Abstract
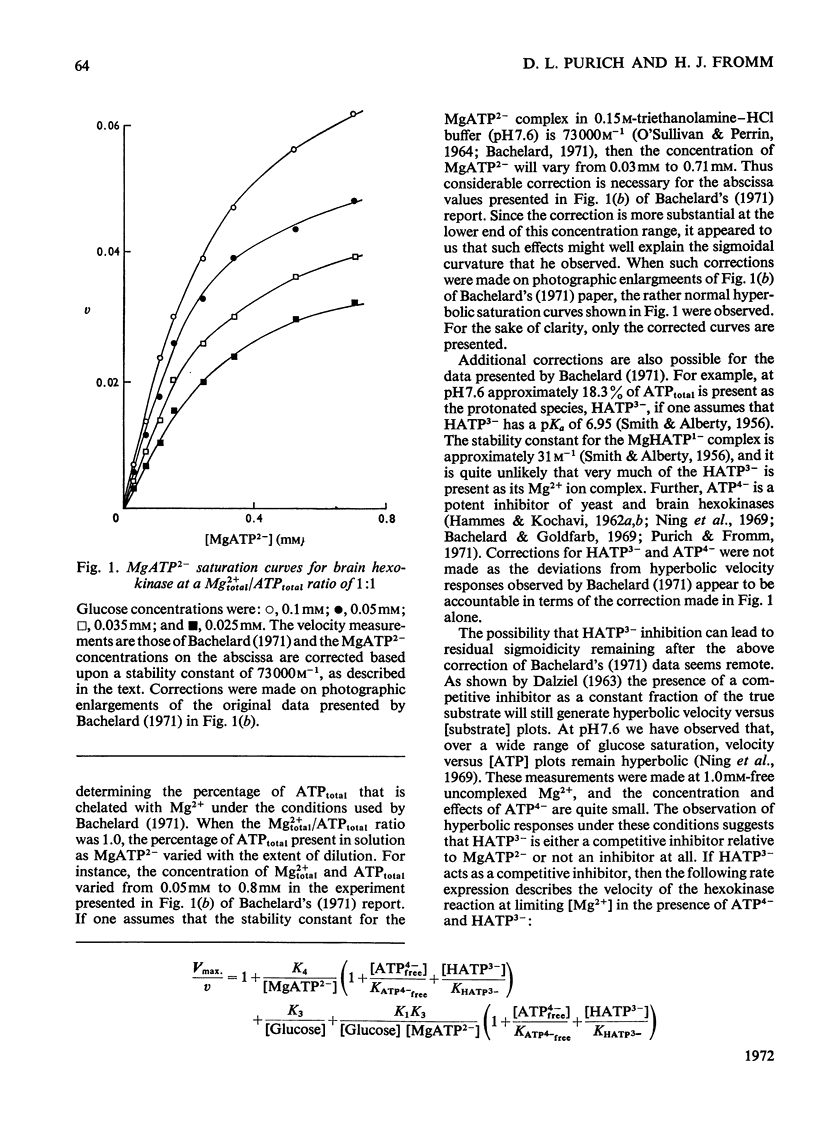
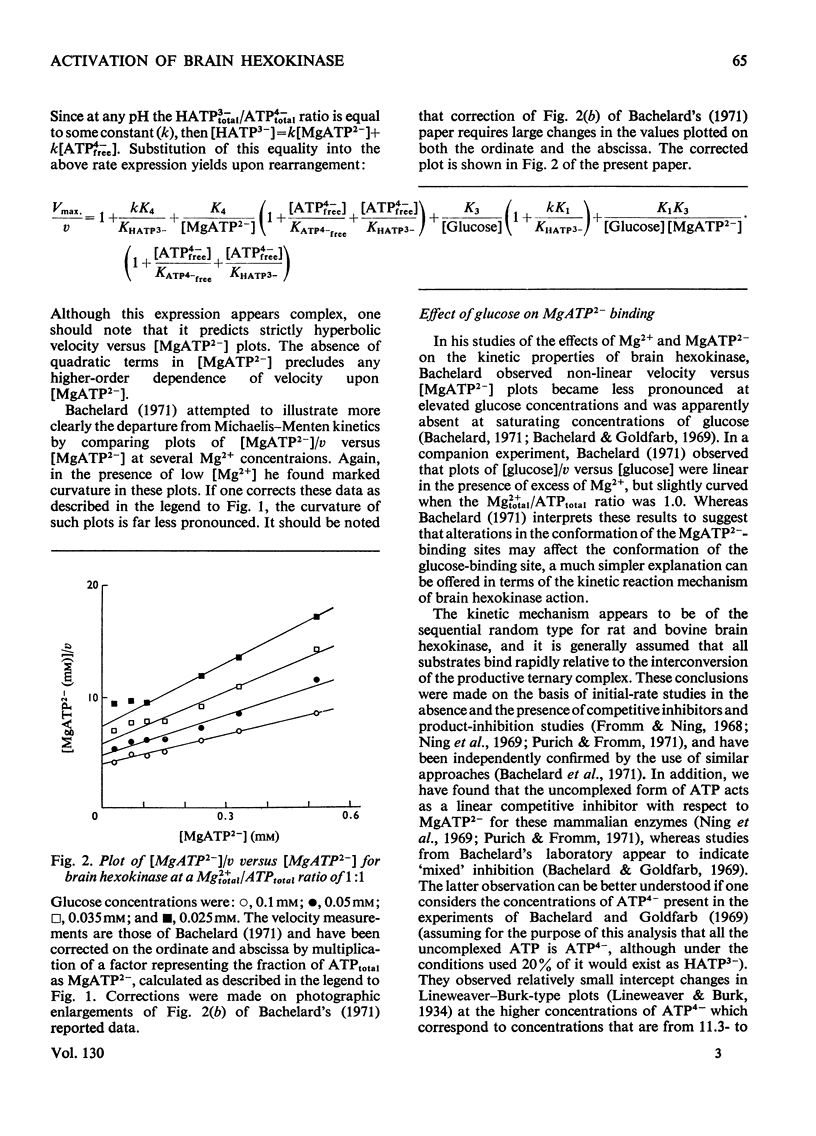
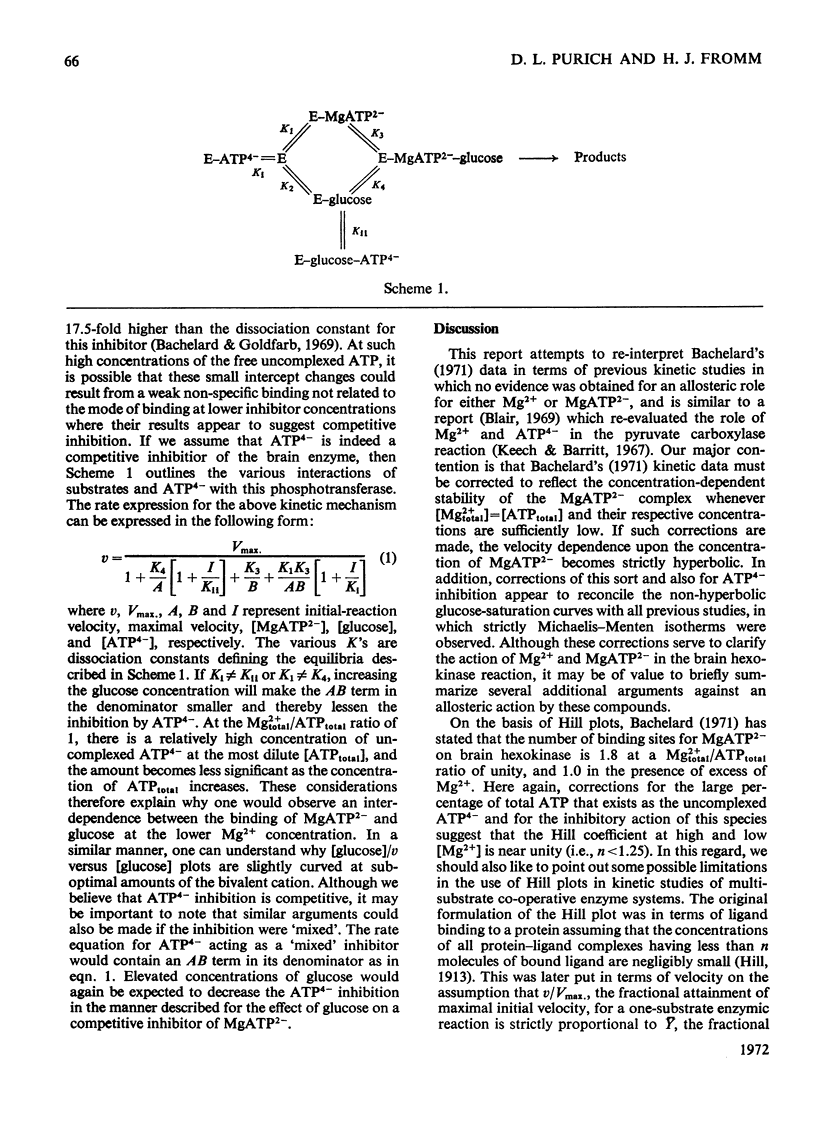
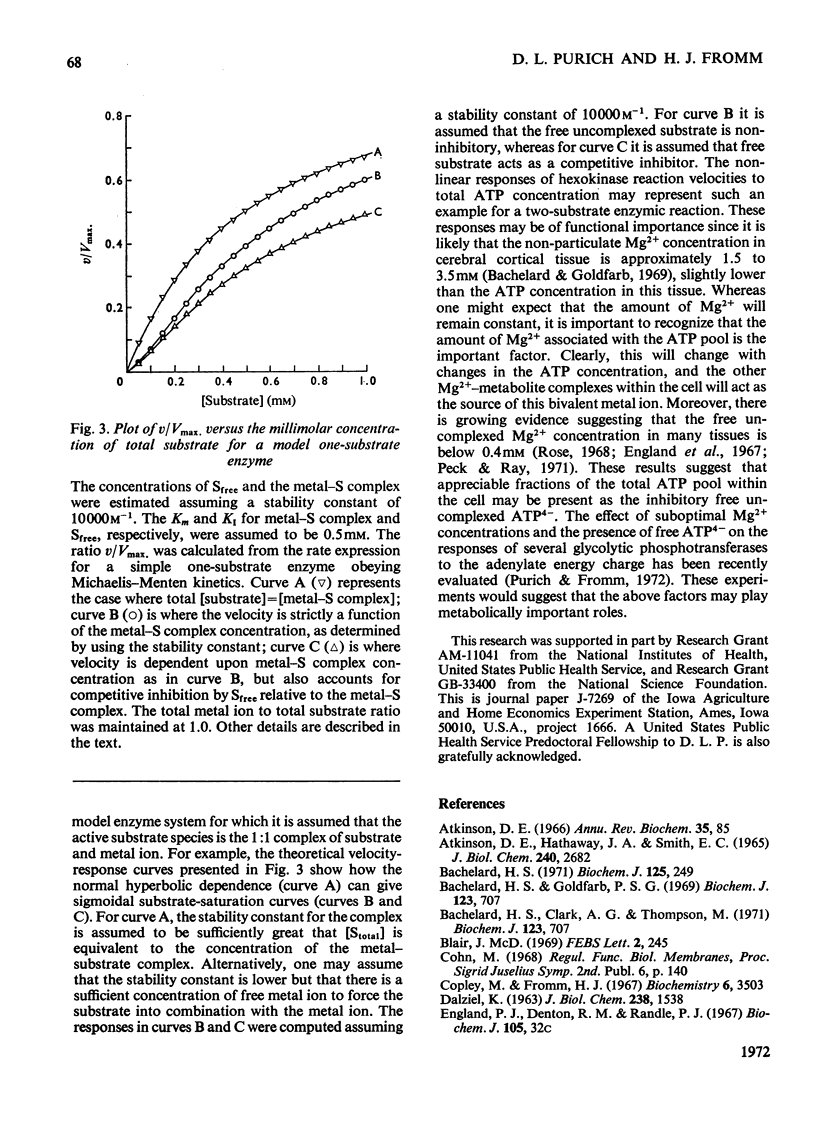
1. An alternative explanation for the kinetic data obtained by Bachelard (1971) for the brain hexokinase reaction is presented. 2. Apparently sigmoidal saturation curves for MgATP2− based upon Bachelard's (1971) studies can be corrected to hyperbolic curves by use of a stability constant for MgATP2− complex formation. 3. A number of other effects related to the concentration-dependent stability of the MgATP2− complex and to the presence of the inhibitory free uncomplexed ATP4− concentration are also explained in terms of a non-allosteric role for either Mg2+ or MgATP2− fully consistent with a number of previous reports on this enzyme. 4. A brief discussion of the validity of Hill plots in studies of multisubstrate co-operative enzymes is presented. 5. A simple model is presented that demonstrates how enzymes obeying Michaelis–Menten kinetics can demonstrate sigmoidal velocity responses if the true substrate of the reaction is the metal–substrate complex.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., HATHAWAY J. A., SMITH E. C. KINETICS OF REGULATORY ENZYMES. KINETIC ORDER OF THE YEAST DIPHOSPHOPYRIDINE NUCLEOTIDE ISOCITRATE DEHYDROGENASE REACTION AND A MODEL FOR THE REACTION. J Biol Chem. 1965 Jun;240:2682–2690. [PubMed] [Google Scholar]
