Abstract
Major depressive disorder is a prevalent mental disorder, yet its pathogenesis remains poorly understood. Accumulating evidence implicates dysregulated immune mechanisms as key contributors to depressive disorders. This review elucidates the complex interplay between peripheral and central immune components underlying depressive disorder pathology. Peripherally, systemic inflammation, gut immune dysregulation, and immune dysfunction in organs including gut, liver, spleen and adipose tissue influence brain function through neural and molecular pathways. Within the central nervous system, aberrant microglial and astrocytes activation, cytokine imbalances, and compromised blood-brain barrier integrity propagate neuroinflammation, disrupting neurotransmission, impairing neuroplasticity, and promoting neuronal injury. The crosstalk between peripheral and central immunity creates a vicious cycle exacerbating depressive neuropathology. Unraveling these multifaceted immune-mediated mechanisms provides insights into major depressive disorder’s pathogenic basis and potential biomarkers and targets. Modulating both peripheral and central immune responses represent a promising multidimensional therapeutic strategy.
Keywords: Major depressive disorder, Neuroimmune interactions, Neuroinflammation, Peripheral-central crosstalk
Introduction
Major depressive disorder(MDD) is a prevalent mental disorder characterized by symptoms including low mood, diminished interest, slowed thinking, poor appetite and sleepless. According to the World Health Organization (WHO), more than 280 million people worldwide currently suffer from MDD, accounting for 3.8% [1] of the global population. MDD is marked by its limited recovery rate and high suicide rate, and projected to become the leading cause of global disease burden by 2030. The pathogenesis of MDD remains unclear, and specific experimental diagnostic markers are not yet identified. Current clinical treatments for MDD primarily focus on medication-based approaches and psychotherapy. Medication typically includes SSRIs, SNRIs, MAOIs, TCAs and anesthetics like ketamine. However, antidepressants face challenges such as slow onset of action, wide individual variation, and inconsistent long-term efficacy. Thus, unraveling the pathogenesis is essential for advancing the diagnosis and treatment of MDD.
Inflammation plays an important role in the pathophysiology of MDD. Patients with chronic inflammatory diseases have an increased risk of MDD [2], which is associated with immune dysfunction and the accumulation of inflammatory factors [3]. Immune cells and associated cytokines interact with the brain through neurological, endocrine, and immune pathways, affecting mood, cognition, and behavior. Patients with MDD also exhibit abnormalities in immune activity [4]. Both peripheral and central immune mechanisms contribute to the onset and progression of MDD through a complex interplay of neuroinflammatory processes. Peripherally, systemic inflammation with elevated cytokines, gut immune dysregulation and dysbiosis, immune dysfunction in liver, spleen and adipose tissue generate inflammatory signals impacting the brain. Neural connections also facilitate bidirectional propagation of peripheral and central inflammation [5–8]. Centrally, the infiltration of peripheral inflammatory mediators, aberrant microglial and astrocytic activation, imbalance of pro/anti-inflammatory cytokines, and subsequent neuroinflammation disrupts neurotransmission, impairs neuroplasticity, and causes neuropathological changes inside the brain [4, 9, 10]. This multifaceted interaction between peripheral immune dysregulation and central neuroinflammation forms the immunological basis of MDD pathogenesis.
In this review, we explore the intricate interactions between peripheral and central immune mechanisms in the pathogenesis of MDD. By examining the role of neuroinflammation and immune dysregulation, we aim to provide a comprehensive understanding of how these processes contribute to depressive symptoms. This exploration highlights potential biomarkers and therapeutic targets, paving the way for more effective, multidimensional treatment strategies for MDD.
Peripheral immune and interactions between peripheral and central immune mechanisms
Systemic inflammation
Numerous factors are intricately associated with the pathogenetic mechanism of MDD. Among these, the systemic inflammatory response elicited by chronic stress, infection, and stress - inducing events occupies a pivotal position [11, 12]. Systemic inflammatory cytokines, mainly including TNF-α, IL-1β, and IL-1 serving as significant indices reflecting the systemic inflammatory status, furnish objective and indispensable criteria for the assessment of the degree of immune activation in patients with MDD [13].
