Abstract
Background:
Longitudinal associations of non-invasive 2-dimensional-phase-contrast magnetic resonance imaging (2D-PC-MRI) velocity markers of the superficial femoral artery (SFA) were analyzed along with characteristics of peripheral artery disease (PAD). We hypothesized that the 2-year differences in MRI-based measures of SFA velocity are associated with longitudinal changes in markers of PAD.
Methods:
A total of 33 (11 diabetic, 22 non-diabetic) PAD patients with baseline and 2-year follow-up MRI scans were included in this secondary analysis of the Effect of Lipid Modification on Peripheral Artery Disease after Endovascular Intervention Trial (ELIMIT). Electrocardiographically-gated 2D-PC-MRI was performed at a proximal and a distal location of the distal SFA territory. SFA lumen, wall, and total vessel volumes, and the normalized wall index (NWI) were analyzed.
Results:
Baseline characteristics did not differ between diabetic and non-diabetic PAD patients. Maximum proximal and distal-SFA velocity measures did not differ between baseline and 2-years (41.98 interquartile range (IQR) (23.58, 72.6) cm/s vs. 40.31 IQR (26.69, 61.29) cm/s; p=0.30). Pooled analysis (N=33) showed that the 24-month change in the NWI was inversely associated with the 24-month change in the proximal maximal SFA velocity (beta= −168.36, R2=0.150, p-value=0.03). The 24-month change of the maximum velocity differences between the proximal and distal SFA locations was inversely associated with the 24-month changes in peak walking distance (beta= −0.003, R2=0.360, p-value=0.011).
Conclusion:
The 2-year change of SFA plaque burden is inversely associated with the 2-year change of proximal peak SFA blood flow velocity. 2D-PC-MRI measured SFA velocity may be of interest in assessing PAD longitudinally.
Keywords: Peripheral artery disease, magnetic resonance imaging, diabetes mellitus, atherosclerosis, superficial femoral artery, arterial blood flow velocity
Introduction
Peripheral artery disease (PAD) is a chronic atherosclerotic disease of the arteries in the extremities, with an approximate burden of 202 million cases globally.1 Recent reports show an approximate 17% increase in prevalence from 2010 to 2015, pushing the burden to 236 million people suffering across the globe from this debilitating disorder worldwide despite advancement in treatment modalities.2 PAD is associated with increased cardiovascular mortality and morbidity along with leg symptoms ranging from intermittent claudication to critical limb ischemia or loss off limb.
Atherosclerosis is a progressive disorder, often with a prolonged period before symptoms arise. Early diagnosis can offer individual risk stratification, targeted intervention and assessment of treatment outcome. Imaging studies provide an avenue to study atherosclerosis disease burden and allows for the longitudinal detection of changes.3 Magnetic resonance imaging (MRI), among other imaging modalities, has been utilized to study PAD and its progression. In addition to the typical measures of plaque burden and plaque characteristics, two dimensional-phase-contrast MRI (2D-PC-MRI), a validated non-invasive rapid imaging technique, can reliably and reproducibly identify atherosclerotic lesions of the superficial femoral artery (SFA).4–7 Here we present the results of a secondary analysis on the Effect of Lipid Modification on Peripheral Artery Disease after Endovascular Intervention Trial (ELIMIT, NCT00687076) utilizing longitudinal 2D-PC-MRI measures of the distal SFA.4 This study aimed to determine associations between 2D-PC-MRI measures of SFA velocity over two-years, as well as detect longitudinal changes in the clinical and functional markers of PAD. We hypothesized that the two-year differences in MRI-based measures of SFA velocity are associated with longitudinal changes in clinical markers of PAD.
Materials and Methods
Study Design
ELIMIT was conducted from 2005 to 2008 at the Ben Taub General Hospital, the Michael E. DeBakey Veterans Affairs Medical Center, and the Houston Methodist Hospital in Houston, TX.4 A total of 102 participants with lifestyle-limiting claudication, consistent with Fontaine stage IIa/IIb, were included. The study obtained approval by the local institutional review board and all participants provided informed consent. The study was a double blind and double-placebo randomized controlled trial in PAD patients. Study participants were randomly divided into two groups: a triple lipid modification therapy group (simvastatin, 40 mg daily; ezetimibe, 10 mg daily; and niacin, 1500 mg daily) and a monotherapy group (simvastatin, 40 mg/day, only). Initial screening of PAD patients was confirmed by an ankle brachial index (ABI) < 0.9 or by imaging studies. ABI measurements and treadmill walking were performed in ELIMIT.
MRI
MRI imaging at the baseline visit with a serial follow up at 6, 12, and 24 months were obtained using a 3.0 T system (Signa Excite, GE Healthcare, Milwaukee, Wisconsin) with a unilateral phased array coil (Pathway Biomedical, Inc.), fixed at the distal SFA, 8cm above the patella. MRI scanning was performed at the same distal SFA location across all participants.4 The SFA plaque burden was measured by fast spin-echo proton-density-weighted (FSE-PDW) MRI scans. Scans were performed on both lower extremities with a total number of 40 slices, slice thickness (ST) of 2 mm and a field of view (FOV) of 22 cm. The repetition time (TR) was 2575 ms, echo time (TE) was 30 ms; in-plane pixel spacing, 0.43 × 0.43 mm; echo train length (ETL), 8; flip angle (FA) = 90°; matrix size, 384 × 224. 2D-PC-MRI scans were acquired with the following setting: TR = 10.6ms, TE = 4.97ms, ST = 6mm, trigger window of 20%, bandwidth = 244 Hz/pixel, and a through-plane encoding phase-contrast encoding velocity (VENC) of 120 cm/s. The ECG-gated 2D-PC-MRI scans were carried out within the field of view, at a proximal and a distal SFA location. Data obtained at the baseline and 2-year follow-up visits were used in this secondary analysis. The SFA vessel volumes for total vessel, wall and lumen volumes were measured from each segment and averaged.
MRI Analysis
In this secondary analysis, we focused on the target limb, defined as the non-intervened or less symptomatic limb of patients.4 MRI scan quality was visually assessed by edge sharpness, amount of blurring, artifacts, and amount of noise.4, 6 Magnitude images were used to trace the SFA lumen boundaries which were then propagated on 2D-phase contrast MRI images to estimate the velocities at the designated proximal and distal locations within the FOV. Each acquired frame of the cardiac cycle was matched with the traced velocity measured within the region of interest (ROI) of the SFA, and thus the maximum of all peak velocities was determined as the maximum velocity. Similarly, the minimum of all lowest velocities across the cardiac cycle was considered the minimum velocity. The average velocity was calculated as the mean velocities averaged over all the frames, while velocity differences were calculated by subtracting the distal values from the proximal values. Background corrections were applied for involuntary muscle twitching, bulk motion, and background noises by subtracting the mean phase information of an adjacent stationary area from the ROI of the SFA. The background area size was at least as large as the luminal ROI and was traced in the vastus medialis or adductor muscles.
