Abstract
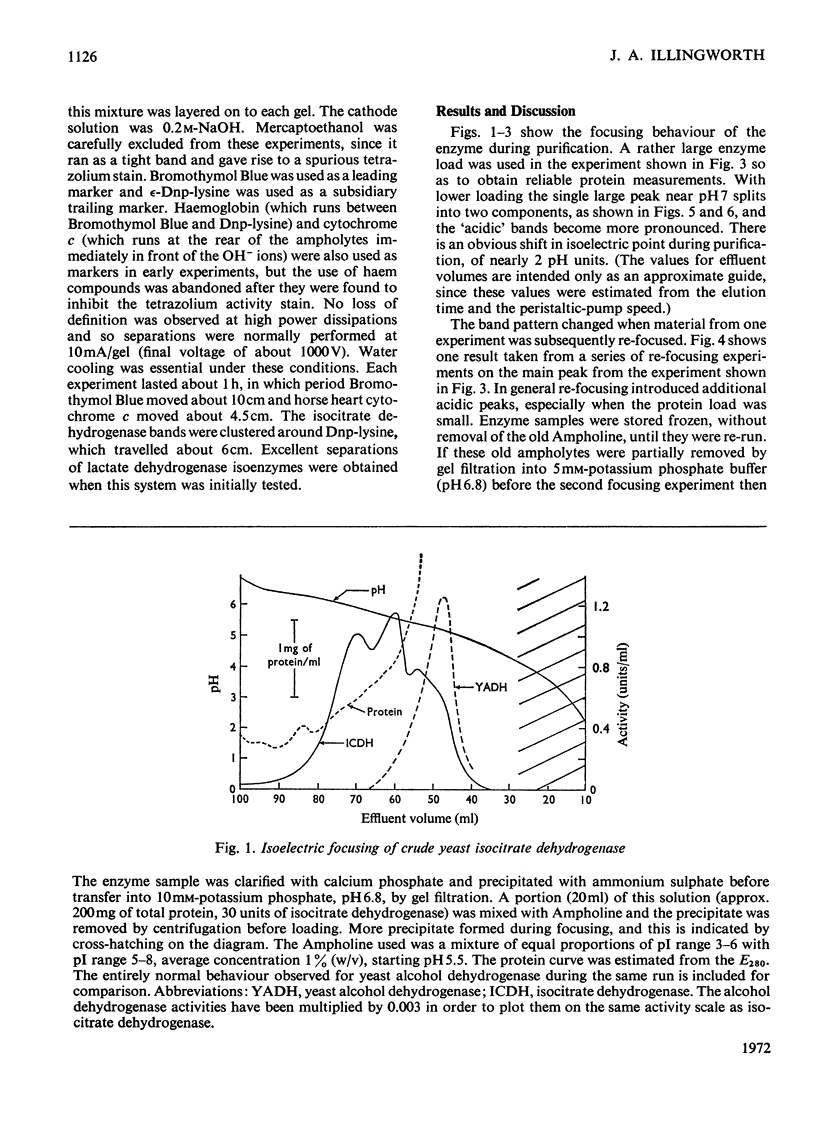
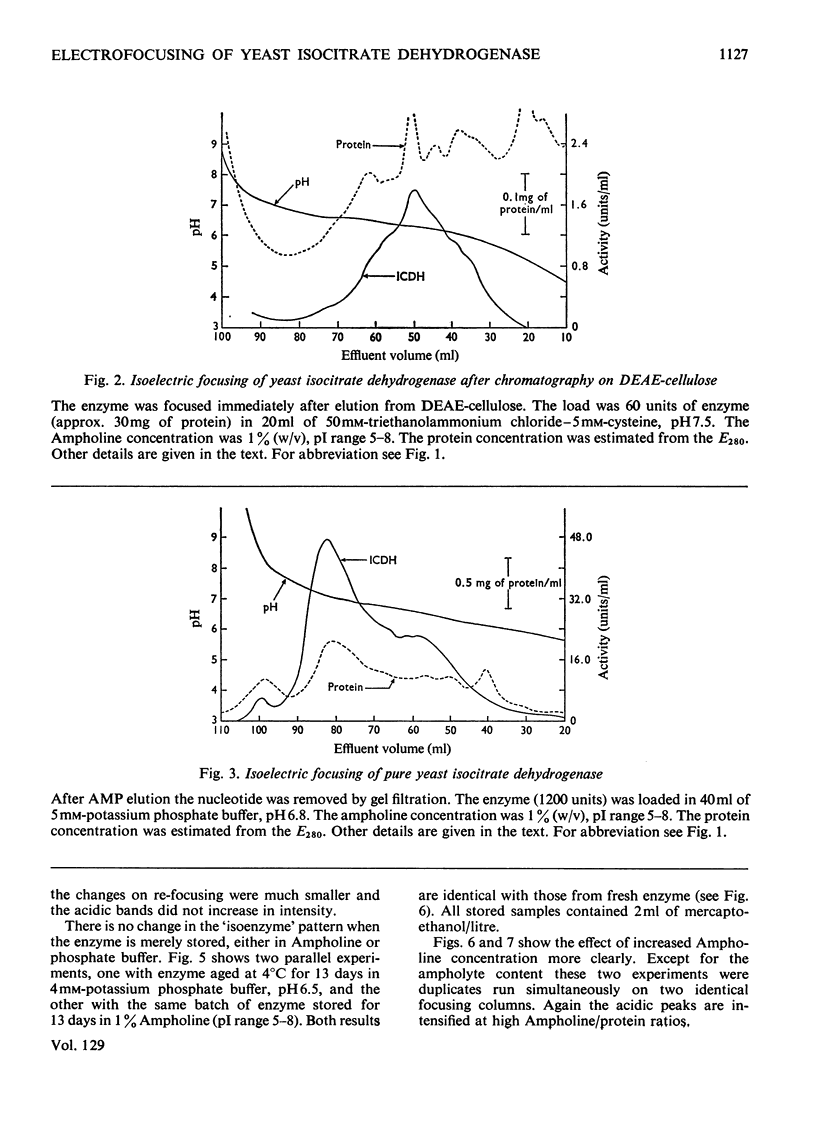
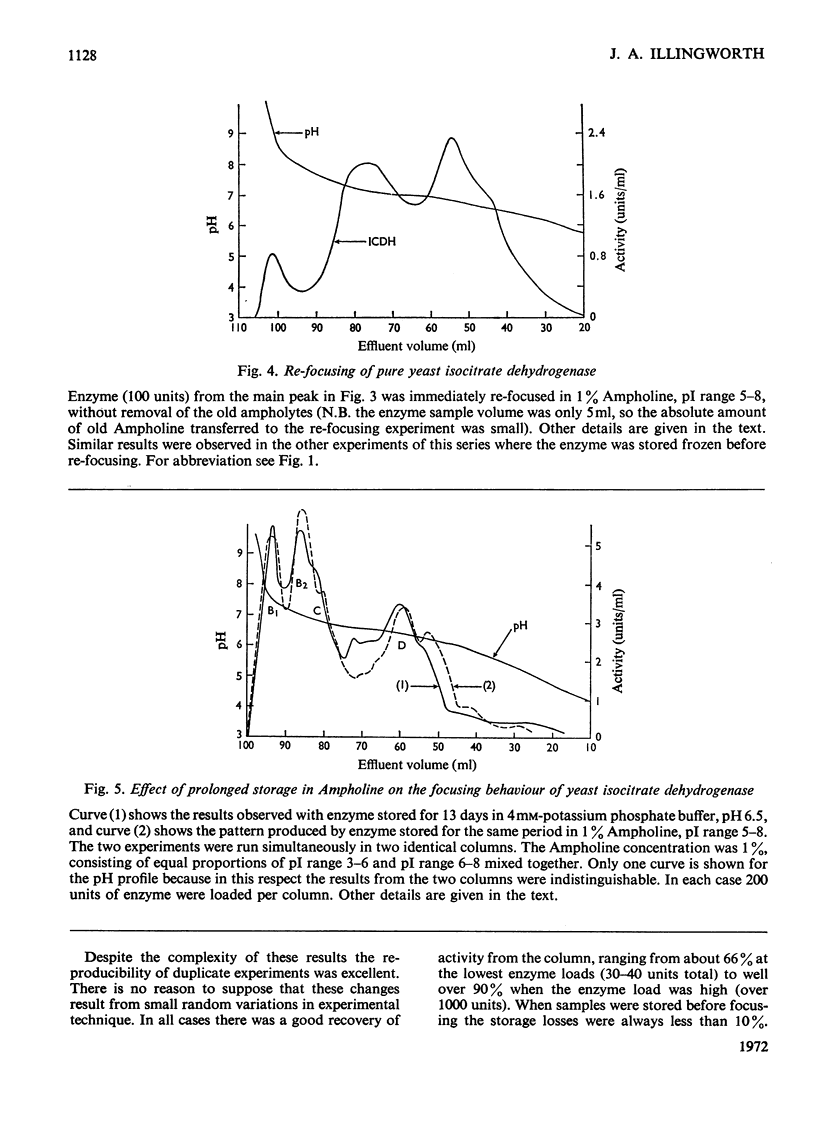
Isoelectric focusing of yeast isocitrate dehydrogenase apparently reveals a number of `isoenzymes'. These have isoelectric points near pH5.5 in crude material, but during purification the mean isoelectric point progressively rises to pH7.0 and the band pattern changes. The shift in isoelectric point during purification is apparently genuine, since it is also manifested in the electrophoretic and chromatographic properties of the enzyme. The multiple forms, however, are an artifact, generated by exposure of the enzyme to Ampholine, since their activities vary with the protein/Ampholine ratio and they cannot be observed in any system from which Ampholine is excluded. There are no detectable isoenzymes of yeast isocitrate dehydrogenase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Illingworth J. A. Purification of yeast isocitrate dehydrogenase. Biochem J. 1972 Oct;129(5):1119–1124. doi: 10.1042/bj1291119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth J. A., Tipton K. F. An automatic apparatus for the study of enzyme kinetics. Biochem J. 1969 Nov;115(3):511–515. doi: 10.1042/bj1150511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejaudier L., Audran R., Steinbuch M. Molecular modification of ceruloplasmin by isoelectric focusing. Biochim Biophys Acta. 1971 Feb 16;229(2):437–439. doi: 10.1016/0005-2795(71)90203-0. [DOI] [PubMed] [Google Scholar]