- Bachelard H. S. Allosteric activation of brain hexokinase by magnesium ions and by magnesium ion--adenosine triphosphate complex. Biochem J. 1971 Nov;125(1):249–254. doi: 10.1042/bj1250249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelard H. S., Clark A. G., Thompson M. F. Cerebral-cortex hexokinase. Elucidation of reaction mechanisms by substrate and dead-end inhibitor kinetic analysis. Biochem J. 1971 Aug;123(5):707–715. doi: 10.1042/bj1230707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelard H. S., Clark A. G., Thompson M. F. Cerebral-cortex hexokinase. Elucidation of reaction mechanisms by substrate and dead-end inhibitor kinetic analysis. Biochem J. 1971 Aug;123(5):707–715. doi: 10.1042/bj1230707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair J. M. A reinterpretation of the kinetics of pyruvate carboxylase. FEBS Lett. 1969 Feb;2(4):245–247. doi: 10.1016/0014-5793(69)80032-3. [DOI] [PubMed] [Google Scholar]
- Copley M., Fromm H. J. Kinetic studies of the brain hexokinase reaction. A reinvestigation with the solubilized bovine enzyme. Biochemistry. 1967 Nov;6(11):3503–3509. doi: 10.1021/bi00863a023. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. The purification of nicotinamide adenine dinucleotide and kinetic effects of nucleotide impurities. J Biol Chem. 1963 Apr;238:1538–1543. [PubMed] [Google Scholar]
