Abstract
This retrospective population-based study investigated the impact of elexacaftor/tezacaftor/ivacaftor (ETI) therapy on inhaled medication adherence in people with cystic fibrosis (pwCF). Prescription refill rate (PRR) for several inhaled medications were compared before and after ETI introduction in three major Italian CF centers. We found a significant decrease in PRR for most inhaled antibiotics and dornase-alpha after ETI implementation.This suggests that patients may be reducing their adherence to inhaled medications, potentially due to improved respiratory symptoms and quality of life. The study highlights the challenges of maintaining adherence to chronic inhaled medications in pwCF, even after the introduction of breakthrough therapies like ETI. Monitoring adherence remains crucial for optimizing patient outcomes, and the PRR emerges as a valuable tool for tracking adherence in real-world settings.
To the editor
The management of cystic fibrosis (CF) necessitates adherence to a multifaceted treatment regimen that includes several chronic medications and a demanding daily routine, oftentimes leading to undesirable lifestyle adjustments. This comprehensive approach, while necessary for slowing disease progression, places a significant burden on people with CF (pwCF) [1].
The success of elexacaftor/tezacaftor/ivacaftor (ETI) in improving respiratory symptoms in pwCF has encouraged the community to seek a simplification of burdensome inhaled therapies. This process has also been reported to occur without medical oversight, with some individuals even reducing or stopping their prescribed medications at their own discretion [1]. Therefore, continuous adherence monitoring is still crucial for overall management of pwCF, but existing tools generate limitations in determining the magnitude of ETI impact on medication compliance.
We conducted a population-based study to retrospectively compare the use of inhaled medications before and after ETI initiation, collecting data from CF pharmacies in Italy.
This study included retrospective data from pharmacies serving three major CF centers in Verona, Rome and Lamezia Terme, Italy. Chronic treatments evaluated were dornase-alpha and several inhaled antibiotics, including colistin, tobramycin, aztreonam, and levofloxacin. Adherence to inhaled medications was indirectly assessed through the calculation of the prescription refill rate (PRR) for each drug. PRR was calculated by comparing the number of prescriptions and the number of drugs actually dispensed yearly. We computed the PRR for each drug in 2019, when ETI was not commercially available in Italy, and in 2021 through 2022, which was after its implementation in clinical practice. Statistical analysis was performed using the SPSS (version-20 IBM-SPSS statistical software, USA).
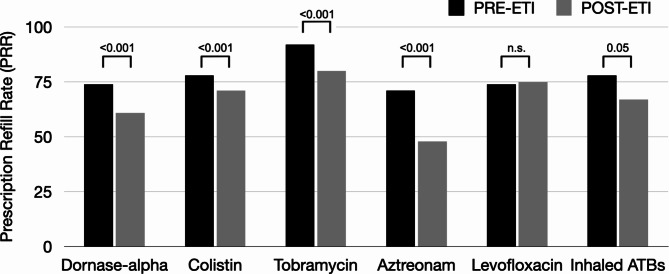
We included a total of four pharmacies serving three major Italian CF centers, encompassing approximately 1,500 pwCF. Our analyses showed that the PRR for dornase-alpha significantly decreased (74% vs. 61%, p < 0.001) in the post-ETI period. As for inhaled antibiotics, the PRR significantly decreased for tobramycin (92% vs. 80%, p < 0.001), aztreonam (71% vs. 48%, p < 0.001), and colistin (78% vs. 71%, p < 0.001), while PRR for levofloxacin did not change (Fig. 1). The overall PRR for inhaled antibiotics dropped from 77.6 to 66.8% after introducing ETI, with a mean difference of 10.8 units (95% CI: -0.1097 to 21.7097, p = 0.05).
Fig. 1.
PRR for chronic inhaled medications before and after ETI initiation
Our study showed that the introduction of ETI has been followed by a significant decline in inhaled medication refills for chronic inhaled treatments. These results mimic findings from earlier studies that used different methods to track medication use, such as the medication possession ratio [2, 3]. In our study, the application to the PRR is especially beneficial due to its reliance on existing administrative data, which minimized data collection and enhanced applicability to healthcare systems.
We showed a decrease in PRR that preceded the introduction of ETI, suggesting that adherence to therapy was already an issue before this new treatment. The decrease in adherence to dornase-alpha could be explained by recent trials on the discontinuation of chronic mucolytics in pwCF with stable lung function after establishment on ETI therapy [4]. Unfortunately, the fact that hypertonic saline is less commonly used and, in many cases, is prepared as a compounded product by pharmacies or patients themselves has limited the ability to assess PRR for this chronic mucoactive. On the contrary, the reduction for inhaled antibiotics is more concerning, as it is not supported by any recommendation or clinical evidence [5]. While this study does not investigate the causes of reduced adherence, we can speculate that improved respiratory outcomes, particularly in terms of symptoms and quality of life, might be a primary factor. Moreover, therapies requiring significant time commitment and multiple doses, such as aztreonam, appear to be most impacted, suggesting pwCF may be simplifying their own regimens in an attempt to decrease overall treatment burden.
The PRR for levofloxacin did not change after the introduction of ETI, aligning with a similar finding a recent multicenter Greek study conducted by Manika and colleagues [3]. While further analysis is warranted, it is plausible that the potential use of levofloxacin as a second-line agent may identify a subset of pwCF with increased frailty and a greater understanding of the role of inhaled therapy. Individual-level data could provide deeper insights into this observation.