Intra-observer intraclass correlation (ICC) analysis was performed to assess the accuracy of the analysis. Tracings for the ICC analysis were done at least one week apart, and the reader was blinded to prior readings. ICC analysis was performed using a two-way random-effects model using the baseline data to assess the intrareader variability for which an ICC > 0.7 was considered an excellent agreement.4, 6, 8–11
Velocity Amplitude
The velocity amplitude was calculated by subtracting the minimum from the maximum velocity at a given point including at the proximal and the distal sites. Differences between velocity amplitudes were calculated by subtracting the 2-year data from the baseline data.
Normalized Wall Index (NWI)
NWI is an established marker for measuring plaque burden in atherosclerotic vessels with a better specificity than lumen volume alone.12 In this study, we calculated the SFA NWI by dividing the wall volume by the total vessel volume. NWI was calculated for both baseline and 2-year data, and the difference was then calculated by subtracting the baseline NWI from the 2-year NWI.
Statistical Analysis
The baseline demographics are expressed as mean (± standard deviation) or median along with interquartile range (IQR, 25% and 75%) for the non-normal variables and frequencies (percentages) of the categorical measures. The normality assumption was confirmed with the Shapiro-Wilk test. Based on the normal distribution of the data, parametric and non-parametric tests were used as appropriate. Nonparametric continuous variables were compared using the Wilcoxon-Mann-Whitney test, while two-sample independent t-tests were used for parametric data. Chi-square tests were used to analyze categorical data. Differences in velocity and other variables were calculated by subtracting the baseline observations from the 2-year observations. Univariate linear regression analyses were performed to determine associations between the 2-year differences in velocities and changes in vessel volumes as well as changes in clinical markers of PAD over two years. A p-value of <0.05 was considered as the statistical significance level. SAS (SAS Institute, Inc., Cary, NC) and Stata Statistical software (College Station, Texas, StataCorp LP) were used for all statistical analysis.
Results
Baseline Characteristics
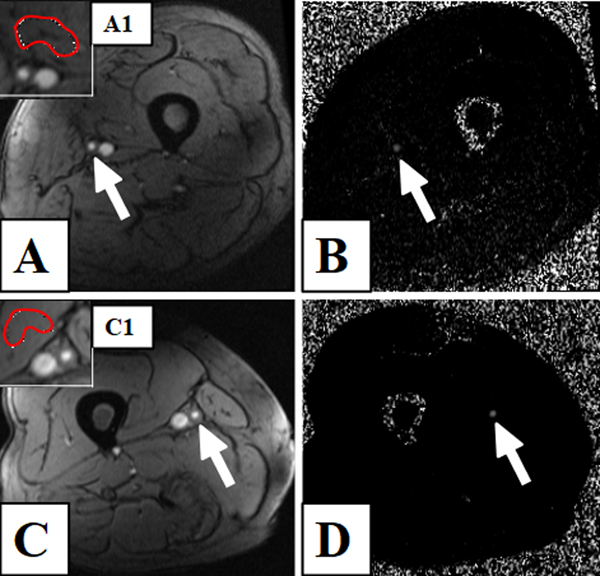
A total of 102 participants were randomized, of whom 87 completed the baseline visit including the primary MRI scanning, 40 individuals returned for the 24-month visit. 2D-PC-MRI scans of suitable quality were available for 70 and 33 participants at the baseline and 24-month follow-up visits, respectively. Therefore, the analysis was performed for 33 individuals, including 11 PAD patients with diabetes (DM-PAD) and 22 non-diabetic PAD patients (NDM-PAD). Figure 1 shows sample 2D-PC-MRI images for a diabetic and a non-diabetic PAD patient. The baseline characteristics and demographics are summarized in Table 1. DM-PAD and NDM-PAD patients did not differ in age, gender, or BMI, and baseline lipid levels were similar. There were no significant differences between the lipid lowering mono-, and triple-therapy groups (data not shown). There were no differences in ABI and treadmill walking parameters between baseline and 2-year follow-up (Table 2).
Figure 1.
Two-dimensional-phase-contrast magnetic resonance imaging (2D-PC-MRI) of the distal superficial femoral artery (SFA) in a peripheral artery disease (PAD) patient with (panels A-B) and without diabetes (panels C-D). Panels A and C: Magnitude images of the SFA (white arrows), along with inserts depicting the background correction regions of interest in the vastus medialis (inserts: A1 and C1, red contours). Panels B and D: Corresponding phase-contrast images showing the SFA (white arrows).
Table 1:
Baseline patient characteristics.
| Variables | All Patients (Total, N=33) |
Diabetic PAD Patients (N=11) |
Non-Diabetic PAD Patients (N=22) |
P-value |
|---|---|---|---|---|
| Age (years) | 62.84 ± 6.2 | 62.97 ± 6.2 | 62.78 ± 6.36 | 0.89 |
| Male sex, n (%) | 29 (87.88) | 8 (24.24) | 21 (63.64) | 0.096 |
| Black race, n (%) | 10 (30.30) | 2 (6.06) | 8 (24.24) | 0.28 |
| Body mass index (kg/m²) | 28.7 (25.2, 34.0) | 29.8 (23.1, 43.3) | 27.15 (25.2, 33.1) | 0.33 |
| Aspirin, n (%) | 33 (100.0) | 11 (33.33) | 22 (66.67) | 1.00 |
| Statin, n (%) | 32 (96.97) | 10 (30.30) | 22 (66.67) | 0.33 |
| Current smoking, n (%) | 15 (45.45) | 3 (9.09) | 12 (36.36) | 0.14 |
| Hypertension, n (%) | 26 (78.79) | 8 (24.24) | 18 (54.55) | 0.66 |
| Hyperlipidemia, n (%) | 30 (93.75) | 10 (31.25) | 20 (62.50) | 1.00 |
| Coronary artery disease, n (%) | 7 (22.58) | 3 (9.68) | 4 (12.90) | 0.65 |
| Triglyceride (mg /dl) | 131 (94, 188) | 140 (94, 192) | 129.5 (91, 188) | 0.96 |
| Non-HDL cholesterol (mg /dl) | 120 (94, 150) | 119 (84, 140) | 125 (106, 151) | 0.39 |
| LDL cholesterol (mg /dl) | 95.5 (71, 111) | 96 (61, 102) | 95 (78, 113) | 0.18 |
| HDL cholesterol (mg /dl) | 39 (34, 45) | 36.0 (33, 51) | 40 (34, 45) | 0.98 |
| Total cholesterol (mg /dl) | 167.0 (134, 187) | 167 (120, 179) | 169 (144, 195) | 0.42 |
Values are expressed as mean (± SD) median (IQR:25%, 75%) and n (%) as appropriate. PAD: peripheral arterial disease; LDL-Low density lipoprotein. HDL= high density lipoprotein.