The release of TNF-α into the bloodstream triggers the activation the immune system and sevretion of other cytokines, such as IL-6, IL-1β, and IL-8, this process exhibits an inherent congruence with relevant reactions within the central nervous system [14]. TNF-α enhances IDO activity, shunting tryptophan metabolism towards kynurenine (KYN) pathway, reducing serotonin synthesis [15] and affecting mood and cognitive function [16]. Concurrently, KYN and its derivatives can activate microglia, thereby triggering a neuroinflammatory response [17]. Moreover, peripheral KYN pathway markers can extend to the cerebrospinal fluid, directly affecting MDD at the central level. Clinically, peripheral TNF-α induces depressive-like symptoms and attenuates brain reward function [18, 19], while anti-TNF-α drugs show efficacy in treating refractory MDD [20].
Under the stimulation of chronic stress, monocytes and macrophages are activated, subsequently releasing IL-1, IL-1β and IL-6, which act in concert to induce a systemic immune response [10, 21]. IL-1 promotes the activation of the hypothalamic- pituitary-adrenal (HPA) axis [22] thus precipitate in the onset of MDD. Additionally, IL-1β can stimulate CD4 + T cells to differentiate into Th17 cells [23], leading to an imbalance in the Th17/Treg ratio and modifying the peripheral immune microenvironment [24]. In this state, peripheral inflammatory factors permeate the BBB, interferere with neurotransmitter metabolism and neural signal transmission, ultimately resulting in depressive and anxiety-like behaviors in chronic unpredictable mild stress (CUMS) mouse model [25]. Clinical investigations have also revealed a significantly elevated serum IL-6 level in patients with MDD [26–29].
Conversely, Treg differentiation factor IL-2 was decreased in MDD patients [30]. Low-dose IL-2 mitigated depressive and anxiety behaviors in CUMS mice by rebalancing hippocampal Th17/Treg ratio, modulating Foxp3+/RORα and IL-6/TGF-β, and suppressing M1 microglial and A1 astrocytic activation [31]. A phase II clinical randomized controlled study has demonstrated that IL-2 enhances the response to antidepressant treatment [32].
Stress - related events can trigger an acute stress response, resulting in a transient activation of the peripheral immune system. The level of C - reactive protein (CRP) is closely correlated with the intensity of the inflammatory response within the body, and an elevation in its level implies an enhanced inflammatory condition. Multiple studies have corroborated that the CRP level is elevated in depressive patients [33–35]. However, no significant disparity in the CRP level has been observed between patients with first-episode major depressive disorder (FEMD) and those with recurrent major depressive disorder (RMD) [36, 37].
The interaction between central and peripheral immune mechanisms is facilitated through both cytokine signaling and neural pathways. Systemic inflammatory cytokines can increase the permeability of the blood-brain barrier, allowing inflammatory mediators to enter the brain, acting on brain immune cells to trigger a neuroinflammatory response. These cytokines also diffuse along nerve fibers to areas in prefrontal cortex, hippocampus, and amygdala, act directly on neurons, initiating NF-κB and JAK-STAT signal transduction pathways [38, 39]. This affects neurotransmitter production, neuroplasticity, and neuronal survival [40].
Gut immune and gut-brain axis
The intestine is not only essential for digestion and nutrient absorption but also represents the largest compartment of the immune system. With a vast number of immune cells and microbial communities, the gut interacts through neural, endocrine, and immune pathways with central nervous system and brain function, known as the gut-brain axis. Gut immunity and gut-brain axis are emerging as a critical component in the development and progression of MDD [41].
Immune responses in the gut elevate the levels of pro-inflammatory cytokines such as IL-6, TNF-α, and IL-1β which trigger systemic inflammation [42], alter brain function by affecting neurotransmitter systems, neuroplasticity, and the integrity of the blood-brain barrier and consequently induce MDD [43]. The activation of intestine immune system also increases gut permeability, allowing endotoxins like LPS to enter the bloodstream and promote neuroinflammation, further exacerbating depressive symptoms [44].
In addition, gut microbiota plays a potential regulatory role in MDD. Studies have indicated patients with MDD are disturbed by both the composition and amount of gut microbiome [45, 46]. Gut microbiota also participates in the development of MDD through interactions with immune cells. A mouse model study reveals Lactobacillus species involved in colonic IL-17-producing γδ T cells differentiation and contributes to MDD by their meningeal accumulation [47].
