Abstract
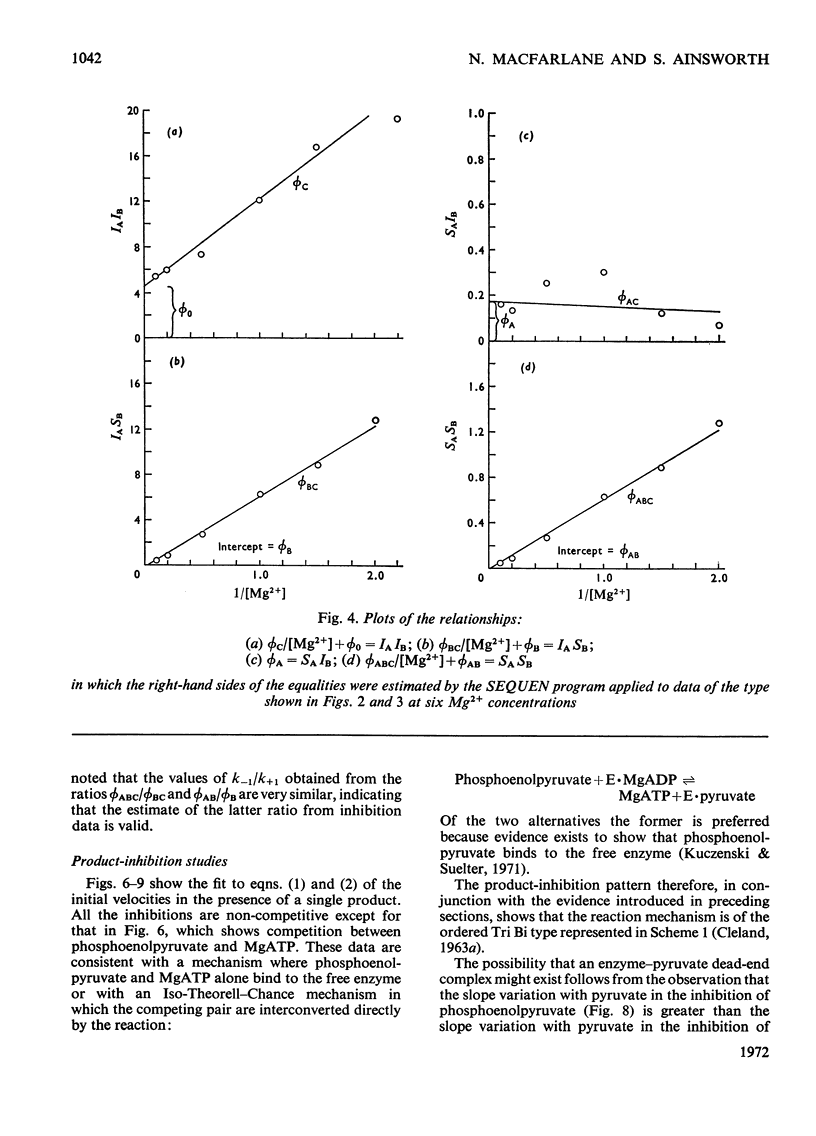
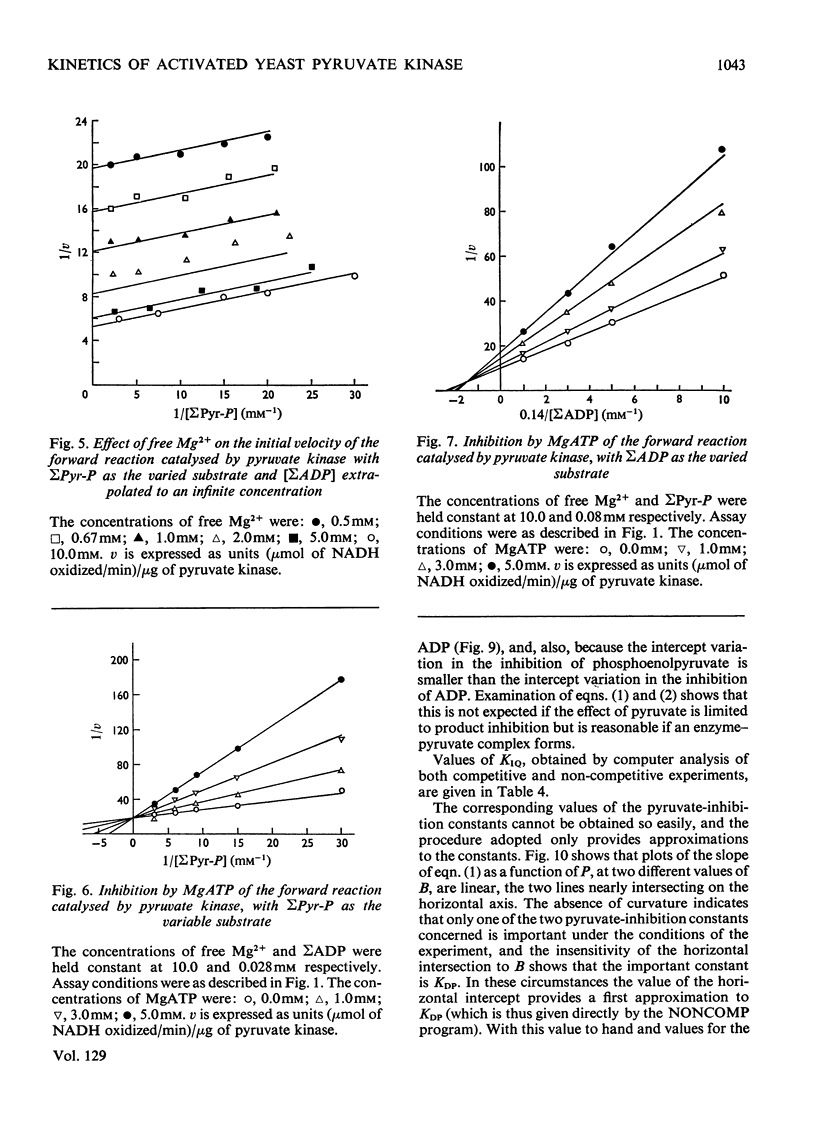
The paper reports a study of the kinetics of the reaction between phosphoenolpyruvate, ADP and Mg2+ catalysed by yeast pyruvate kinase when activated by fructose 1,6-diphosphate and K+. The experimental results indicate that the reaction mechanism is of the Ordered Tri Bi type with the substrates binding in the order phosphoenolpyruvate, ADP and Mg2+. Direct phosphoryl transfer takes place in the quaternary complex, with pyruvate released before MgATP. A dead-end enzyme–pyruvate complex is also indicated. Values have been determined for the Michaelis, dissociation and inhibition constants of the reaction. Several of the rate constants involved have also been evaluated.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOCK R. M., LING N. S., MORELL S. A., LIPTON S. H. Ultraviolet absorption spectra of adenosine-5'-triphosphate and related 5'-ribonucleotides. Arch Biochem Biophys. 1956 Jun;62(2):253–264. doi: 10.1016/0003-9861(56)90123-0. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. Computer programmes for processing enzyme kinetic data. Nature. 1963 May 4;198:463–465. doi: 10.1038/198463a0. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- COHN M., HUGHES T. R., Jr Nuclear magnetic resonance spectra of adenosine di- and triphosphate. II. Effect of complexing with divalent metal ions. J Biol Chem. 1962 Jan;237:176–181. [PubMed] [Google Scholar]
- COHN M., HUGHES T. R., Jr Phosphorus magnetic resonance spectra of adenosine di- and triphosphate. I. Effect of pH. J Biol Chem. 1960 Nov;235:3250–3253. [PubMed] [Google Scholar]