Table 2:
Clinical markers of PAD at baseline and 2-year follow-up.
| Clinical Markers of PAD | N | Baseline | N | Two-year | P-value |
|---|---|---|---|---|---|
| Pooled data | |||||
|
| |||||
| Peak walking time, (min) | 23 | 3.02 (2.03, 4.05) | 24 | 3.2 (2.11, 4.77) | 0.81 |
| Claudication onset time, (min) | 23 | 1.37 (1.15, 2.32) | 22 | 1.4 (1.0, 2.39) | 1.00 |
| Absolute distance walked, (miles) | 23 | 0.09 (0.06, 0.13) | 23 | 0.1 (0.06, 0.16) | 1.00 |
| Initial distance walked, (miles) | 23 | 0.04 (0.02, 0.07) | 22 | 0.04 (0.02, 0.06) | 0.58 |
| Ankle brachial index (ABI) | 26 | 0.80 (0.73, 0.97) | 28 | 0.72 (0.54, 0.95) | 0.38 |
|
| |||||
| Diabetic PAD patients | |||||
|
| |||||
| Peak walking time, (min) | 8 | 4.2 (3.15, 2.54) | 8 | 3.02 (2.3, 5.22) | 0.45 |
| Claudication onset time, (min) | 8 | 1.67 (0.73, 2.17) | 7 | 2.05 (0.46, 2.2) | 1.00 |
| Absolute distance walked, (miles) | 8 | 0.1 (0.09, 0.13) | 8 | 0.14 (0.07, 0.17) | 1.00 |
| Initial distance walked, (miles) | 8 | 0.03 (0.01, 0.06) | 7 | 0.05 (0.001, 0.06) | 1.00 |
| Ankle brachial index (ABI) | 9 | 0.8 (0.75, 0.89) | 8 | 0.83 (0.6, 1.0) | 1.00 |
|
| |||||
| Non-diabetic PAD patients | |||||
|
| |||||
| Peak walking time, (min) | 15 | 3.0 (1.5, 4.0) | 16 | 3.2 (2.0, 3.9) | 1.00 |
| Claudication onset time, (min) | 15 | 1.37 (1.2, 2.46) | 15 | 1.4 (1.0, 2.5) | 1.00 |
| Absolute distance walked, (miles) | 15 | 0.08 (0.05, 0.13) | 15 | 0.1 (0.06, 0.15) | 0.75 |
| Initial distance walked, (miles) | 15 | 0.04 (0.03, 0.08) | 15 | 0.04 (0.02, 0.08) | 0.51 |
| Ankle brachial index (ABI) | 17 | 0.8 (0.73, 1.0) | 20 | 0.67 (0.49, 0.9) | 0.18 |
|
| |||||
Values are expressed as median (IQR: 25%, 75%). SFA: superficial femoral artery. PAD: peripheral arterial disease. SFA: superficial femoral artery. Pooled data: For peak walking time, claudication onset time, absolute distance walked, and initial distance walked, n=23 (diabetics, n=8; and nondiabetics, n=15) at baseline. ABI, n=26 (diabetics, n=9, and nondiabetics, n=17) at baseline; and n=28 (diabetics, n=8, and nondiabetics, n=20) at 24-months. At 2-year follow up, both claudication onset time and initial distance walked, n =22 (diabetics, n=7, and nondiabetics, n=15), whereas n=24 for peak walking time (diabetics, n=8, and nondiabetics, n=16) and n=23 for the absolute distance walked (diabetics, n=8, and nondiabetics, n=15), respectively. The group means of the corresponding subgroups were compared with the Wilcoxon-Mann Whitney test.
MRI-Based Measures of SFA velocity
Pooled analysis (n=33) showed that the SFA velocity amplitude and the average and maximum SFA velocities decreased significantly between the proximal and distal SFA locations at both baseline and at 2-years (all p<0.006), whereas the minimum SFA velocities were similar (p=0.73; Table 3). There were no significant changes in SFA velocity measures between baseline and the 2-year follow-up (Table 4).
Table 3:
Magnetic resonance imaging measured velocity parameters in the distal SFA.
| Pooled Data (N=33) | Proximal SFA Location | Distal SFA Location | N | P-value |
|---|---|---|---|---|
| Average velocity, cm/s | 21.92 (14.91, 37.0) | 17.66 (12.0, 23.83) | 33 | 0.005 |
| Maximum velocity, cm/s | 40.31 (26.69, 61.29) | 28.25 (15.61, 40.53) | 33 | 0.001 |
| Minimum velocity, cm/s | 4.97 (2.11, 12.05) | 4.55 (1.79, 10.63) | 33 | 0.73 |
|
| ||||
| Proximal to distal | Baseline | P-value | 2-years | P-value |
|
| ||||
| Delta of Velocity Amplitude | 8.2 (0.9, 31.4) | 0.0003 | 9.6 (2.3, 23.7) | <0.0001 |
|
| ||||
| Diabetic PAD Patients | ||||
|
| ||||
| Average velocity, cm/s | 27.28 (18.77, 46.17) | 22.43 (14.74, 32.87) | 11 | 0.55 |
| Maximum velocity, cm/s | 41.38 (26.69, 87.67) | 34.23 (24.52, 48.78) | 11 | 0.23 |
| Minimum velocity, cm/s | 7.58 (2.38, 22.79) | 10.63 (0.85, 18.32) | 11 | 1.00 |
|
| ||||
| Proximal to distal | Baseline | P-value | 2-years | P-value |
|
| ||||
| Delta of Velocity Amplitude | 12.2 (2.3, 20.1) | 0.065 | 7.7 (2.0, 31.5) | 0.012 |
|
| ||||
| Non-Diabetic PAD Patients | ||||
|
| ||||
| Average velocity, cm/s | 21.42 (14.91, 36.86) | 16.19 (10.29, 23.95) | 22 | 0.004 |
| Maximum velocity, cm/s | 38.54 (27.3, 61.29) | 28.96 (15.61, 40.53) | 22 | 0.004 |
| Minimum velocity, cm/s | 4.41 (1.62, 7.84) | 3.95 (1.716, 6.71) | 22 | 0.83 |
|
| ||||
| Proximal to distal | Baseline | P-value | 2-years | P-value |
|
| ||||
| Delta of Velocity Amplitude | 5.9 (0.5, 34.1) | 0.0043 | 9.65 (2.3, 23.7) | 0.0001 |
Values are expressed as median (IQR:25%, 75%). SFA: superficial femoral artery. PAD: peripheral arterial disease. Level of significance is set at <0.05.