Moreover, metabolites and neuroactive substances produced by gut microbiota are involved in the pathophysiology of MDD by regulating immune responses and neural functions. Short-chain fatty acids (SCFAs) modulate central activity through fatty acid receptor 2 (FFA2) and free fatty acid receptor 3 (FFA3) [48]. SCFAs can directly target microglia without passing through receptors and exert inhibitory effects on histone deacetylase (HDAC) activity, nuclear factor-kappa B (NF-κB) activity, and lipopolysaccharide (LPS)-induced neuroinflammation [49]. Moreover, some SCFAs can cross the blood-brain barrier (BBB) by diffusion [50, 51] or via monocarboxylate transporter proteins located on endothelial cells and inhibit inflammatory responses [52]. Neuroactive substances produced by gut microbiota, such as serotonin (5-HT) and gamma-aminobutyric acid (GABA), are also involved in the regulation of mood and behavior. In depressive states, the gut microbiota induce alterations in 5-HT [53–55]and GABA [56, 57] and activate the vagus nerve (VN), passing inflammation signal to the brainstem and hippocampus, exacerbating depressive conditions [58]. Additionally, gut hormones also regulate physiological functions and cognitive processes in the brain. GLP-1 secreted by intestinal enteroendocrine L-cells bind with receptors distributed in the hippocampus and hypothalamus, participating in learning, memory, and emotional regulation [59]. Research demonstrate GLP-1 receptor agonists can alleviate depressive symptoms by inhibiting the release of pro-inflammatory cytokines and reducing neuroinflammation, suggesting potential applications of gut hormones in the treatment of MDD [60]. Studies indicate that cholecystokinin(CCK) secreted by secretory cells in the mucosal lining of the small intestine play a role in synaptic plasticity changes related to learning and memory [61]. In CSDS-induced depressive mouse models, excitatory synaptic transmission in the CCKBLA-D2NAc glutamate aversion circuit is significantly enhanced, inhibition of this circuit can effectively overcome depressive symptoms [62]. These molecules mediate bidirectional communication between the gut and the brain, revealing key links in the pathogenesis of MDD.
Pathological changes in MDD in turn contribute to dysbiosis by altering the gut environment [63, 64]. Empirical evidence from clinical studies reveals that individuals afflicted with MDD demonstrate heightened levels of Bacteroidaceae, Desulfovibrionaceae, and Enterococcaceae, in contrast to individuals with robust health [65]. This imbalance in microbial composition facilitates the overproduction of pro-inflammatory cytokines, including TNF-α [66, 67], thereby intensifying the inflammatory response within the gut.
Liver immune
The liver is a multifunctional organ, performing critical physiological functions such as immunity, metabolism, and detoxification. Changes in its structure and function can impact brain activity [68, 69]. Studies have found that mood disorders are prevalent among patients with chronic liver disease [70], suggesting that alterations in liver structure and function may trigger or exacerbate MDD.
As liver function declines, neurotoxic compounds accumulate in the body, leading to elevated blood ammonia levels. Once ammonia enters the brain through the bloodstream, it is taken up by astrocytes and metabolized into glutamine, which activates NMDA receptors. This activation results in intracellular calcium overload, affecting synaptic function and consequently, brain function [71, 72]. Elevated levels of ammonia and glutamate also promote microglial activation, leading to the release of inflammatory cytokines and the induction of neuroinflammation, ultimately contributing to the onset of MDD [73].
In pathological states, the number of immune cells in the liver and their production of inflammatory cytokines increase. These cytokines can enter the brain via neuroendocrine pathways or the bloodstream [42], altering the permeability of the BBB and inducing neuroinflammation, which affects neurotransmission and neuronal function, thereby triggering or worsening depressive symptoms [74].
Beyond directly impacting brain function, the liver can influence the brain through the gut. The liver, gut, and brain form a communication network known as the gut-liver-brain axis [75]. The liver can alter the composition and quantity of gut microbiota and their metabolites, which, through the VN or activation of the hypothalamic-pituitary-adrenal (HPA) axis, can modify neural signals in various brain regions. These changes affect reward, motivation, mood, and stress responses [76, 77], thereby promoting the development of MDD.