- Dalziel K. The interpretation of kinetic data for enzyme-catalysed reactions involving three substrates. Biochem J. 1969 Sep;114(3):547–556. doi: 10.1042/bj1140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeckel R., Hess B., Lauterborn W., Wüster K. H. Purification and allosteric properties of yeast pyruvate kinase. Hoppe Seylers Z Physiol Chem. 1968 May;349(5):699–714. doi: 10.1515/bchm2.1968.349.1.699. [DOI] [PubMed] [Google Scholar]
- Hunsley J. R., Suelter C. H. Yeast pyruvate kinase. I. Purification and some chemical properties. J Biol Chem. 1969 Sep 25;244(18):4815–4818. [PubMed] [Google Scholar]
- Hunsley J. R., Suelter C. H. Yeast pyruvate kinase. II. Kinetic properties. J Biol Chem. 1969 Sep 25;244(18):4819–4822. [PubMed] [Google Scholar]
- KACHMAR J. F., BOYER P. D. Kinetic analysis of enzyme reactions. II. The potassium activation and calcium inhibition of pyruvic phosphoferase. J Biol Chem. 1953 Feb;200(2):669–682. [PubMed] [Google Scholar]
- Kuczenski R. T., Suelter C. H. Effect of temperature and effectors on the conformations of yeast pyruvate kinase. Biochemistry. 1970 Feb 17;9(4):939–945. doi: 10.1021/bi00806a033. [DOI] [PubMed] [Google Scholar]
- Kuczenski R. T., Suelter C. H. Interactions of Fructose 1,6-diphosphate, substrates, and monovalent cations with yeast pyruvate kinase monitored by changes in enzyme fluorescence. Biochemistry. 1971 Jul 20;10(15):2862–2866. doi: 10.1021/bi00791a010. [DOI] [PubMed] [Google Scholar]
- Kuczenski R. T., Suelter C. H. Yeast pyruvate kinase. Native and subunit molecular weight. Biochemistry. 1970 May 12;9(10):2043–2047. doi: 10.1021/bi00812a003. [DOI] [PubMed] [Google Scholar]
