P45) the critical period (3, 36) were perfused transcardially with PBS under Nembutal anesthesia, followed by 3.5% paraformaldehyde, 1% picric acid, and 0.05% glutaraldehyde in 0.1 M PBS (pH 7.3). Brains were further fixed (3 days, 4°C) in 2% formaldehyde/1% picric acid/0.1 M PBS (pH 7.3) (38). After cryoprotection (20% sucrose/0.1 M PBS; overnight, 4°C), tissue sections (50 μm) were cut on a freezing microtome. Sections were incubated (overnight, 15°C) in PBS containing 2% normal goat serum and 0.1% Triton X-100 with 0.5–1.0 μg/ml mAb (mG2Na-5) raised against putative extracellular amino acid residues 87–134 of mGluR2, as described (38). After PBS wash, sections were incubated in biotinylated goat anti-mouse IgG (Vector Laboratories), rewashed, and then reacted with ABC kit (Vector Laboratories) and 0.05% diaminobenzidine/0.0006% H2O2 for chromogen formation.
Results
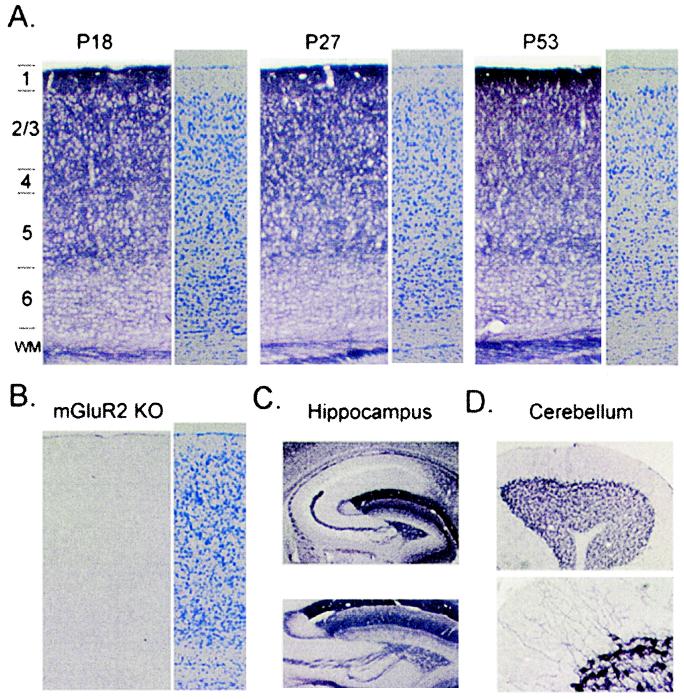
We first determined the cellular localization of mGluR2 by using a mAb that recognizes only this subtype of group II mGluRs coupled to adenylate cyclase (38). As originally reported for rats, most intense mGluR2 immunoreactivity in WT mouse brain was observed in supragranular layers of neocortex. In the binocular zone of primary visual cortex, no apparent changes in labeling intensity were observed across laminae before (<P23), during (P24–35), or after (>P45) the critical period for experience-dependent plasticity (Fig. 1A) (3, 36). Staining specificity was confirmed by the complete absence of signal in mGluR2 KO mice, which maintained normal gross cortical morphology (Fig. 1B). The densely positive cortical neuropil may express mGluR2 at both pre- and postsynaptic sites. In other regions, either pre- (hippocampal mossy fibers, Fig. 1C) or postsynaptic (cerebellar Golgi cells, Fig. 1D) elements were visualized in isolation, as reported earlier for rats (38, 39). Notably, some areas (e.g., CA1 of hippocampus) were entirely devoid of mGluR2 (Fig. 1C), predicting a differential involvement of this receptor in synaptic function across brain regions.
Figure 1.
mGluR2 expression in mouse visual cortex. (A) Upper layers of binocular zone densely labeled across late postnatal development: before (P18), during (P27), or after (P53) the critical period for MD effects (3). Alternate sections, Nissl stain (three mice per age group). (B) No signal in mGluR2 KO mice confirms Ab specificity with no gross change of laminar organization because of deletion (Nissl). (C) Complete absence of mGluR2 in area CA1 of WT mice (Upper). Presynaptic mGluR2 in mossy fiber axons emerging from hippocampal dentate gyrus (Lower). (D) Postsynaptic mGluR2 in somata and dendrites of cerebellar Golgi cells.
