Abstract
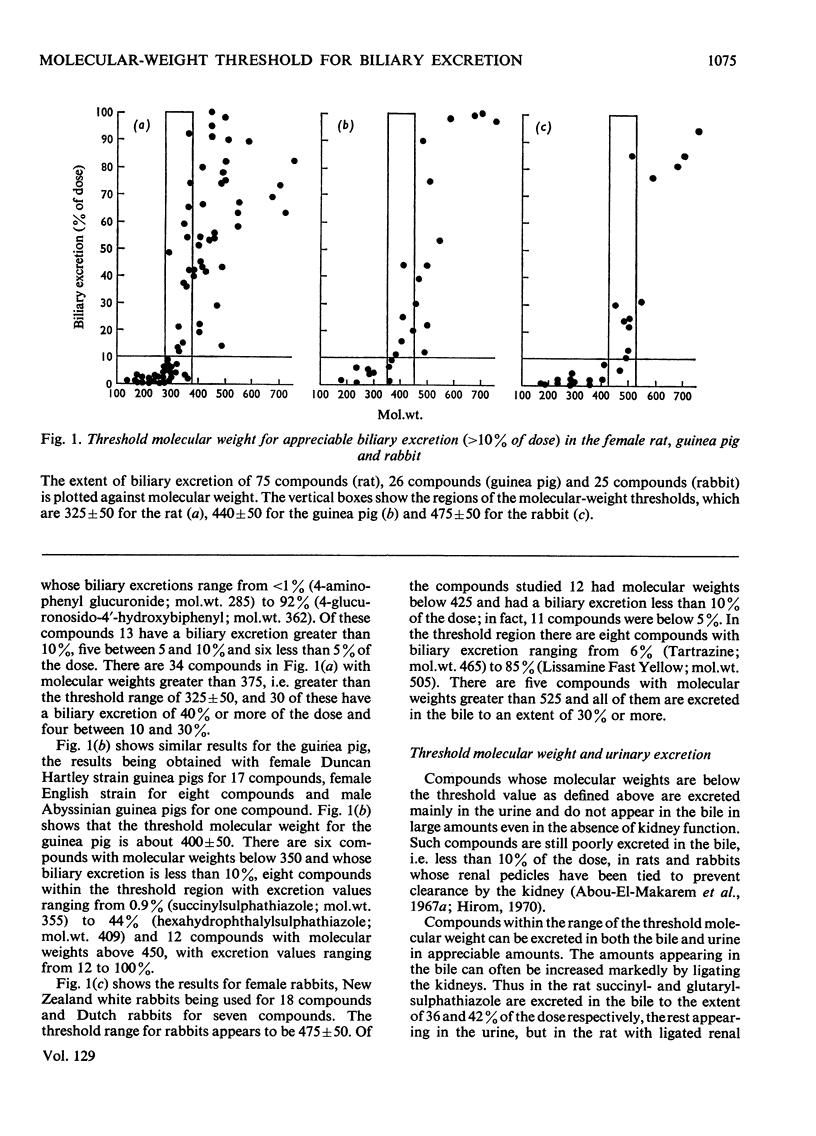
1. The excretion in the bile and urine after intravenous injection of 16 organic anions having molecular weights between 355 and 752 was studied in female rats, guinea pigs and rabbits. 2. These compounds were mostly excreted unchanged, except for three of them, which were metabolized to a slight extent (<7% of dose). 3. The rat excreted all the compounds extensively (22–90% of dose) in the bile. 4. In guinea pigs four of the compounds with mol.wt. 355–403 were excreted in the bile to the extent of 7–16% of the dose, four with mol.wt. 407–465 to the extent of 25–44% and eight compounds with mol.wt. 479–752 to the extent of 44–100%. 5. In rabbits four compounds with mol.wt. 355–465 were excreted in the bile to the extent of 1–8% of the dose, two compounds with mol.wt. 479 and 495 to the extent of 24 and 22%, and six compounds with mol.wt. 505–752 to the extent of 31–94%. 6. These results, together with those of other investigations from this laboratory, are discussed and the conclusion is reached that there is a threshold molecular weight for appreciable biliary excretion (i.e. more than 10% of dose) of anions, which varies with species: about 325±50 for the rat, 400±50 for the guinea pig and 475±50 for the rabbit. 7. Anions with molecular weights greater than about 500 are extensively excreted in the bile of all three species. 8. That proportion of the dose of these compounds which is not excreted in the bile is excreted in the urine, and in the three species, bile and urine are complementary excretory pathways, urinary excretion being greatest for the compounds of lowest molecular weight and tending to decrease with increasing molecular weight. 9. Some implications of this interspecies variation in the molecular-weight requirement for extensive biliary excretion are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-El-Makarem M. M., Millburn P., Smith R. L., Williams R. T. Biliary excretion in foreign compounds. Species difference in biliary excretion. Biochem J. 1967 Dec;105(3):1289–1293. doi: 10.1042/bj1051289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-El-Makarem M. M., Millburn P., Smith R. L., Williams R. T. Biliary excretion of foreign compounds. Benzene and its derivatives in the rat. Biochem J. 1967 Dec;105(3):1269–1274. doi: 10.1042/bj1051269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertagni P., Hirom P. C., Millburn P., Osiyemi F. O., Smith R. L., Turbert H. B., Williams R. T. Sex and species differences in the biliary excretion of tartrazine and lissamine fast yellow in the rat, guinea-pig and rabbit. The influence of sex hormones on tartrazine excretion in the rat. J Pharm Pharmacol. 1972 Aug;24(8):620–624. doi: 10.1111/j.2042-7158.1972.tb09073.x. [DOI] [PubMed] [Google Scholar]
- CAESAR J., SHALDON S., CHIANDUSSI L., GUEVARA L., SHERLOCK S. The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci. 1961 Aug;21:43–57. [PubMed] [Google Scholar]
- CHERRICK G. R., STEIN S. W., LEEVY C. M., DAVIDSON C. S. Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960 Apr;39:592–600. doi: 10.1172/JCI104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX I. J., BROOKER L. G., HESELTINE D. W., ESSEX H. E., WOOD E. H. A tricarbocyanine dye for continuous recording of dilution curves in whole blood independent of variations in blood oxygen saturation. Proc Staff Meet Mayo Clin. 1957 Sep 4;32(18):478–484. [PubMed] [Google Scholar]
