Abstract
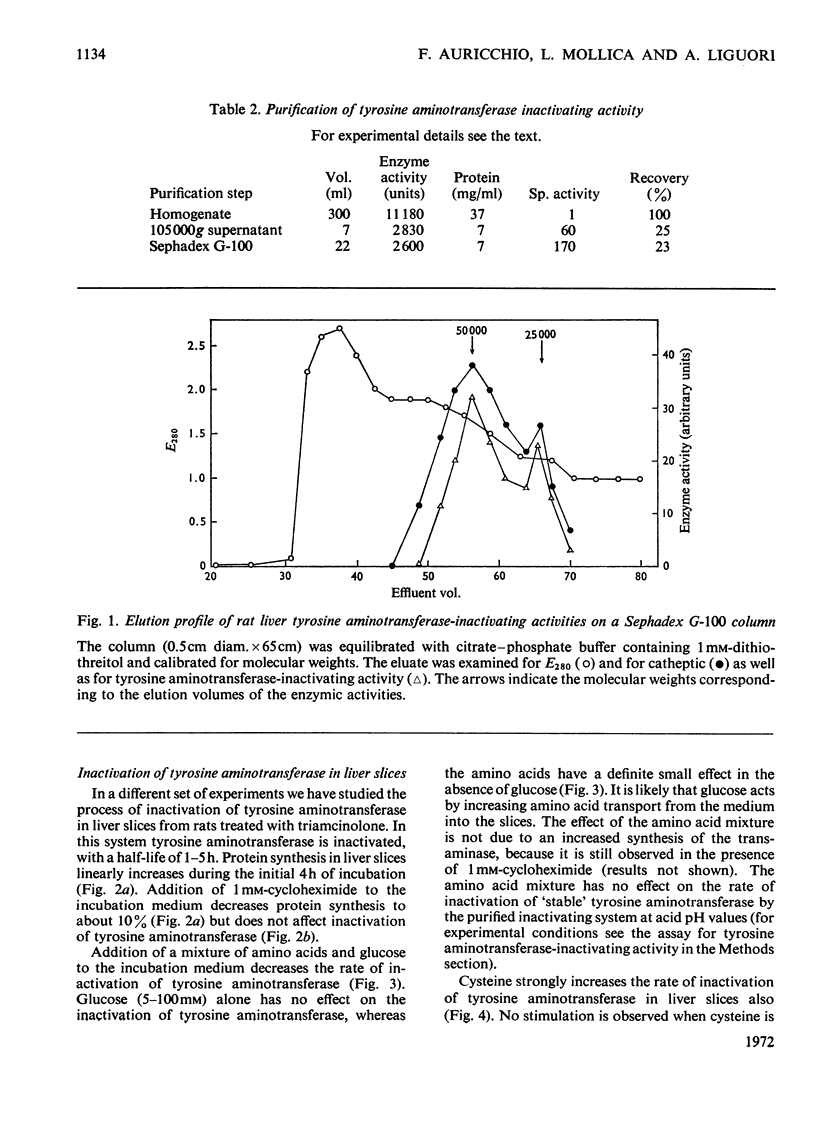
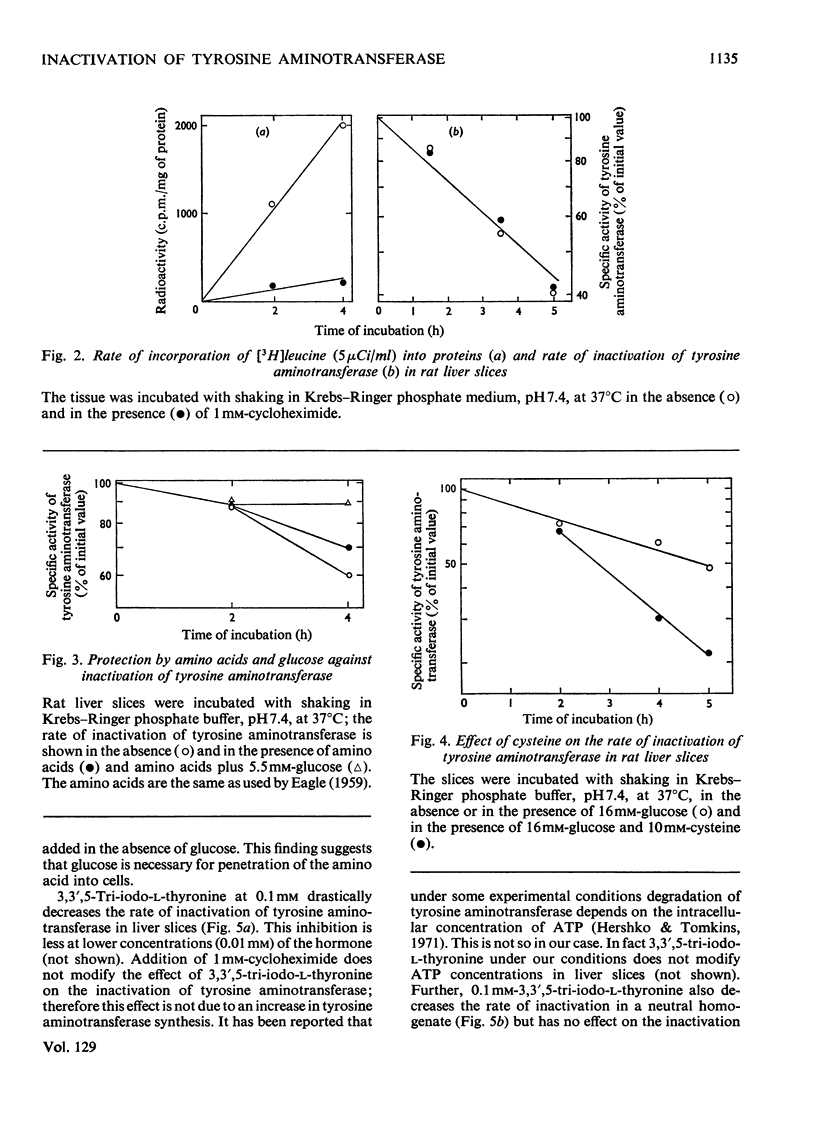
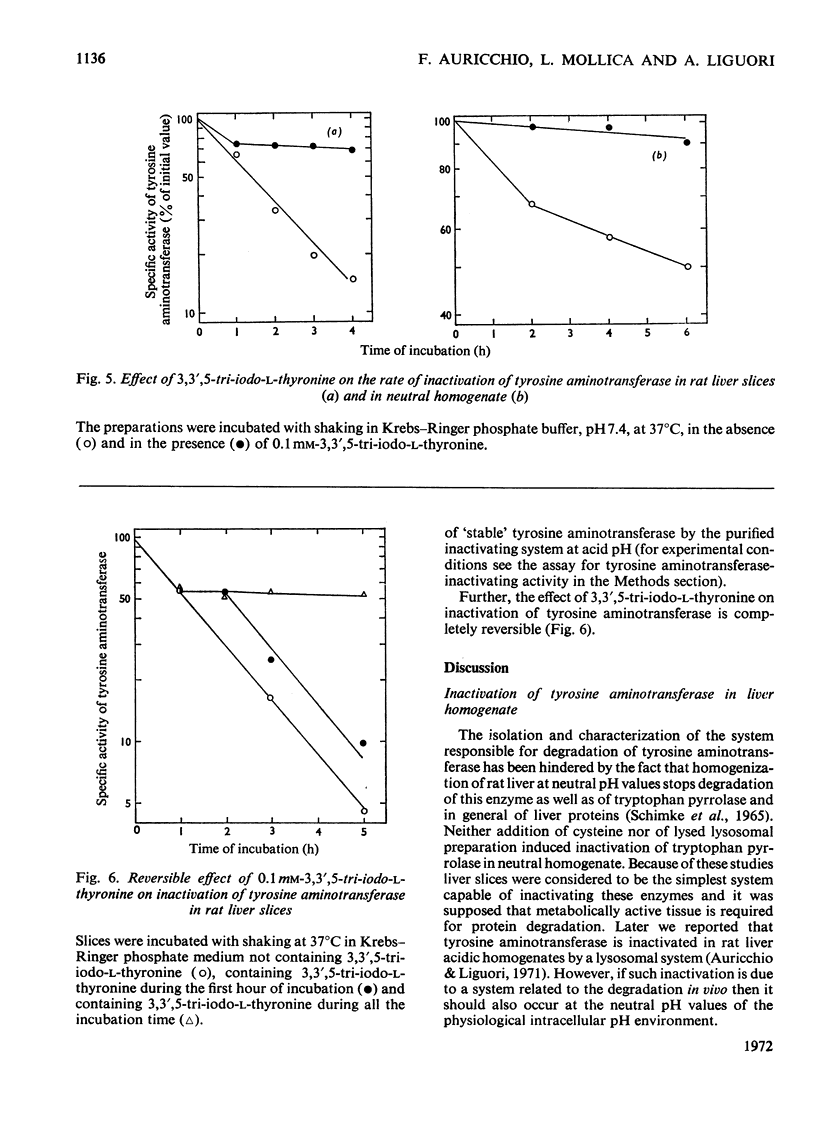
Inactivation of tyrosine aminotransferase induced in vivo by triamcinolone was studied in a homogenate incubated at neutral pH values. The integrity and the presence of subcellular particles together with a compartment of acidic pH are necessary for inactivation of tyrosine aminotransferase. It is suggested that tyrosine aminotransferase is inactivated inside lysosomes. The system responsible for inactivation of tyrosine aminotransferase was partially purified and identified with lysosomal cathepsins B and B1. Inactivation of tyrosine aminotransferase in liver slices is controlled by the amino acid concentration and strongly stimulated by cysteine. 3,3′,5-Tri-iodo-l-thyronine reversibly and strongly decreases the rate of inactivation of tyrosine aminotransferase. The effect is not due to an increased rate of tyrosine aminotransferase synthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auricchio F., Bruni C. B. Dextran-gel filtration of enzymes in the presence of their high-molecular-weight substrates. Biochem J. 1966 Jan;98(1):290–292. doi: 10.1042/bj0980290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auricchio F., Liguori A. A study of a tyrosine aminotransferase inactivating system in rat liver homogenate. FEBS Lett. 1971 Feb 9;12(6):329–332. doi: 10.1016/0014-5793(71)80007-8. [DOI] [PubMed] [Google Scholar]