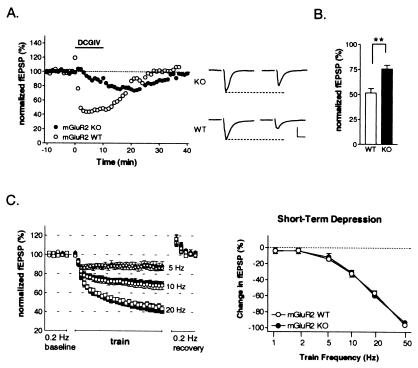
Direct stimulation of mGluR2 in slices of visual cortex with the group II mGluR-selective agonist DCG-IV (1 μM) (28, 29) produced a rapid and powerful depression of synaptic transmission (Fig. 2A), consistent with a presynaptic locus (26). Depressed responses stabilized within a 10-min application period and recovered by 20 min after drug washout. In slices lacking mGluR2, only a small, gradual response decrement was observed, reflecting DCG-IV affinity for mGluR3 (29). Maximal depression and onset time course differed significantly between KO and WT (Fig. 2 A and B). Bursts of electrical stimuli also produce a rapid, presynaptic STD of synaptic transmission within neocortex (2, 30, 40–42). To determine a role for mGluR2 in this process, we compared STD induction over a range of burst intervals (1–50 Hz) in KO and WT slices. Steady-state depression levels during a 30-pulse train were indistinguishable across frequencies between KO and WT (Fig. 2C). Posttrain augmentation also did not differ between groups (17 ± 2% vs. 15 ± 1% for frequencies >5 Hz, 6–8 slices WT and KO, respectively; P > 0.3, t test).
Figure 2.
Presynaptic depression without mGluR2 autoreceptors in visual cortex. (A) Layer 2/3 synaptic field potentials (fEPSP) decrease rapidly and reversibly during brief DCG-IV (1 μM) application to WT slices. Note slower, weaker profile of mGluR2 KO. Sample traces before and after drug (scale bar = 500 μV, 5 msec). (B) Reduced depression in KO vs. WT (mean ± SEM; six slices, three mice each; **, P < 0.01). (C) Similar STD during brief physiological bursts of synaptic activity in KO and WT over a range of stimulus trains (mean ± SEM; six to eight slices, four mice per frequency). Some error bars smaller than symbols.
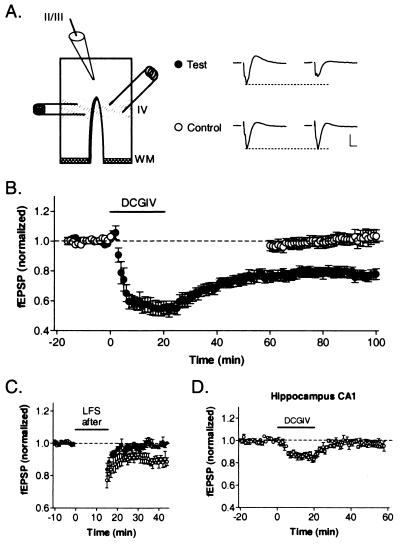
Interestingly, extended exposure (>15 min) to mGluR2 agonist produced a depression of field responses in WT slices that did not recover to baseline levels upon washout. To systematically explore this observation, a two-pathway experiment was designed in which standard coronal slices were split from white matter up to layer 4 (Fig. 3A). The independence of inputs stimulated from either side of the cut in layer 4 was confirmed by additive responses in layer 2/3 that showed no interaction when closely paired stimuli were presented across the two sites. For each experiment, one pathway (test) was continuously stimulated at baseline frequency (0.1 Hz), whereas the other (control) was switched off during drug application and washout. Only the test pathway was persistently depressed by DCG-IV (●, Fig. 3B), revealing a form of coincidence detection requiring both synaptic activity and mGluR2 stimulation. Moreover, unaltered control responses confirmed the independence of the two pathways as well as complete washout of drug, eliminating the possibility that prolonged depression of the test input was because of the continued presence and agonist action of DCG-IV (○, Fig. 3B).
Figure 3.
LTD by prolonged mGluR2 activation in visual cortex but not CA1. (A) Two-pathway recording configuration with representative traces before and after DCG-IV. Control input was stopped during drug application, whereas test stimulation continued at baseline frequency (0.1 Hz). (Scale bar = 500 μV, 5 ms.) (B) Only test input (●, six slices, six mice) is persistently depressed after DCG-IV (1 μM, 20 min) washout from bath. Note unaltered control path (○). (C) Subsequent LFS fails to induce LTD along previously depressed test path (●, five slices), whereas control input is still susceptible (○, four slices). (D) Prolonged DCG-IV (1 μM, 20 min) exposure does not persistently depress synaptic transmission in WT hippocampal area CA1 (six slices).
To determine whether this depression shared mechanisms in common with conventional LTD produced by electrical LFS (5), a stimulus train (900 pulses, 1 Hz) was further delivered to either pathway once a stable depression had been established (>40 min) by DCG-IV. Only control inputs that had not previously been affected by the drug underwent LTD by LFS (Fig. 3C). Prolonged mGluR2 stimulation in conjunction with synaptic activity thus produced a saturating LTD converging upon mechanisms triggered by electrical LFS.