- FROMM H. J., SILVERSTEIN E., BOYER P. D. EQUILIBRIUM AND NET REACTION RATES IN RELATION TO THE MECHANISM OF YEAST HEXOKINASE. J Biol Chem. 1964 Nov;239:3645–3652. [PubMed] [Google Scholar]
- Fromm H. J., Ning J. Kinetic studies of solubilized brain hexokinase with D-fructose as a substrate. Biochem Biophys Res Commun. 1968 Aug 21;32(4):672–677. doi: 10.1016/0006-291x(68)90291-x. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Hill A. V. The Combinations of Haemoglobin with Oxygen and with Carbon Monoxide. I. Biochem J. 1913 Oct;7(5):471–480. doi: 10.1042/bj0070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keech B., Barritt G. J. Allosteric activation of sheep kidney pyruvate carboxylase by the magnesium ion (Mg2+) and the magnesium adenosine triphosphate ion (MgATP2-). J Biol Chem. 1967 May 10;242(9):1983–1987. [PubMed] [Google Scholar]
- Kosow D. P., Rose I. A. Activators of yeast hexokinase. J Biol Chem. 1971 Apr 25;246(8):2618–2625. [PubMed] [Google Scholar]
- Ning J., Purich D. L., Fromm H. J. Studies on the kinetic mechanism and allosteric nature of bovine brain hexokinase. J Biol Chem. 1969 Jul 25;244(14):3840–3846. [PubMed] [Google Scholar]