- Auricchio F., Martin D., Jr, Tompkins G. Control of degradation and synthesis of induced tyrosine aminotransferase studied in hepatoma cells in culture. Nature. 1969 Nov 22;224(5221):806–808. doi: 10.1038/224806b0. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. A role of aminoacyl-tRNA in the regulation of protein breakdown in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Feb;68(2):362–366. doi: 10.1073/pnas.68.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S. I., Granner D. K., Tomkins G. M. Tyrosine aminotransferase. Purificaton and characterization. J Biol Chem. 1967 Sep 25;242(18):3998–4006. [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Hershko A., Tomkins G. M. Studies on the degradation of tyrosine aminotransferase in hepatoma cells in culture. Influence of the composition of the medium and adenosine triphosphate dependence. J Biol Chem. 1971 Feb 10;246(3):710–714. [PubMed] [Google Scholar]
- KENNEY F. T. Induction of tyrosine-alpha-ketoglutarate transaminase in rat liver. IV. Evidence for an increase in the rate of enzyme synthesis. J Biol Chem. 1962 Nov;237:3495–3498. [PubMed] [Google Scholar]
- LIN E. C., KNOX W. E. Adaptation of the rat liver tyrosine-alpha-ketoglutarate transaminase. Biochim Biophys Acta. 1957 Oct;26(1):85–88. doi: 10.1016/0006-3002(57)90057-4. [DOI] [PubMed] [Google Scholar]
- LIN E. C., KNOX W. E. Specificity of the adaptive response to tyrosine-alpha-ketoglutarate transaminase in the rat. J Biol Chem. 1958 Nov;233(5):1186–1189. [PubMed] [Google Scholar]
- Nath K., Koch A. L. Protein degradation in Escherichia coli. I. Measurement of rapidly and slowly decaying components. J Biol Chem. 1970 Jun 10;245(11):2889–2900. [PubMed] [Google Scholar]
- Otto K. Uber ein neues Kathepsin. Reinigung aus Rindermilz, Eigenschaften, sowie Vergleich mit Kathepsin B. Hoppe Seylers Z Physiol Chem. 1967 Nov;348(11):1449–1460. [PubMed] [Google Scholar]