Table 4:
Two-years differences in magnetic resonance imaging measured velocity parameters in the SFA.
| Pooled Data (N=34) | Baseline | 2-year | N | P-value |
|---|---|---|---|---|
| Velocity measures | Proximal SFA Location | |||
|
| ||||
| Maximum velocity, cm/s | 41.98 (23.58, 72.6) | 40.31 (26.69, 61.29) | 33 | 0.30 |
| Minimum velocity, cm/s | 4.89 (1.55, 9.18) | 4.41 (1.62, 9.23) | 33 | 0.73 |
| Average velocity, cm/s | 26.1 (16.92, 38.75) | 21.92 (14.91, 37.0) | 33 | 0.16 |
| Velocity amplitude | 37.4 (14.4, 67.7) | 31.2 (13.6, 60.4) | 33 | 0.49 |
|
| ||||
| Distal SFA Location | ||||
|
| ||||
| Maximum velocity, cm/s | 30.66 (16.87, 43.39) | 28.25 (15.61, 40.53) | 33 | 0.49 |
| Minimum velocity, cm/s | 4.31 (2.6, 8.89) | 4.0 (1.51, 8.89) | 33 | 0.30 |
| Average velocity, cm/s | 15.86 (11.11, 28.41) | 17.66 (12.0, 23.83) | 33 | 0.49 |
| Velocity amplitude | 20.5 (8.4, 36.3) | 21.1 (6.5, 34.6) | 33 | 0.16 |
|
| ||||
| Diabetic PAD Patients | Baseline | 2-year | N | P-value |
|
| ||||
| Velocity | Proximal SFA Location | |||
|
| ||||
| Maximum velocity, cm/s | 48.91 (38.73, 89.43) | 40.31 (22.09, 93.73) | 11 | 1.00 |
| Minimum velocity, cm/s | 4.9 (2.13, 9.22) | 7.58 (1.215, 22.79) | 11 | 0.52 |
| Average velocity, cm/s | 27.98 (23.82, 44.83) | 24.74 (13.74, 47.13) | 11 | 0.52 |
| Velocity amplitude | 45 (29.2, 88.0) | 30.1 (7.4 ,80.9) | 11 | 0.07 |
|
| ||||
| Distal SFA Location | ||||
|
| ||||
| Maximum velocity, cm/s | 38.06 (16.92 ,69.05) | 28.25 (15.24, 41.06) | 11 | 0.29 |
| Minimum velocity, cm/s | 3.38 (0.42, 7.4) | 5.4 (0.47, 18.32) | 11 | 1.00 |
| Average velocity, cm/s | 27.22 (8.67, 34.05) | 21.37 (14.55, 23.83) | 11 | 0.83 |
| Velocity amplitude | 23.4 (12.7, 70.0) | 19.1 (2.7, 42.1) | 11 | 0.23 |
|
| ||||
| Non-Diabetic PAD Patients | Baseline | 2-year | N | P-value |
|
| ||||
| Velocity | Proximal SFA Location | |||
|
| ||||
| Maximum velocity, cm/s | 39.71 (18.68, 68.19) | 38.54 (27.3, 61.29) | 22 | 0.065 |
| Minimum velocity, cm/s | 4.5 (1.38, 9.18) | 4.41 (1.62, 7.84) | 22 | 1.00 |
| Average velocity, cm/s | 21.8 (14.53, 38.75) | 21.42 (14.91, 36.86) | 22 | 0.23 |
| Velocity amplitude | 34.5 (9.1, 51.0) | 35.6 (20.4, 60.4) | 22 | 0.83 |
|
| ||||
| Distal SFA Location | ||||
|
| ||||
| Maximum velocity, cm/s | 26.54 (12.54, 41.56) | 28.96 (15.61, 40.53) | 22 | 1.00 |
| Minimum velocity, cm/s | 4.53 (2.76, 9.62) | 3.95 (1.716, 6.71) | 22 | 0.065 |
| Average velocity, cm/s | 15.72 (11.24, 26.14) | 16.19 (10.29, 23.95) | 22 | 0.55 |
| Velocity amplitude | 19.95 (6.5, 34.5) | 21.3 (10.9, 33.8) | 22 | 0.53 |
Values are expressed as median (IQR:25%, 75%). SFA: superficial femoral artery. PAD: peripheral arterial disease. Level of significance is set at <0.05.
Results of a subgroup analysis show that SFA velocity amplitude and SFA maximum and average velocities were significantly higher in the proximal SFA location than those in the distal SFA locations at baseline and after 2 years in NDM-PAD patients (maximum SFA velocity: 38.54 (IQR: 27.3, 61.29) cm/s vs. 28.96 (IQR: 15.61, 40.53) cm/s, p = 0.004), but not in DM-PAD participants (maximum SFA velocity: 41.38 (26.69,87.67) cm/s vs. 34.23 (24.52,48.78) cm/s, p = 0.23; Table 3).
Sub-group analyses did not show any significant changes in SFA velocity measures between baseline and 2 years. Among NDM-PAD patients, the differences between maximum and minimum SFA velocities from baseline to 2-year follow-up were non-significant (proximal SFA location: 39.71 (18.68, 68.19) cm/s vs. 38.54 (27.3, 61.29) cm/s, p=0.065; Table 3).
The ICC analysis was performed for 10 randomly selected baseline scans and was reported previously.6 Briefly, the intrareader reproducibility of SFA mean and maximum velocities was excellent, respectively (0.996 (confidence interval [CI]: 0.996, 0.997); 0.999 (CI: 0.999, 0.999)).6
SFA Plaque Burden Measures
SFA measures of plaque burden including SFA wall, lumen, and total vessel volumes, and NWI did not differ between baseline and data at 2 years (Table 5). In a sub-group analysis, no significant differences over 2-years were observed for SFA lumen volumes in non-diabetic PAD patients (0.01465 (0.01, 0.02) cc. vs. 0.0185 (+0.0100) cc., p=0.078), nor in DM-PAD patients (0.0210 (0.0143) cc. vs. 0.0198 (+0.0097) cc., p=0.065; Table 5).