- O'SULLIVAN W. J., PERRIN D. D. THE STABILITY CONSTANTS OF METAL-ADENINE NUCLEOTIDE COMPLEXES. Biochemistry. 1964 Jan;3:18–26. doi: 10.1021/bi00889a005. [DOI] [PubMed] [Google Scholar]
- Peck E. J., Jr, Ray W. J., Jr Metal complexes of phosphoglucomutase in vivo. Alterations induced by insulin. J Biol Chem. 1971 Feb 25;246(4):1160–1167. [PubMed] [Google Scholar]
- Purich D. L., Fromm H. J. Studies on factors influencing enzyme responses to adenylate energy charge. J Biol Chem. 1972 Jan 10;247(1):249–255. [PubMed] [Google Scholar]
- Purich D. L., Fromm H. J. The kinetics and regulation of rat brain hexokinase. J Biol Chem. 1971 Jun 10;246(11):3456–3463. [PubMed] [Google Scholar]
- Rose I. A. The state of magnesium in cells as estimated from the adenylate kinase equilibrium. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1079–1086. doi: 10.1073/pnas.61.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph F. B., Fromm H. J. Initial rate studies of adenylosuccinate synthetase with product and competitive inhibitors. J Biol Chem. 1969 Jul 25;244(14):3832–3839. [PubMed] [Google Scholar]
- Uyeda K. Studies on the reaction mechanism of skeletal muscle phosphofructokinase. J Biol Chem. 1970 May 10;245(9):2268–2275. [PubMed] [Google Scholar]