Hippocampus and neocortex have been proposed to share common forms of synaptic plasticity in vitro (43). We, therefore, examined whether 20-min exposure to DCG-IV would induce a similar LTD in area CA1 of WT mouse hippocampus. As expected from the complete absence of mGluR2 in this region (Fig. 1C), Schaeffer collateral responses in stratum radiatum decreased only gradually and modestly, recovering quickly after drug washout (Fig. 3D). This result was reminiscent of DCG-IV action through mGluR3 in the neocortex of mGluR2 KO mice (Fig. 2A). Conversely, LFS in area CA1 of mGluR2 KO mice produced LTD (84 ± 2% 25 min after LFS, n = 7).
A fundamental feature of LTD induction by LFS in rats is dependence on NMDA receptor activation (5). We confirmed a disruption of LTD in slices of WT mouse visual cortex exposed to the NMDA receptor antagonist d-APV (50 μM; Fig. 4A). In contrast, LTD produced by prolonged DCG-IV application was enhanced by d-APV (Fig. 4B vs. Fig. 3B; 71 ± 3% vs. 79 ± 3%, respectively; P < 0.01, t test). These results rule out the possibility that the mGluR2 ligand induced LTD by means of NMDA receptors, for which DCG-IV is a partial agonist at higher concentrations (29, 44). We, therefore, determined whether mGluR2 is an independent or essential component of neocortical LTD by standard LFS. Depression was reversibly blocked in WT slices exposed to an mGluR2-selective antagonist, MCCG (100 μM) (30), recovering after drug washout (Fig. 4C). Similarly, no LTD by LFS was observed in slices of visual cortex from mGluR2 KO mice studied blind to genotype and compared to WT (Fig. 4D).
Figure 4.
LFS-induced LTD in visual cortex by mGluR2. (A) LTD by LFS (900 pulses, 1 Hz) blocked by d-APV (50 μM, seven slices, five WT mice). (B) Robust LTD by DCG-IV (1 μM, 20 min) in d-APV (50 μM, 10 slices, five WT mice). (C) LTD by LFS in WT (six slices, three mice) blocked by mGluR2 antagonist, MCCG (100 μM, left), reversibly (>40-min wash, right). (D) No LTD by LFS in mGluR2 KO (●, 10 slices, four mice) vs. WT (○, nine slices, four mice). (E) Weak, decaying depression by LFS when NMDA receptor function is enhanced (50:1 Ca2+/Mg2+ ratio, 10 μM d-cycloserine) in mGluR2 KO (○, five slices, three mice). Further group I mGluR blockade unmasks potentiation by LFS (●, 500 μM AIDA; four slices, two mice).
A recent report of LTD in perirhinal cortex (45) further suggests that enhanced NMDA receptor function may override a requirement for mGluR2 activation, while maintaining a synergistic role for mGluR1. We, therefore, applied LFS to slices from mGluR2 KO mice bathed in elevated calcium (5 mM), reduced magnesium (0.1 mM) solution containing d-cycloserine (10 μM) (35), a positive allosteric modulator of the NMDA receptor (Fig. 4E). Under these conditions, a weak and variable depression was observed that tended to gradually decay back to baseline over 35 min. Additional blockade of group I mGluRs with AIDA (500 μM) (34) yielded potentiation of responses after LFS. This result is consistent with the earlier observation of unmasked potentiation when multiple mGluRs are blocked by the broad spectrum antagonist MCPG (13).
A specific role for individual mGluR subtypes in experience-dependent plasticity of the visual cortex has never been examined directly. To address this issue, we obtained extracellular, single-unit recordings blind to genotype from mGluR2 KO and WT mice in vivo. In the absence of mGluR2, the distribution of input strength from the two eyes (ocular dominance) developed normally (Fig. 5 A and B, Upper), exhibiting a contralateral bias within the binocular zone typical of mature mouse visual cortex (3, 11, 12, 36). MD during the developmental critical period shifts ocular dominance distributions in favor of the open eye (3, 37). Suturing one eyelid shut for 4 days (at P25–28) yielded similarly robust loss of deprived-eye input in both mGluR2 KO and WT mice (Fig. 5 A and B, Lower).
Figure 5.
Ocular dominance plasticity in the absence of mGluR2. (A) Typical contralateral eye bias (ocular dominance <4) of WT (Upper, 96 cells, four mice) shifts toward open, ipsilateral eye after brief MD during critical period (Lower, 82 cells, four mice). (B) mGluR2 KO shift similarly after MD (Upper, nondeprived: 93 cells, four mice; Lower, MD: 185 cells, eight mice). The contralateral bias index (range 0–1, upper right corner) decreases from typically high values in mice when MD is effective (3, 11, 12, 36). (C) Ocular dominance plasticity is unimpaired in mGluR2 KO mice treated during MD with vehicle (Upper, 50 mM NaOH intracerebroventricularly; 115 cells, five mice) or group I mGluR antagonist (Lower, 50 mM AIDA, intracerebroventricularly; 133 cells, six mice).