Table 5:
Two years differences in SFA wall, lumen and total volumes and normalized wall index.
| SFA Volumes | Baseline | 2-year | 2-Year Difference | P-value |
|---|---|---|---|---|
| Pooled Data | (N=33) | (N=32) | (N=32) | |
|
| ||||
| SFA wall volume, cc | 0.0406 (±0.0114) | 0.0398 (±0.0118) | 0.0012 (−0.005, 0.008) | 0.86 |
| SFA lumen volume, cc | 0.0178 (0.0097, 0.0232) | 0.0189 (±0.0098) | 0.0010 (±0.006) | 0.86 |
| SFA total volume, cc | 0.0574 (0.0430, 0.0688) | 0.0588 (±0.0203) | 0.0005 (±0.0168) | 0.60 |
| Normalized wall index | 0.706 (±0.103) | 0.692 (±0.085) | 0.0053 (−0.05, 0.038) | 1.00 |
|
| ||||
| Diabetic PAD Patients | (N=11) | (N=11) | (N=11) | |
|
| ||||
| SFA wall volume, cc | 0.0409 (±0.0146) | 0.0379 (±0.0123) | −0.0030 (±0.0134) | 0.55 |
| SFA lumen volume, cc | 0.0210 (±0.0143) | 0.0198 (±0.0097) | −0.0012 (±0.0091) | 0.065 |
| SFA total volume, cc | 0.0620 (±0.0275) | 0.0578 (±0.0199) | −0.0042 (±0.0208) | 0.55 |
| Normalized wall index | 0.684 (±0.097) | 0.667 (±0.104) | 0.0185 (−0.0465, 0.0365) | 0.27 |
|
| ||||
| Non-Diabetic PAD Patients | (N=22) | (N=21) | (N=21) | |
|
| ||||
| SFA wall volume, cc | 0.0405 (±0.0099) | 0.0408 (±0.0118) | 0.0008 (±0.0124) | 0.38 |
| SFA lumen volume, cc | 0.01465 (0.01, 0.02) | 0.0185 (±0.0100) | 0.0022 (±0.0048) | 0.08 |
| SFA total volume, cc | 0.0577 (±0.0163) | 0.0594 (±0.0209) | 0.0030 (±0.0142) | 0.19 |
| Normalized wall index | 0.717 (±0.106) | 0.705 (±0.073) | −0.019 (±0.070) | 0.38 |
Values are expressed as mean (± SD) and median (IQR:25%, 75%) as appropriate. SFA: superficial femoral artery. PAD: peripheral arterial disease. Values are expressed up to 4 decimal places for being very small numbers.
Associations between measures of SFA plaque burden and SFA velocities
The 24-month change in NWI was inversely associated with the 24-month change in proximal maximal SFA velocity (beta= −168.36, R2=0.150, p-value=0.03; Table 6). Similarly, a significant inverse association was observed in a sub-group analysis among diabetic (beta=−345.99, R2=0.52, p-value=0.01) but not among non-diabetic PAD patients (beta=−35.34, R2=0.01, p-value=0.7; Table 6) The 24-month change in the maximum velocity differences between the proximal and distal SFA locations was inversely associated with the 24-month changes in NWI (beta=−268.48, R2=0.489, p-value=0.017; Table 6) in the DM-PAD group, but not in the NDM-PAD patients.
Table 6:
Association between 24 months changes in normalized wall ratio and 24 months differences in velocity at the proximal SFA location.
| Variable | N | Beta | SE | R2 | Adj R2 | P-value | |
|---|---|---|---|---|---|---|---|
| Pooled Data | |||||||
|
| |||||||
| Delta NWI | 24Mo Δ of proximal SFA maximum velocity, cm/s | 32 | −168.36 | 74.29 | 0.15 | 0.12 | 0.03 |
| 24Mo Δ of proximal SFA average velocity, cm/s | 32 | −84.19 | 34.97 | 0.16 | 0.13 | 0.02 | |
| 24Mo Δ of proximal SFA minimum velocity, cm/s | 32 | −0.02 | 19.84 | <0.01 | −0.030 | 1.00 | |
| 24Mo Δ of distal SFA maximum velocity, cm/s | 32 | −17.50 | 21.59 | 0.02 | −0.01 | 0.42 | |
| 24Mo Δ of distal SFA average velocity, cm/s | 32 | −44.72 | 23.40 | 0.11 | 0.08 | 0.07 | |
| 24Mo Δ of distal SFA minimum velocity, cm/s | 32 | −71.94 | 51.39 | 0.06 | 0.03 | 0.17 | |
| 24Mo Δ of Delta SFA maximum velocity, cm/s | 32 | −96.42 | 49.25 | 0.11 | 0.08 | 0.06 | |
| 24Mo Δ of Delta SFA average velocity, cm/s | 32 | −39.47 | 26.19 | 0.07 | 0.04 | 0.14 | |
| 24Mo Δ of Delta SFA minimum velocity, cm/s | 32 | −0.70 | 6.01 | 0.00 | −0.03 | 0.91 | |
|
| |||||||
| Diabetic PAD patients | |||||||
|
| |||||||
| Delta NWI | 24Mo Δ of proximal SFA maximum velocity, cm/s | 11 | −345.99 | 111.24 | 0.52 | 0.46 | 0.01 |
| 24Mo Δ of proximal SFA average velocity, cm/s | 11 | −181.30 | 41.22 | 0.68 | 0.65 | <0.01 | |
| 24Mo Δ of proximal SFA minimum velocity, cm/s | 11 | −16.60 | 41.33 | 0.02 | −0.09 | 0.7 | |
| 24Mo Δ of distal SFA maximum velocity, cm/s | 11 | −39.13 | 50.55 | 0.06 | −0.04 | 0.46 | |
| 24Mo Δ of distal SFA average velocity, cm/s | 11 | −58.32 | 31.38 | 0.27 | 0.20 | 0.096 | |
| 24Mo Δ of distal SFA minimum velocity, cm/s | 11 | −77.51 | 79.79 | 0.10 | −0.01 | 0.36 | |
| 24Mo Δ of Delta SFA maximum velocity, cm/s | 11 | −268.48 | 91.51 | 0.49 | 0.43 | 0.017 | |
| 24Mo Δ of Delta SFA average velocity, cm/s | 11 | −122.98 | 52.43 | 0.38 | 0.31 | 0.044 | |
| 24Mo Δ of Delta SFA minimum velocity, cm/s | 11 | 14.57 | 6.08 | 0.39 | 0.32 | 0.04 | |
|
| |||||||
| Non-Diabetic PAD patients | |||||||
|
| |||||||
| Delta NWI | 24Mo Δ of proximal SFA maximum velocity, cm/s | 21 | −35.34 | 91.15 | 0.01 | −0.04 | 0.7 |
| 24Mo Δ of proximal SFA average velocity, cm/s | 21 | −12.00 | 45.89 | <0.01 | −0.05 | 0.8 | |
| 24Mo Δ of proximal SFA minimum velocity, cm/s | 21 | 11.35 | 18.62 | 0.02 | −0.03 | 0.55 | |
| 24Mo Δ of distal SFA maximum velocity, cm/s | 21 | −2.77 | 11.02 | <0.01 | −0.05 | 0.80 | |
| 24Mo Δ of distal SFA average velocity, cm/s | 21 | −34.42 | 33.75 | 0.05 | <0.01 | 0.32 | |
| 24Mo Δ of distal SFA minimum velocity, cm/s | 21 | −66.07 | 67.72 | 0.05 | <−0.01 | 0.34 | |
| 24Mo Δ of Delta SFA maximum velocity, cm/s | 21 | 30.73 | 41.51 | 0.03 | −0.02 | 0.47 | |
| 24Mo Δ of Delta SFA average velocity, cm/s | 21 | 22.43 | 21.15 | 0.06 | 0.01 | 0.30 | |
| 24Mo Δ of Delta SFA minimum velocity, cm/s | 21 | −12.29 | 7.67 | 0.12 | 0.07 | 0.13 | |
Delta NWI= two years’ differences in Normalized Wall Index (NWI). Delta SFA (maximum, minimum, and average) velocity: refers to the respective SFA velocity differences between the proximal and distal locations in the SFA. SE= Standard Error; 24Mo Δ= 24-month difference in velocity measures. BETA=parameters estimate; SFA: superficial femoral artery. Adj R2 = adjusted R2. Level of significance is set at <0.05.