Traumatic brain injury induces alterations in cerebral lipids, with lysophosphatidyl choline (LPC) and cholesterol esters (CE) being the most impacted lipid families in the hippocampus. Concurrently, it perturbs lipid metabolism and inflammatory markers in the liver, exacerbating hepatic inflammation [78]. Clinical studies have revealed fluctuations in leptin levels among depressed patients [79]. Leptin mitigates hepatic lipid levels by augmenting the secretion of triglycerides and diminishing lipogenesis [80], thereby alleviating inflammation triggered by lipid overload and lipotoxicity [81].
Spleen immune
The spleen, the largest lymphatic organ in the human body, performs crucial functions in immunity, hematopoiesis, and inflammation. With the physical function of filtering pathogens and abnormal cells from the blood, spleen facilitate interactions between antigen-presenting cells (APCs) and homologous lymphocytes [82]. Zhang X et al. have demonstrated a direct connection between CRH neurons and the splenic nerve, which regulates adaptive immunity, providing further evidence of a direct link between MDD and immune organs [83].
Further studies reveal that imbalance in regulatory B cells and plasma cells cause increased psychosocial stress. Splenic-derived B cells may influence behavior by modulating meningeal myeloid cell activation and meningeal interferon responses leading to MDD [84]. Additionally, a clinical study showed that high levels of CD8 + T cells can induce MDD [85]. Repeated stimulation of neurons expressing D1 receptors in the nucleus accumbens of tumor-implanted mice significantly increases the number of CD8 + T cells in the spleen [86]. Therefore, inhibiting the exhaustion of splenic CD8 + T cells and improving immune system function may become novel therapeutic targets for treating MDD.
Research indicates that corticosterone (CORT)-induced depressive-like mice exhibited impaired splenic function, immunity, and differential expression of genes and brain/splenic proteins, suggesting an interplay between splenic immune dysregulation and MDD pathogenesis [87]. As was shown in mouse model, stimulation of the ventral tegmental area of the brain, part of the brain’s reward circuitry, boosted splenic activity and consequently enhanced the adaptive immune response [88]. Moreover, under chronic stress, there is an escalation in the secretion of catecholamines [89], which, upon interaction with α and β adrenergic receptors in the spleen, can precipitate a reduction in splenic volume and induce immunosuppression [90, 91].
Adipose tissue immune
In addition to the liver, gut, and spleen, adipose tissue also plays a role in the development of MDD by regulating brain function. Adipocytes secrete cytokines such as TNF-α, IL-6, and chemokine MCP-1, leading to the accumulation of inflammatory cells in adipose tissue and creating a chronic inflammatory environment [92]. Studies have shown that individuals with obesity have higher levels of free fatty acids and immune cells compared to healthy individuals. These substances cause abnormal proliferation of hypothalamic microglia, subsequently affecting the functions of the hippocampus, amygdala, and reward centers [93], regions whose dysfunction is closely associated with the onset of MDD. Furthermore, dietary studies indicate that a high-fat diet can affect synaptic plasticity, insulin signaling, and corticosterone levels, thereby induce MDD [94].
Neural networks
Recently, emerging evidence suggested that neural networks connecting the brain with key peripheral immune organs influence both immunological responses and depressive behaviors [95], mainly through vagus nerve (VN) and sympathetic nervous system [96, 97].
Bone marrow receives dense sympathetic innervation [98] from PVN, medulla and VLM, these fibers suppress expression of CXCL1233 in the bone marrow, inhibits hematopoiesis, retains neutrophils and monocytes [99]. Neurons from NTS, DMV, and PVN, innervate the spleen. These neural connections influence splenic macrophages and T cells, modulating cytokine production and systemic inflammation. Restraint stress activates the cholinergic pathway via the sympathetic system, triggering anti-inflammatory programs in splenic cells [100, 101]. Neural circuits connecting the brain to the liver involve both sympathetic and parasympathetic pathways [102]. Hypothalamic regions, such as the PVN, and brainstem nuclei innervate the liver [103], regulating hepatic glucose production, lipid metabolism, and immune responses [104]. Stress-induced activation of these pathways can lead to hepatic inflammation, macrophage infiltration and metabolic syndrome, conditions frequently associated with MDD [105, 106]. Neurons in the arcuate nucleus (ARC) and PVN project to adipose tissue, influencing lipolysis, glucose metabolism, and immune cell activity within the adipose tissue, through sympathetic and parasympathetic pathways. Dysregulation of these pathways promoting inflammation and metabolic disturbances that are often comorbid with MDD.