Given that NMDA receptor function dominates visual responsiveness in vivo (46), LTD machinery may have been modestly engaged in the absence of mGluR2 (Fig. 4E). To eliminate potential synergistic effects of enhanced NMDA receptor function, we further injected the group I mGluR antagonist AIDA (50 mM, intracerebroventricularly) (47) into mGluR2 KO mice throughout the MD period. At this dose (see Methods), LTD is blocked in vitro (Fig. 4E). Recorded blind to drug or vehicle infusion, both groups still exhibited normal ocular dominance plasticity (Fig. 5C).
Discussion
In visual cortex, experience-dependent modifications occur only during a discrete time window in early life (3, 37). Subtype-specific immunostaining revealed no alteration in mGluR2 expression before, during, or after the critical period for plasticity (Fig. 1). Using a nonselective Ab in kittens, a previous study reported intense layer 4 staining at critical period onset followed by upper layers (25) that may rather depict the other group II subtype, mGluR3. Sustained activation of mGluR3 could be thought to substitute for mGluR2 to produce LTD in KO mice, but this possibility seems unlikely, given the primary localization of mGluR3 to glial processes (48). Functional changes in mGluR efficacy have also been correlated with critical period time course (24, 49), but do not appear to determine MD effects (Fig. 5) (13). Synergistic action of multiple mGluRs in kitten visual cortex may, for instance, reflect fluctuations of other factors during the critical period (19), such as endogenous cAMP levels or adenosine (50, 51).
Our findings clearly demonstrate that hippocampal area CA1 is an inaccurate model of visual cortical plasticity, contrary to previous proposals (43). Basic mechanisms for inducing long-term changes in synaptic efficacy can differ strikingly across brain regions. Group II mGluRs are especially important for triggering LTD in septal (52) and neocortical areas, including barrel (53), perirhinal (45, 54), prefrontal (55), and now visual cortex. In hippocampus, only LTD of dentate gyrus granule cells and their axons is reported to use mGluR2 (31, 56, 57). In area CA1 (58–61) or cerebellar Purkinje cells (62, 63) where mGluR2 is not expressed (Fig. 1 C and D), LTD is triggered by group I mGluRs. Similarly, long-term potentiation (LTP) and short-term plasticity may be distinct at excitatory synapses of hippocampus and neocortex (64, 65).
In the neocortex, LTD can be induced by two very different stimulus protocols. Although bursts of HFS are effective only when NMDA receptors are blocked (4, 32, 33, 66), NMDA receptors must be activated by LFS to produce LTD (5–7). These fundamentally contradictory paradigms could reflect nonoverlapping forms of plasticity as in hippocampus (60), but an mGluR component may offer a unified view of neocortical LTD mechanism. Intracellular calcium thresholds dictate the direction of plasticity within cortical neurons (67–70). A delicate homeostatic balance between LTP and LTD may exist (71), perhaps through distinct kinase cascades for bidirectional modification of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (56, 57, 72, 73). During HFS, strong calcium influx via NMDA receptors is prone to activate Ca2+/calmodulin-dependent protein kinase II, leading to LTP (72, 74). Only when counteracting efforts of enhanced NMDA currents are blocked, either by d-APV (4, 32, 33, 66) or weak intracellular calcium chelation (75, 76), can depression by mGluRs be revealed after tetanic stimulation. Such a pathological condition may explain why highly active, open eye input is paradoxically lost in the presence of d-APV after MD in vivo (10).
Electron microscopy reveals mGluRs to lie in the periphery of the postsynaptic density (77). Glutamate released by LFS, unlike for HFS (27, 78), will diffuse poorly and bind primarily with high-affinity NMDA receptors directly opposed to the terminal bouton, only weakly stimulating mGluR2. Synergistic interaction with group I mGluRs coupled to phosphoinositide hydrolysis (45, 79–81), or modulation by NMDA receptors through postsynaptic density proteins (82, 83), may allow mGluR2 to promote LTD induction at lower calcium levels. Here, direct potent stimulation of mGluR2 obviated the need for NMDA receptor activation (Fig. 4B). Conversely, unopposed NMDA action unmasks LTP when mGluR2 and all group I mGluRs are blocked (Fig. 4E) (13), while in the absence of mGluR5 alone, remaining mGluR1 and -2 subtypes adequately support LTD (84). Moreover, mGluR2 itself down-regulates protein kinase A activity to maintain dephosphorylated sites on α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors underlying LTD expression (73). In the absence of its most sensitive cAMP-regulatory subunit (RIβ), protein kinase A mutant mice do not exhibit LTD by LFS (11).