Associations between measures of SFA velocities and Clinical markers of PAD
The 24-month change of the maximum velocity differences between the proximal and distal SFA locations was inversely associated with the 24-month changes in the peak walking distance (beta= −0.003, R2=0.360, p-value=0.011; Table 7). A similar inverse association persisted in a sub-group analysis in the DM-PAD group (n=7, beta= −0.004, r2=0.651, p= 0.028), but not in the NDM-PAD group (n=10, beta= 0.001 r2=0.014, p= 0.75; Table 7).
Table 7.
Associations between the 2-year difference in velocity changes from proximal to distal SFA locations with the 2-year changes in clinical markers of PAD.
| Variable | Independent Variable | N | Beta | SE | R2 | Adj. R 2 | P-value |
|---|---|---|---|---|---|---|---|
| Pooled Data | |||||||
|
| |||||||
| 24-Mo Δ of maximum vel. difference (proximal to distal) | 24Mo Δ of absolute distance walked, (miles) | 17 | −0.003 | 0.001 | 0.36 | 0.32 | 0.01 |
| 24Mo Δ of Initial distance walked, (miles) | 16 | <0.01 | 0.001 | 0.01 | −0.07 | 0.78 | |
| 24Mo Δ of Ankle brachial index | 22 | 7.778 | 21.553 | 0.01 | −0.04 | 0.72 | |
| 24Mo Δ of Peak walking time, (min) | 18 | −0.040 | 0.035 | 0.08 | 0.02 | 0.26 | |
| 24Mo Δ of Claudication onset time, (min) | 16 | 0.027 | 0.025 | 0.08 | 0.01 | 0.30 | |
|
| |||||||
| 24-Mo Δ of minimum vel. difference (proximal to distal) | 24Mo Δ of absolute distance walked, (miles) | 17 | −0.001 | 0.005 | 0.01 | −0.07 | 0.90 |
| 24Mo Δ of Initial distance walked, (miles) | 16 | 0.001 | 0.002 | 0.03 | −0.04 | 0.55 | |
| 24Mo Δ of Ankle brachial index | 22 | 5.637 | 6.537 | 0.04 | −0.01 | 0.40 | |
| 24Mo Δ of Peak walking time, (min) | 18 | 0.034 | 0.121 | 0.01 | −0.06 | 0.78 | |
| 24Mo Δ of Claudication onset time, (min) | 16 | 0.048 | 0.080 | 0.03 | −0.05 | 0.56 | |
|
| |||||||
| Diabetic PAD Patients | |||||||
|
| |||||||
| 24-Mo Δ of maximum vel. difference (proximal to distal) | 24Mo Δ of absolute distance walked, (miles) | 7 | −0.004 | 0.001 | 0.65 | 0.58 | 0.03 |
| 24Mo Δ of Initial distance walked, (miles) | 6 | <0.01 | <0.01 | 0.08 | −0.16 | 0.60 | |
| 24Mo Δ of Ankle brachial index | 7 | 1.616 | 61.093 | <0.01 | −0.20 | 0.98 | |
| 24Mo Δ of Peak walking time, (min) | 7 | −0.055 | 0.059 | 0.15 | −0.02 | 0.39 | |
| 24Mo Δ of Claudication onset time, (min) | 6 | 0.035 | 0.014 | 0.60 | 0.50 | 0.07 | |
|
| |||||||
| 24-Mo Δ of minimum vel. difference (proximal to distal) | 24Mo Δ of absolute distance walked, (miles) | 7 | −0.001 | 0.010 | <0.01 | −0.20 | 0.89 |
| 24Mo Δ of Initial distance walked, (miles) | 6 | 0.002 | 0.002 | 0.19 | −0.02 | 0.39 | |
| 24Mo Δ of Ankle brachial index | 7 | 9.29 | 3.30 | 0.61 | 0.54 | 0.04 | |
| 24Mo Δ of Peak walking time, (min) | 7 | 0.143 | 0.260 | 0.06 | −0.13 | 0.61 | |
| 24Mo Δ of Claudication onset time, (min) | 6 | 0.052 | 0.072 | 0.12 | −0.11 | 0.51 | |
|
| |||||||
| Non-Diabetic PAD Patients | |||||||
|
| |||||||
| 24-Mo Δ of maximum vel. difference (proximal to distal) | 24Mo Δ of absolute distance walked, (miles) | 10 | 0.001 | 0.002 | 0.01 | −0.11 | 0.75 |
| 24Mo Δ of Initial distance walked, (miles) | 10 | <0.01 | 0.002 | <0.01 | −0.13 | 0.96 | |
| 24Mo Δ of Ankle brachial index | 15 | 15.590 | 19.620 | 0.05 | −0.03 | 0.44 | |
| 24Mo Δ of Peak walking time, (min) | 11 | −0.011 | 0.042 | 0.01 | −0.10 | 0.80 | |
| 24Mo Δ of Claudication onset time, (min) | 10 | 0.006 | 0.055 | <0.01 | −0.12 | 0.92 | |
|
| |||||||
| 24-Mo Δ of minimum vel. difference (proximal to distal) | 24Mo Δ of absolute distance walked, (miles) | 10 | −0.002 | 0.004 | 0.02 | −0.10 | 0.68 |
| 24Mo Δ of Initial distance walked, (miles) | 10 | 0.001 | 0.005 | <0.01 | −0.12 | 0.87 | |
| 24Mo Δ of Ankle brachial index | 15 | −7.444 | 3.88 | 0.22 | 0.16 | 0.07 | |
| 24Mo Δ of Peak walking time, (min) | 11 | −0.135 | 0.103 | 0.16 | 0.07 | 0.23 | |
| 24Mo Δ of Claudication onset time, (min) | 10 | 0.017 | 0.151 | <0.01 | −0.12 | 0.91 | |
24Mo Δ= 24 month change of the velocity difference between the proximal to the distal locations; Vel.= Velocity; SE= standard error; SFA= superficial femoral artery. Adj R2 = adjusted R 2. Significance level: p<0.05. Pooled data: For both claudication onset time and initial distance walked, n=16 (PAD patients with diabetes: n=6; PAD patients without diabetes: n=10). Absolute distance walked: n=17 (PAD patients with diabetes: n=7, PAD patients without diabetes: n=10), and peak walking time: n=18 (PAD patients with diabetes: n=7, PAD patients without diabetes: n=11). ABI: n=22 (PAD patients with diabetes: n=7, PAD patients without diabetes: n=15). N was variable based on patient’s ability to perform different clinical tests during the visits or due to loss to follow-up.