- FOX I. J., WOOD E. H. Applications of dilution curves recorded from the right side of the heart or venous circulation with the aid of a new indicator dye. Proc Staff Meet Mayo Clin. 1957 Sep 18;32(19):541–550. [PubMed] [Google Scholar]
- GRADY H. J., ELLIOTT W. H., DOISY E. A., Jr, BOCKLAGE B. C., DOISY E. A. Synthesis and metabolic studies of progesterone-21-C14. J Biol Chem. 1952 Apr;195(2):755–762. [PubMed] [Google Scholar]
- Gregson R. H., Hirom P. C., Millburn P., Smith R. L., Turbert H. B., Williams R. T. The biliary excretion of tartrazine. Sex differences in the rat and species differences in the rat, guinea-pig and rabbit. J Pharm Pharmacol. 1972 Jan;24(1):20–24. doi: 10.1111/j.2042-7158.1972.tb08859.x. [DOI] [PubMed] [Google Scholar]
- HUNTON D. B., BOLLMAN J. L., HOFFMAN H. N. Studies of hepatic function with indocyanine green. Gastroenterology. 1960 Dec;39:713–724. [PubMed] [Google Scholar]
- Hirom P. C., Millburn P., Smith R. L., Williams R. T. Molecular weight and chemical structure as factors in the biliary excretion of sulphonamides in the rat. Xenobiotica. 1972 May;2(3):205–214. doi: 10.3109/00498257209111051. [DOI] [PubMed] [Google Scholar]
- Levine W. G., Millburn P., Smith R. L., Williams R. T. The role of the hepatic endoplasmic reticulum in the biliary excretion of foreign compounds by the rat. The effect of phenobarbitone and SKF 525-A (diethylaminoethyl diphenylpropylacetate). Biochem Pharmacol. 1970 Jan;19(1):235–244. doi: 10.1016/0006-2952(70)90344-8. [DOI] [PubMed] [Google Scholar]
- Millburn P., Smith R. L., Williams R. T. Biliary excretion in foreign compounds. Sulphonamide drugs in the rat. Biochem J. 1967 Dec;105(3):1283–1287. doi: 10.1042/bj1051283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millburn P., Smith R. L., Williams R. T. Biliary excretion of foreign compounds. Biphenyl, stilboestrol and phenolphthalein in the rat: molecular weight, polarity and metabolism as factors in biliary excretion. Biochem J. 1967 Dec;105(3):1275–1281. doi: 10.1042/bj1051275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg A. A., Kirdani R. Y., Back N., Weyman P., Slaunwhite W. R., Jr Biliary excretion and enterohepatic circulation of estrone and estriol in rodents. Am J Physiol. 1967 Nov;213(5):1138–1142. doi: 10.1152/ajplegacy.1967.213.5.1138. [DOI] [PubMed] [Google Scholar]
- Symchowicz S., Staub M. S., Wong K. K. A comparative study of griseofulvin-14C metabolism in the rat and rabbit. Biochem Pharmacol. 1967 Dec;16(12):2405–2411. doi: 10.1016/0006-2952(67)90225-0. [DOI] [PubMed] [Google Scholar]
- Taylor W., Scratcherd T. Steroid metabolism in the rabbit. Biliary and urinary excretion of metabolites of [4-C]progesterone. Biochem J. 1965 Oct;97(1):89–94. doi: 10.1042/bj0970089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. The excretion of steroid hormone metabolites in bile and feces. Vitam Horm. 1971;29:201–285. doi: 10.1016/s0083-6729(08)60050-3. [DOI] [PubMed] [Google Scholar]
- WHEELER H. O., CRANSTON W. I., MELTZER J. I. Hepatic uptake and biliary excretion of indocyanine green in the dog. Proc Soc Exp Biol Med. 1958 Oct;99(1):11–14. doi: 10.3181/00379727-99-24229. [DOI] [PubMed] [Google Scholar]