We propose a postsynaptic locus for long-term mGluR2 effects that remains to be tested. First, interaction among group I and II mGluRs and NMDA receptors is most easily achieved within the same intracellular compartment (85). Second, cytoplasmic signaling cascades are enhanced when group I and II mGluRs are costimulated (19, 45, 51, 80, 81). Third, postsynaptic injection of either the G-protein inhibitor guanosine 5′-[β-thio]diphosphate (33, 53) or depolarizing holding current (4, 45, 68) blocks LTD induction in neocortex. Fourth, prolonged exposure to DCG-IV was required to induce LTD, perhaps reflecting constrained access to postsynaptic mGluR2. Finally, no difference in presynaptic STD was observed across a range of stimulus frequencies in mGluR2 KO and WT mice (Fig. 2C). Depletion of vesicles during brief bursts of activity is generally believed to underlie STD (41, 42, 86, 87). Exogenous manipulation of release probability (Fig. 2A) (1, 42) can alter dynamics, but neither endogenous γ-aminobutyric acid type B receptors (data not shown) (2) nor the major glutamatergic autoreceptor mGluR2 is required (Fig. 2C) (41, 86), possibly reflecting its distant, preterminal localization (39). Indeed, it is imprudent to dismiss efficacy of mGluR antagonists on LTD on the basis of presynaptic phenomena (15, 16, 84).
Given the fundamental role for mGluRs, homosynaptic LTD may well be induced by gross perturbation of neuronal activity such as MD, as theory suggests (9). Our findings demonstrate on mechanistic grounds that it does not drive the subsequent loss of sensory input in vivo. Previous work with mGluR2 KO mice yields a similar dissociation between mossy fiber LTD and hippocampal spatial learning (31). Conversely, in an animal model with impaired ocular dominance plasticity, LTP and LTD are preserved (12). Interestingly, STD is altered at selected stimulus frequencies in these same mice (3). Presynaptic short-term plasticity can influence learning (88) and may efficiently scale bursts of sensory input in mGluR2 KO mice (40, 89). How local circuit dynamics might encode sensory deprivation in vivo (2, 3), and whether development of other receptive field properties relies more directly on LTP or LTD mechanisms, warrants further investigation.
Acknowledgments
We thank Dr. R. Shigemoto (Kyoto University) for kindly providing mGluR2 mAbs, S. Fujishima for genotyping and maintenance of mGluR2 KO mice, and Drs. M. Fagiolini and H. Katagiri for experimental assistance and critical comments.
Abbreviations
- STD
short-term depression
- mGluR
metabotropic glutamate receptor
- NMDA
N-methyl-d-aspartate
- d-APV
d-amino-5-phosphonovalerate
- MCPG
α-methyl-4-carboxyphenylglycine
- DCG-IV
(2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine
- LFS
low-frequency stimulation
- LTD
long-term depression
- LTP
long-term potentiation
- MD
monocular deprivation
- WT
wild type
- KO
knockout
- AIDA
(R,S)-1-aminoindan-1,5-dicarboxylic acid
- ACSF
artificial cerebrospinal fluid
- HFS
high-frequency stimulation
- Pn
postnatal day n
References
- 1.Ramoa A S, Sur M. Cereb Cortex. 1996;6:640–646. doi: 10.1093/cercor/6.4.640. [DOI] [PubMed] [Google Scholar]
- 2.Finnerty G T, Roberts L S E, Connors B W. Nature (London) 1999;400:367–371. doi: 10.1038/22553. [DOI] [PubMed] [Google Scholar]
- 3.Fagiolini M, Hensch T K. Nature (London) 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- 4.Artola A, Brocher S, Singer W. Nature (London) 1990;347:69–72. doi: 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- 5.Kirkwood A, Bear M F. J Neurosci. 1994;14:3404–3412. doi: 10.1523/JNEUROSCI.14-05-03404.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudek S M, Friedlander M J. Neuron. 1996;16:1097–1106. doi: 10.1016/s0896-6273(00)80136-1. [DOI] [PubMed] [Google Scholar]
- 7.Feldman D E, Nicoll R A, Malenka R C, Isaac J T R. Neuron. 1998;21:347–357. doi: 10.1016/s0896-6273(00)80544-9. [DOI] [PubMed] [Google Scholar]