Associations between 2-year changes in SFA lumen volumes and SFA velocities
There was a significant association between the 24-month changes in SFA lumen volume and the 24-month changes in the maximum proximal SFA velocity in diabetic but not in non-diabetic PAD patients (beta=0.00019, r2=0.762, p< 0.001 vs. beta=−0.00003, r2=0.03, p= 0.45; Supplementary Table 1).
Discussion
This study found a significant decrease in blood flow velocity, measured by 2D-PC-MRI, from the proximal to the distal locations of the distal SFA both at baseline and at 2-year follow-up. The 2-year change of the NWI, a measure of plaque burden, was inversely associated with the 2-year change of the SFA blood flow velocity. The 2-year change in peak walking distance was inversely associated with the 2-year change of the SFA blood flow velocity difference between the proximal and the distal location among diabetic PAD patients but not in non-diabetics.
PAD patients are known to have a decrease in blood flow velocity to the lower extremities, as evidenced by previous reports and confirmed by the findings of this study.13 A prior study analyzing the baseline data also confirmed the similar pattern of decreased velocity distally.6 In a subgroup analysis, the non-diabetic PAD group had shown a similar pattern while the diabetic PAD patients did not show any significant decrease in velocity distally. Impaired vascular compliance or poor arterial compressibility secondary to medial calcification has been previously noted in patients with diabetes.14 Attenuated vascular compliance could in part explain our findings of similar distal SFA velocities in diabetic PAD patients.15
PAD and diabetes are known to be associated with an elevation of inflammatory markers like CRP, which has been found to enhance endothelial cell apoptosis and colocalization of oxidized LDL in atherosclerotic plaques. CRP also mediates the production of leukocyte adhesion molecules and chemotactic substances and inhibits endothelial cell nitric oxide (NO) synthase (eNOS) leading to dysregulation of vascular tone along with impairing fibrinolysis by producing plasminogen activator inhibitor (PAI)-1. These pathophysiological attributes may have contributed in part to the observed longitudinal vascular changes in DM-PAD patients.16 The inverse association between 2-year changes in plaque burden and SFA velocity markers at the proximal site may be attributable to the higher risk profile of PAD patients with diabetes compared to those without diabetes.
Taniwaki et. al. reported that lower extremity symptoms are closely associated with the stiffness index of the femoral artery, irrespective of femoral artery intima-media thickness in PAD patients with type 2 diabetes.17, 18 Our study agrees with previous reports that attributed an increased wall stiffening or loss of vascular compliance to advanced sclerotic changes in the large arteries in diabetics.18–20
According to Dolan et al., diabetic PAD patients are more likely to report classic symptoms of intermittent claudication, exertional leg pain and a lesser mean of 6-minute walking distance in comparison to the matched non-diabetic PAD group.21 Our findings agree with the results from a report by Dolan et al. and we further found that the 2-year change in peak walking distance is inversely associated with the 2-year change in the SFA maximum velocity (between the proximal to the distal SFA segment) among diabetic PAD patients. Taken together, these findings by others and our results could possibly help explain how progressive arterial stiffness and resultant changes in blood flow to the lower extremities could impact clinical markers of PAD including absolute distance walked.
Hodnett et al. reported that symptomatic PAD patients with diabetes can be diagnosed accurately with a non-contrast based quiescent-interval single-shot (QISS) MR angiography (MRA) technique compared with standard contrast-enhanced MRA.22 The 2D-PC-MRI technique utilized in this study does not use a gadolinium-based-contrast agent, and also was shown to be reproducible. Recent reports from the literature compared MR angiography and color-guided duplex US for evaluating stenoses and occlusions in PAD and found a better discriminatory power of MRA compared with duplex US.23 The sensitivity of duplex US is lower compared with MRA, although both have high specificity.23, 24 In this study we found that 2D-PC-MRI can reliably measure longitudinal changes in SFA blood flow velocities in PAD patients, and imaging measures are associated with markers of PAD which is in agreement with previous reports from the literature.21
Limitations
This study has limitations. This is a secondary analysis utilizing the ELIMIT data and all limitations of the primary analyses apply, including but not limited to that most of the participants were male.4 The sample size is limited, and a significant amount of baseline participants did not return for the 2-year follow-up visit, further reducing the amount of analyzed datasets. Future studies are recommended to assess the generalizability of our findings. The distal SFA territory, a common location of atherosclerotic plaque development was selected for MR imaging across all patients. However, study participants may have had substantial plaque formation in other regions of the SFA outside the imaged FOV. This study was not powered to assess longitudinal differences in 2D-PC-MRI based SFA blood flow velocities between diabetic and non-diabetic PAD patients. Phase-contrast MR imaging technologies have substantially improved since ELIMIT participants were imaged. Newer sequences including 4D-flow MRI may provide additional insights in SFA blood flow velocities of PAD patients and should be considered in future imaging studies.25
Conclusion
2D-PC-MRI measures of SFA blood flow velocities decreased significantly between the proximal and distal SFA imaging locations at both baseline and at 2-years, whereas the minimum SFA velocities were similar. The 2-year change of SFA plaque burden is inversely associated with the 2-year change of proximal peak SFA blood flow velocity. 2D-PC-MRI measured SFA velocity may be of interest in assessing PAD longitudinally.
Supplementary Material
Supplemental Table 1. Associations between 2-year differences in lumen volume and the 2-year differences in magnetic resonance imaging measured velocity parameters in the distal SFA.
Acknowledgments
We thank all study participants for their cooperation.
Funding
This work was supported in part by the National Institutes of Health (R01HL137763 and K25HL121149, R01HL075824, and the American Heart Association (13BGIA16720014).
Footnotes
Competing Interests
The authors declare that there is no conflict of interest.