- Peterkofsky B. The inactivation of chick tyrosine aminotransferase by acid and alkaline phosphatases. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1171–1178. doi: 10.1016/0006-291x(71)90586-9. [DOI] [PubMed] [Google Scholar]
- ROODYN D. B., FREEMAN K. B., TATA J. R. THE STIMULATION BY TREATMENT IN VIVO WITH TRI-IODOTHYRONINE OF AMINO ACID INCORPORATION INTO PROTEIN BY ISOLATED RAT-LIVER MITOCHONDRIA. Biochem J. 1965 Mar;94:628–641. doi: 10.1042/bj0940628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIBKO S., TAPPEL A. L. RAT-KIDNEY LYSOSOMES: ISOLATION AND PROPERTIES. Biochem J. 1965 Jun;95:731–741. doi: 10.1042/bj0950731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T., Sweeney E. W., Berlin C. M. Studies of the stability in vivo and in vitro of rat liver tryptophan pyrrolase. J Biol Chem. 1965 Dec;240(12):4609–4620. [PubMed] [Google Scholar]
- Tata J. R., Widnell C. C. Ribonucleic acid synthesis during the early action of thyroid hormones. Biochem J. 1966 Feb;98(2):604–620. doi: 10.1042/bj0980604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. B., Tomkins G. M., Curran J. F. Induction of tyrosine alpha-ketoglutarate transaminase by steroid hormones in a newly established tissue culture cell line. Proc Natl Acad Sci U S A. 1966 Jul;56(1):296–303. doi: 10.1073/pnas.56.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeriote F. A., Auricchio F., Tomkins G. M., Riley D. Purification and properties of rat liver tyrosine aminotransferase. J Biol Chem. 1969 Jul 10;244(13):3618–3624. [PubMed] [Google Scholar]