- 8.Blais B S, Shouval H Z, Cooper L N. Proc Natl Acad Sci USA. 1999;96:1083–1087. doi: 10.1073/pnas.96.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rittenhouse C D, Shouval H Z, Paradiso M A, Bear M F. Nature (London) 1999;397:347–350. doi: 10.1038/16922. [DOI] [PubMed] [Google Scholar]
- 10.Bear M F, Kleinschmidt A, Gu Q A, Singer W. J Neurosci. 1990;10:909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hensch T K, Gordon J A, Brandon E P, McKnight G S, Idzerda R L, Stryker M P. J Neurosci. 1998;18:2108–2117. doi: 10.1523/JNEUROSCI.18-06-02108.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hensch T K, Fagiolini M, Mataga N, Stryker M P, Baekkeskov S, Kash S F. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hensch T K, Stryker M P. Science. 1996;272:554–557. doi: 10.1126/science.272.5261.554. [DOI] [PubMed] [Google Scholar]
- 14.Haruta H, Kamishita T, Hicks T P, Takahashi M P, Tsumoto T. NeuroReport. 1994;5:1829–1832. doi: 10.1097/00001756-199409080-00036. [DOI] [PubMed] [Google Scholar]
- 15.Huber K M, Sawtell N B, Bear M F. J Neurosci. 1998;18:1–9. doi: 10.1523/JNEUROSCI.18-01-00001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selig D K, Lee H K, Bear M F, Malenka R C. J Neurophysiol. 1995;74:1075–1082. doi: 10.1152/jn.1995.74.3.1075. [DOI] [PubMed] [Google Scholar]
- 17.Conn P J, Pin J-P. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 18.Nakanishi S, Masu M. Annu Rev Biophys Biomol Struct. 1994;23:319–348. doi: 10.1146/annurev.bb.23.060194.001535. [DOI] [PubMed] [Google Scholar]
- 19.Reid S N M, Daw N W, Gregory D S, Flavin H. J Neurosci. 1996;16:7619–7626. doi: 10.1523/JNEUROSCI.16-23-07619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiler I J, Greenough W T. Proc Natl Acad Sci USA. 1993;90:7168–7171. doi: 10.1073/pnas.90.15.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferraguti F, Baldani-Guerra B, Corsi M, Nakanishi S, Corti C. Eur J Neurosci. 1999;11:2073–2082. doi: 10.1046/j.1460-9568.1999.00626.x. [DOI] [PubMed] [Google Scholar]
- 22.Antonini A, Fagiolini M, Stryker M P. J Neurosci. 1999;19:4388–4406. doi: 10.1523/JNEUROSCI.19-11-04388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Cristo G, Berardi N, Cancedda L, Pizzorusso T, Putignano E, Ratto G M, Maffei L. Science. 2001;292:2337–2340. doi: 10.1126/science.1059075. [DOI] [PubMed] [Google Scholar]
- 24.Beaver C J, Ji Q-H, Daw N W. J Neurophysiol. 1999;82:86–93. doi: 10.1152/jn.1999.82.1.86. [DOI] [PubMed] [Google Scholar]
- 25.Reid S N M, Romano C. J Comp Neurol. 2001;429:270–276. doi: 10.1002/1096-9861(20000108)429:2<270::aid-cne7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 26.Sladeczek F, Momiyama A, Takahashi T. Proc R Soc London Ser B. 1993;253:297–303. doi: 10.1098/rspb.1993.0117. [DOI] [PubMed] [Google Scholar]
- 27.Scanziani M, Salin P A, Vogt K E, Malenka R C, Nicoll R A. Nature (London) 1997;385:630–634. doi: 10.1038/385630a0. [DOI] [PubMed] [Google Scholar]
- 28.Ohfune Y, Shimamoto K, Ishida M, Shinozaki H. Bioorg Med Chem Lett. 1993;3:15–18. [Google Scholar]
- 29.Hayashi Y, Momiyama A, Takahashi T, Ohishi H, Ogawa-Meguro R, Shigemoto R, Mizuno N, Nakanishi S. Nature (London) 1993;366:687–690. doi: 10.1038/366687a0. [DOI] [PubMed] [Google Scholar]
- 30.Knopfel T, Lukic S, Leonard T, Flor P J, Kuhn R, Gasparini F. Neuropharmacology. 1995;34:1099–1102. doi: 10.1016/0028-3908(95)00111-i. [DOI] [PubMed] [Google Scholar]
- 31.Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, et al. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch J C, Crepel F. Exp Brain Res. 1991;85:621–624. doi: 10.1007/BF00231747. [DOI] [PubMed] [Google Scholar]
- 33.Kato N. Proc Natl Acad Sci USA. 1993;90:3650–3654. doi: 10.1073/pnas.90.8.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pellicciari R, Luneia R, Costantino G, Marinozzi M, Natalini B, Jakobsen P, Kanstrup A, Lombardi G, Moroni F, Thomsen C. J Med Chem. 1995;38:3717–3719. doi: 10.1021/jm00019a002. [DOI] [PubMed] [Google Scholar]