Ethics Approval and Consent to Participate
The study obtained approval by the local institutional review board and all participants provided informed consent.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics−-2015 update: a report from the American Heart Association. Circulation 2015; 131: e29–322. 2014/12/19. DOI: 10.1161/cir.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013; 382: 1329–1340. 2013/08/07. DOI: 10.1016/s0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 3.Gimnich OA, Zil EAA and Brunner G. Imaging Approaches to the Diagnosis of Vascular Diseases. Curr Atheroscler Rep 2022; 24: 85–96. 2022/01/27. DOI: 10.1007/s11883-022-00988-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunner G, Yang EY, Kumar A, et al. The Effect of Lipid Modification on Peripheral Artery Disease after Endovascular Intervention Trial (ELIMIT). Atherosclerosis 2013; 231: 371–377. DOI: 10.1016/j.atherosclerosis.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Aidi H, Mani V, Weinshelbaum KB, et al. Cross-sectional, prospective study of MRI reproducibility in the assessment of plaque burden of the carotid arteries and aorta. Nat Clin Pract Cardiovasc Med 2009; 6: 219–228. 2009/01/29. DOI: 10.1038/ncpcardio1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinharoy A, Reddy N, Lin JK, et al. Magnetic resonance imaging based superficial femoral artery velocity measurements in peripheral artery disease. Magn Reson Imaging 2022; 93: 128–134. 2022/08/09. DOI: 10.1016/j.mri.2022.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamran H, Nambi V, Negi S, et al. Magnetic Resonance Venous Volume Measurements in Peripheral Artery Disease (from ELIMIT). Am J Cardiol 2016; 118: 1399–1404. 2016/10/25. DOI: 10.1016/j.amjcard.2016.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrout PE and Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979; 86: 420–428. 1979/03/01. [DOI] [PubMed] [Google Scholar]
- 9.Nunnally JC and Bernstein IH. Psychometric theory. New York: McGraw-Hill Inc, 1994. [Google Scholar]
- 10.Carod-Artal FJ, Ferreira Coral L, Stieven Trizotto D, et al. Self- and proxy-report agreement on the Stroke Impact Scale. Stroke; a journal of cerebral circulation 2009; 40: 3308–3314. 2009/08/08. DOI: STROKEAHA.109.558031 [pii] 10.1161/STROKEAHA.109.558031 [doi]. [DOI] [PubMed] [Google Scholar]
- 11.Etherington J, Innes G, Christenson J, et al. Development, implementation and reliability assessment of an emergency physician performance evaluation tool. CJEM 2000; 2: 237–245. 2007/07/07. DOI: F2C406BCF38444C5A771BCDCC054F63A [pii]. [DOI] [PubMed] [Google Scholar]
- 12.Singh J, Brunner G, Morrisett JD, et al. Patient-Specific Flow Descriptors and Normalized wall index in Peripheral Artery Disease: a Preliminary Study. Comput Methods Biomech Biomed Eng Imaging Vis 2018; 6: 119–127. 20161012. DOI: 10.1080/21681163.2016.1184589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zemaitis MR, Boll JM and Dreyer MA. Peripheral Arterial Disease. StatPearls. Treasure Island (FL): StatPearls Publishing; Copyright © 2022, StatPearls Publishing LLC., 2022. [PubMed] [Google Scholar]
- 14.Edmonds ME, Morrison N, Laws JW, et al. Medial arterial calcification and diabetic neuropathy. Br Med J (Clin Res Ed) 1982; 284: 928–930. 1982/03/27. DOI: 10.1136/bmj.284.6320.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santoro L, Flex A, Nesci A, et al. Association between peripheral arterial disease and cardiovascular risk factors: role of ultrasonography versus ankle-brachial index. Eur Rev Med Pharmacol Sci 2018; 22: 3160–3165. 2018/06/05. DOI: 10.26355/eurrev_201805_15076. [DOI] [PubMed] [Google Scholar]
- 16.Jude EB, Eleftheriadou I and Tentolouris N. Peripheral arterial disease in diabetes--a review. Diabet Med 2010; 27: 4–14. 2010/02/04. DOI: DME2866 [pii] 10.1111/j.1464-5491.2009.02866.x [doi]. [DOI] [PubMed] [Google Scholar]
- 17.Kimoto E, Shoji T, Shinohara K, et al. Preferential stiffening of central over peripheral arteries in type 2 diabetes. Diabetes 2003; 52: 448–452. [DOI] [PubMed] [Google Scholar]
- 18.Taniwaki H, Shoji T, Emoto M, et al. Femoral artery wall thickness and stiffness in evaluation of peripheral vascular disease in type 2 diabetes mellitus. Atherosclerosis 2001; 158: 207–214. [DOI] [PubMed] [Google Scholar]
- 19.Emoto M, Nishizawa Y, Kawagishi T, et al. Stiffness indexes β of the common carotid and femoral arteries are associated with insulin resistance in NIDDM. Diabetes care 1998; 21: 1178–1182. [DOI] [PubMed] [Google Scholar]
- 20.Ahlgren AR, Sundkvist G, Wollmer P, et al. Increased aortic stiffness in women with type 1 diabetes mellitus is associated with diabetes duration and autonomic nerve function. Diabet Med 1999; 16: 291–297. 1999/04/29. DOI: 10.1046/j.1464-5491.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 21.Dolan NC, Liu K, Criqui MH, et al. Peripheral artery disease, diabetes, and reduced lower extremity functioning. Diabetes care 2002; 25: 113–120. 2002/01/05. DOI: 10.2337/diacare.25.1.113. [DOI] [PubMed] [Google Scholar]
- 22.Hodnett PA, Ward EV, Davarpanah AH, et al. Peripheral arterial disease in a symptomatic diabetic population: prospective comparison of rapid unenhanced MR angiography (MRA) with contrast-enhanced MRA. AJR American journal of roentgenology 2011; 197: 1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visser K and Hunink M. Peripheral arterial disease: gadolinium-enhanced MR angiography versus color-guided duplex US--a meta-analysis. Radiology 2000. [DOI] [PubMed] [Google Scholar]
- 24.Collins RT and Weiss RS. Vanishing point calculation as a statistical inference on the unit sphere. Proc Third Int Conf on Computer Vision. Osaka, Japan 1990. [Google Scholar]
- 25.Bissell MM, Raimondi F, Ait Ali L, et al. 4D Flow cardiovascular magnetic resonance consensus statement: 2023 update. J Cardiovasc Magn Reson 2023; 25: 40. 2023/07/21. DOI: 10.1186/s12968-023-00942-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Associations between 2-year differences in lumen volume and the 2-year differences in magnetic resonance imaging measured velocity parameters in the distal SFA.