The vagus nerve is the primary neural conduit linking the gut to the brain. Alterations in vagal tone can affect gut permeability and microbiota composition, leading to immune activation and production of pro-inflammatory cytokines [107]. Within the gut, the neural signals relayed by the brain are received by the enteric nervous system, consisting of approximately 2 to 6 million neurons, glia and extrinsic ganglia [108]. ENS system play a crucial role in maintaining gut homeostasis and preventing excessive immune activation [109], and contribute significantly to the progression of MDD by mediating the exacerbation of intestinal inflammation induced by chronic stress [110].
Vagus nerve stimulation (VNS) has been explored as a therapeutic intervention for MDD, highlighting its potential to modulate immune responses and improve mood. VNS can activate α7 nicotinic acetylcholine receptors, reducing TNF-α production induced by LPS in microglia, restoring balance in the immune system, thus providing neuroprotection [111]. This therapeutic approach underscores the importance of the vagus nerve in the neural regulation of immune functions and its potential in treating MDD.
In conclusion, neural networks linking the CNS to peripheral immune organs are integral to the bidirectional communication between the brain and the immune system. Neural signals potentially precipitate in MDD by increasing circulating immune cell numbers, altering immune cell chemotaxis, or making them more responsive to systemic inflammatory signals.
HPA axis
Stress significantly impacts the immune response and the pathological process of MDD through the HPA axis. The HPA axis regulates physiological functions by affecting hormone production and the activity and distribution of immune cells [112].
Patients with MDD typically exhibit increased secretion of adrenocorticotropic hormone (ACTH), which stimulates the adrenal glands to produce more glucocorticoids (GCs). Under chronic stress, elevated blood GC levels and reduced GR expression impair the normal feedback regulation of GRs. This results in persistent hyperactivity of the HPA axis, which maintains high GC levels [113] and upregulates inflammatory pathways, triggering an inflammatory response in hippocampal cells [114]. It also activates microglia [115], leading to neuroinflammation and persistent depressive symptoms. Studies have shown that using GR antagonists, such as mifepristone, can modulate cortisol and ACTH levels and improve clinical manifestations in depressed patients [116, 117].
Both chronic stress stimuli and peripheral inflammatory states are capable of leading to sustained activation of the hypothalamic-pituitary-adrenal axis (HPA axis), resulting in increased secretion of stress hormones such as cortisol. Cortisol directly promotes the release of pro-inflammatory cytokines and inhibits the synthesis and release of serotonin, dopamine, norepinephrine and anti-inflammatory cytokine IL-10. This imbalance of cytokines and changes in neurotransmitters further promote the spread of inflammatory responses throughout the body, leading to depressed mood, anxiety, and sleep disturbances. At the same time, cortisol can affect the balance of neurotransmitters and neuronal plasticity in the prefrontal cortex, hippocampus, and amygdala [118].