- 35.Hood W F, Compton R P, Monahan J B. Neurosci Lett. 1989;98:91–95. doi: 10.1016/0304-3940(89)90379-0. [DOI] [PubMed] [Google Scholar]
- 36.Gordon J A, Stryker M P. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiesel T, Hubel D. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 38.Neki A, Ohishi H, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Neurosci Lett. 1996;202:197–200. doi: 10.1016/0304-3940(95)12248-6. [DOI] [PubMed] [Google Scholar]
- 39.Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor P J, Neki A, Abe T, Nakanishi S, Mizuno N. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abbot L F, Varela J A, Sen K, Nelson S B. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- 41.Markram H, Tsodyks M. Nature (London) 1996;382:807–810. doi: 10.1038/382807a0. [DOI] [PubMed] [Google Scholar]
- 42.Varela J A, Sen K, Gibson J, Fost J, Abbot L F, Nelson S B. J Neurosci. 1997;17:7926–7940. doi: 10.1523/JNEUROSCI.17-20-07926.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirkwood A, Dudek S, Gold J T, Aizenman C D, Bear M F. Science. 1993;260:1518–1521. doi: 10.1126/science.8502997. [DOI] [PubMed] [Google Scholar]
- 44.Breakwell N A, Huang L Q, Rowan M J, Anwyl R. Eur J Pharmacol. 1997;322:173–178. doi: 10.1016/s0014-2999(97)00015-0. [DOI] [PubMed] [Google Scholar]
- 45.Cho K, Kemp N, Noel J, Aggleton J P, Brown M W, Bashir Z I. Nat Neurosci. 2000;3:150–156. doi: 10.1038/72093. [DOI] [PubMed] [Google Scholar]
- 46.Miller K D, Chapman B, Stryker M P. Proc Natl Acad Sci USA. 1989;86:5183–5187. doi: 10.1073/pnas.86.13.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nielsen K S, MacPhail E M, Riedel G. Eur J Pharmacol. 1997;326:105–108. doi: 10.1016/s0014-2999(97)85402-7. [DOI] [PubMed] [Google Scholar]
- 48.Petralia R S, Wang Y X, Niedzielski A S, Wenthold R J. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- 49.Dudek S, Bear M F. Science. 1989;246:673–675. doi: 10.1126/science.2573152. [DOI] [PubMed] [Google Scholar]
- 50.Cartmell J, Kemp J A, Alexander S P H, Shinozaki H, Kendall D A. Br J Pharmacol. 1994;111:364–369. doi: 10.1111/j.1476-5381.1994.tb14069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schoepp D D, Johnson B G, Monn J A. J Neurochem. 1996;66:1981–1985. doi: 10.1046/j.1471-4159.1996.66051981.x. [DOI] [PubMed] [Google Scholar]
- 52.Tolchard S, Clarke G C, Collingridge G L, Fitzjohn S M. Eur J Neurosci. 2000;12:1843–1847. doi: 10.1046/j.1460-9568.2000.00080.x. [DOI] [PubMed] [Google Scholar]
- 53.Egger V, Feldmeyer D, Sakmann B. Nat Neurosci. 1999;2:1098–1105. doi: 10.1038/16026. [DOI] [PubMed] [Google Scholar]
- 54.McCaffery B, Cho K, Bortolotto Z A, Aggleton J P, Brown M W, Conquet F, Collingridge G L, Bashir Z I. Neuroscience. 1999;93:977–984. doi: 10.1016/s0306-4522(99)00205-5. [DOI] [PubMed] [Google Scholar]
- 55.Otani S, Auclair N, Desce J-M, Roisin M-P, Crepel F. J Neurosci. 1999;19:9788–9802. doi: 10.1523/JNEUROSCI.19-22-09788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang L Q, Rowan M J, Anwyl R. NeuroReport. 1997;10:687–693. doi: 10.1097/00001756-199702100-00022. [DOI] [PubMed] [Google Scholar]
- 57.Manahan-Vaughan D. Neuropharmacology. 1998;37:1459–1464. doi: 10.1016/s0028-3908(98)00150-6. [DOI] [PubMed] [Google Scholar]
- 58.Bashir Z I, Jane D E, Sunter D C, Watkins J C, Collingridge G L. Eur J Pharmacol. 1993;239:265–266. doi: 10.1016/0014-2999(93)91009-c. [DOI] [PubMed] [Google Scholar]
- 59.Bolshakov V Y, Siegelbaum S A. Science. 1994;264:1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- 60.Oliet S H R, Malenka R C, Nicoll R A. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 61.Huber K M, Kayser M S, Bear M F. Science. 2000;288:1254–1256. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 62.Shigemoto R, Abe T, Nomura S, Nakanishi S, Hirano T. Neuron. 1994;12:1245–1255. doi: 10.1016/0896-6273(94)90441-3. [DOI] [PubMed] [Google Scholar]