It is important to note that our results are limited by the retrospective design and the lack of individual patient data, which precluded a patient-level assessment of adherence. Unlike the medication possession ratio, traditionally employed in adherence studies for its ability to provide patient-specific insights, the PRR operates at a population or cohort level.
Our study relies solely on medication dispensing data, which may not accurately reflect actual medication usage. Early refills or stockpiling could potentially skew the results. Moreover, the data did not consider factors like changes in medication regimens, hospitalisations, or the quality of adherence (e.g. correct timing and dosage). However, by including data from three leading CF centers across Italy, our findings are likely more applicable to a wider population of CF patients.
In conclusion, our findings indicate that ETI has significantly contributed to a decline in adherence to chronic inhaled medications among individuals with cystic fibrosis. Supporting this patient population in maintaining treatment adherence has become increasingly challenging, emphasising the ongoing importance of adherence monitoring. Given these challenges, PRR emerges as a valuable and accessible tool for most centers. While generating robust evidence in this field requires further investigation, PRR can serve as a practical method for tracking adherence in real-world settings.
Acknowledgements
This study was endorsed by the Italian Respiratory Society - Società Italiana di Pneumologia (IRS/SIP) and by the Società Italiana per lo studio della Fibrosi Cistica (SIFC).
Author contributions
Conceptualization: AG, GC, MC, MAC, FB. Data curation and formal analysis: AG, FP, GG, AP. Methodology: AG, FP. Writing - original draft: AG, FP. Writing - review and editing: AG, GC, MC, MAC, FB. All authors read and approved the final manuscript.
Funding
This study was supported by an unrestricted grant supplied by Chiesi Italia SpA.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by local Institutional Ethical Committee, followed by the approval of the other local ethical committees (CE Milano Area B, parere n. 594_2016bis). All participants provided written informed consent to take part in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Southern KW, Addy C, Bell SC, Bevan A, Borawska U, Brown C, Burgel PR, Button B, Castellani C, Chansard A, Chilvers MA, Davies G, Davies JC, De Boeck K, Declercq D, Doumit M, Drevinek P, Fajac I, Gartner S, Georgiopoulos AM, Gursli S, Gramegna A, Hansen CM, Hug MJ, Lammertyn E, Landau EEC, Langley R, Mayer-Hamblett N, Middleton A, Middleton PG, Mielus M, Morrison L, Munck A, Plant B, Ploeger M, Bertrand DP, Pressler T, Quon BS, Radtke T, Saynor ZL, Shufer I, Smyth AR, Smith C, van Koningsbruggen-Rietschel S. Standards for the care of people with cystic fibrosis; establishing and maintaining health. J Cyst Fibros. 2024;23(1):12–28. 10.1016/j.jcf.2023.12.002. Epub 2023 Dec 21. PMID: 38129255. [DOI] [PubMed] [Google Scholar]
- 2.Girón RM, Peláez A, Ibáñez A, Martínez-Besteiro E, Gómez-Punter RM, Martínez-Vergara A, Ancochea J, Morell A. Longitudinal study of Therapeutic Adherence in a cystic fibrosis unit: identifying potential factors Associated with medication possession ratio. Antibiot (Basel). 2022;11(11):1637. 10.3390/antibiotics11111637. PMID: 36421281; PMCID: PMC9687051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manika K, Diamantea F, Tsakona A, Kakolyris A, Sopiadou A, Kotoulas SC, Sionidou M, Kirvasili SS, Hadji-Mitrova M, Papadaki E, Chrysochoou EA, Hatziagorou E. Use of cystic fibrosis inhaled medication before and after elexacaftor/tezacaftor/ivacaftor initiation. J Cyst Fibros. 2024;23(1):29–31. Epub 2023 May 9. PMID: 37169616. [DOI] [PubMed] [Google Scholar]
- 4.Mayer-Hamblett N, Ratjen F, Russell R, Donaldson SH, Riekert KA, Sawicki GS, Odem-Davis K, Young JK, Rosenbluth D, Taylor-Cousar JL, Goss CH, Retsch-Bogart G, Clancy JP, Genatossio A, O’Sullivan BP, Berlinski A, Millard SL, Omlor G, Wyatt CA, Moffett K, Nichols DP, Gifford AH, SIMPLIFY Study Group. Discontinuation versus continuation of hypertonic saline or dornase alfa in modulator treated people with cystic fibrosis (SIMPLIFY): results from two parallel, multicentre, open-label, randomised, controlled, non-inferiority trials. Lancet Respir Med. 2023;11(4):329–40. 10.1016/S2213-2600(22)00434-9. Epub 2022 Nov 4. PMID: 36343646; PMCID: PMC10065895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gramegna A, Aliberti S, Calderazzo MA, Casciaro R, Ceruti C, Cimino G, Fabrizzi B, Lucanto C, Messore B, Pisi G, Taccetti G, Tarsia P, Blasi F, Cipolli M. The impact of elexacaftor/tezacaftor/ivacaftor therapy on the pulmonary management of adults with cystic fibrosis: An expert-based Delphi consensus. Respir Med. 2023;220:107455. 10.1016/j.rmed.2023.107455. Epub 2023 Nov 3. PMID: 37926181. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.