- Mildvan A. S., Cohn M. Kinetic and magnetic resonance studies of the pyruvate kinase reaction. II. Complexes of enzyme, metal, and substrates. J Biol Chem. 1966 Mar 10;241(5):1178–1193. [PubMed] [Google Scholar]
- PHILLIPS R. C., GEORGE P., RUTMAN R. J. POTENTIOMETRIC STUDIES OF THE SECONDARY PHOSPHATE IONIZATIONS OF AMP, ADP, AND ATP, AND CALCULATIONS OF THERMODYNAMIC DATA FOR THE HYDROLYSIS REACTIONS. Biochemistry. 1963 May-Jun;2:501–508. doi: 10.1021/bi00903a019. [DOI] [PubMed] [Google Scholar]
- Phillips R. C., George P., Rutman R. J. Thermodynamic studies of the formation and ionization of the magnesium(II) complexes of ADP and ATP over the pH range 5 to 9. J Am Chem Soc. 1966 Jun 20;88(12):2631–2640. doi: 10.1021/ja00964a002. [DOI] [PubMed] [Google Scholar]
- Pon N. G., Bondar R. J. A direct spectrophotometric assay for pyruvate kinase. Anal Biochem. 1967 May;19(2):272–279. doi: 10.1016/0003-2697(67)90163-7. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Harano Y., Sue F., Morimura H. Crystallization, characterization and metabolic regulation of two types of pyruvate kinase isolated from rat tissues. J Biochem. 1967 Jul;62(1):71–91. doi: 10.1093/oxfordjournals.jbchem.a128639. [DOI] [PubMed] [Google Scholar]
- Taylor C. B., Morris H. P., Weber G. A comparison of the properties of pyruvate kinase from hepatoma 3924-A, normal liver and muscle. Life Sci. 1969 Jun 15;8(12):635–644. doi: 10.1016/0024-3205(69)90220-3. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLD F., BALLOU C. E. Studies on the enzyme enolase. I. Equilibrium studies. J Biol Chem. 1957 Jul;227(1):301–312. [PubMed] [Google Scholar]