- 63.Conquet F, Bashir Z I, Davies C H, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Conde F, et al. Nature (London) 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- 64.Buonomano D V. J Neurosci. 1999;19:6748–6754. doi: 10.1523/JNEUROSCI.19-16-06748.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castro-Alamancos M A, Connors B W. Proc Natl Acad Sci USA. 1997;94:4161–4166. doi: 10.1073/pnas.94.8.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kato N. Brain Res. 1994;665:158–160. doi: 10.1016/0006-8993(94)91168-1. [DOI] [PubMed] [Google Scholar]
- 67.Artola A, Singer W. Trends Neurosci. 1993;11:480–487. doi: 10.1016/0166-2236(93)90081-v. [DOI] [PubMed] [Google Scholar]
- 68.Artola A, Hensch T, Singer W. J Neurophysiol. 1996;76:984–994. doi: 10.1152/jn.1996.76.2.984. [DOI] [PubMed] [Google Scholar]
- 69.Yasuda H, Tsumoto T. Neurosci Res. 1996;24:265–274. doi: 10.1016/0168-0102(95)01001-7. [DOI] [PubMed] [Google Scholar]
- 70.Hansel C, Artola A, Singer W. Eur J Neurosci. 1997;9:2309–2322. doi: 10.1111/j.1460-9568.1997.tb01648.x. [DOI] [PubMed] [Google Scholar]
- 71.Nishiyama M, Hong K, Mikoshiba K, Poo M-M, Kato K. Nature (London) 2000;408:584–588. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- 72.Barria A, Muller D, Derkach V, Griffith L C, Soderling T R. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 73.Lee H-K, Barbarosie M, Kameyama K, Bear M F, Huganir R L. Nature (London) 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- 74.Kirkwood A, Silva A, Bear M F. Proc Natl Acad Sci USA. 1997;94:3380–3383. doi: 10.1073/pnas.94.7.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kimura F, Tsumoto T, Nishigori A, Yoshimura Y. NeuroReport. 1990;1:65–68. doi: 10.1097/00001756-199009000-00018. [DOI] [PubMed] [Google Scholar]
- 76.Yoshimura Y, Tsumoto T, Nishigori A. NeuroReport. 1991;2:393–396. doi: 10.1097/00001756-199107000-00010. [DOI] [PubMed] [Google Scholar]
- 77.Lujan R, Roberts J D, Shigemoto R, Ohishi H, Somogyi P. J Chem Neuroanat. 1997;13:219–241. doi: 10.1016/s0891-0618(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 78.Batchelor A M, Garthwaite J. Nature (London) 1997;385:74–77. doi: 10.1038/385074a0. [DOI] [PubMed] [Google Scholar]
- 79.Rahman S, Neuman R S. Br J Pharmacol. 1996;117:675–683. doi: 10.1111/j.1476-5381.1996.tb15243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nicoletti F, Casabona G, Genazzani A A, L'Episcopo M R, Shinozaki H. Eur J Pharmacol. 1993;245:297–298. doi: 10.1016/0922-4106(93)90111-l. [DOI] [PubMed] [Google Scholar]
- 81.Mistry R, Golding N, Challiss R A. Br J Pharmacol. 1998;123:581–589. doi: 10.1038/sj.bjp.0701626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Husi H, Ward M A, Choudhary J S, Blackstock W P, Grant S G N. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- 83.Migaud M, Charlesworth P, Dempster M, Webster L C, Watabe A M, Makhinson M, He Y, Ramsay M F, Morris R G, Morrison J H, et al. Nature (London) 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- 84.Sawtell N B, Huber K M, Roder J C, Bear M F. J Neurophysiol. 1999;82:3594–3597. doi: 10.1152/jn.1999.82.6.3594. [DOI] [PubMed] [Google Scholar]
- 85.Fagni L, Chavis P, Ango F, Bockaert J. Trends Neurosci. 2000;23:80–88. doi: 10.1016/s0166-2236(99)01492-7. [DOI] [PubMed] [Google Scholar]
- 86.Dobrunz L E, Stevens C F. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 87.Wang L-Y, Kaczmarek L K. Nature (London) 1998;394:384–388. doi: 10.1038/28645. [DOI] [PubMed] [Google Scholar]
- 88.Silva A C, Rosahl T W, Chapman P F, Marowitz Z, Friedman E, Frankland P W, Cestari V, Cioffi D, Sudhof T C, Bourtchuladze R. Curr Biol. 1996;6:1509–1518. doi: 10.1016/s0960-9822(96)00756-7. [DOI] [PubMed] [Google Scholar]
- 89.Turrigiano G G, Nelson S B. Curr Opin Neurobiol. 2000;10:358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]