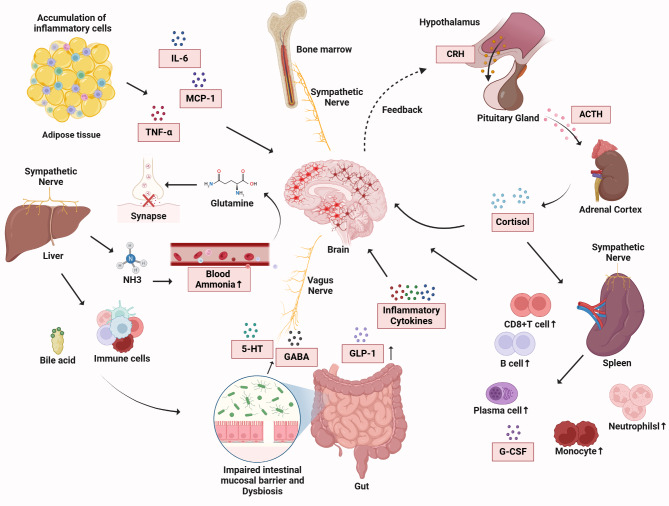
In conclusion, peripheral inflammatory mediators are capable of traversing the blood-brain barrier and entering the brain, where they activate microglia and astrocytes, eliciting a neuroinflammatory response. Functional alterations in immune organs, including the gut, liver, spleen, and adipose tissue, can also influence brain activity, leading to aberrations in neurotransmitter metabolism and neuronal damage. Neural networks, including the vagus nerve and sympathetic nervous system, establish connections between peripheral immune organs and the brain, modulating immune responses and depressive behaviors. Furthermore, stress can impact the hypothalamic-pituitary-adrenal (HPA) axis, altering hormone levels and cytokine balance, ultimately triggering a systemic inflammatory reaction that manifests as depressive symptoms. These mechanisms are illustrated in Fig. 1.
Fig. 1.
Peripheral immune and interactions between peripheral and central immune mechanisms
The figure shows the complex interaction between peripheral and central immune mechanisms during inflammation. Inflammatory cells in adipose tissue secrete cytokines (IL − 6, MCP − 1, TNF - α), activating sympathetic nerves, the HPA axis, and bone marrow, causing the release of hormones (CRH, ACTH, cortisol). Inflammatory cytokines increase brain glutamine, activate the vagus nerve, and elevated blood ammonia from liver metabolism damages the blood - brain barrier. Also, cytokines, bile acid, and immune cells disrupt the intestinal barrier, increasing bacterial translocation and the inflammatory response. This activates immune cells (B cells, plasma cells, neutrophils, monocytes) and cause the releasing of inflammatory mediators (cytokines, GABA, GLP − 1, G - CSF). The figure emphasizes the complex interaction involving various systems, cytokines, hormones, and neurotransmitters in inflammatory conditions
Central immune mechanisms in MDD
As discussed, peripheral immune activation affect the central nervous system through various pathways, precipitating and exacerbating depressive symptoms. Moreover, once peripheral inflammatory molecules gain access to the brain, they inevitably activate the resident immune cells of the central nervous system, initiating a cascade of neuroinflammatory responses. Central immune mechanisms centered around the blood-brain barrier breach, microglial and astrocytic activation, and propagation of inflammatory signaling pathways leads to neuronal injury, neurotransmitter dysregulation, and functional impairment of brain regions, ultimately manifesting in the clinical symptoms of MDD.
Blood-brain barrier (BBB) integrity
The blood-brain barrier (BBB) is a highly selective, semi-permeable barrier that separates the peripheral circulatory system from the brain and the central nervous system (CNS). It restricts the entry of non-specific immune cells and harmful substances into the brain, protecting the nervous system from external threats. However, when the organism is attacked by inflammation, the permeability and integrity of the BBB is compromised [119], allowing inflammatory factors to enter, exacerbating the neuroinflammatory response and participating in the onset of various neurological diseases including Alzheimer’s disease, multiple sclerosis, Parkinson’s disease and MDD [6, 120–122].
In patients with MDD, the HPA axis got activated, leading to release of glucocorticoids [123]. Glucocorticoids bind to glucocorticoid receptors (GR) and mineralocorticoid receptors (MR) located in the prefrontal cortex and limbic system [124], degrading tight junction proteins [125], enabling the passage of immune cells and inflammation mediators through the BBB, which affect brain function and cause depressive symptoms.
Moreover, under chronic stress, altered neurotrophic factor expression dysregulates tight junction proteins like Cldn5, compromising BBB structural integrity [126, 127]. Inflammatory stimuli like LPS also activate brain endothelial caspase-4/11-GSDMD signaling, leading to inflammatory BBB damage [128].
Studies in chronic restraint stress (CRS) and CSDS mouse models have have demonstrated BBB disruption as a key factor contributing to depressive-like behaviors [129–131]. The excessive release of peripheral pro-inflammatory factors directly acts on endothelial cells, leading to cytoskeletal remodeling and a decrease in the stability of tight junctions, resulting in the increased of BBB leakage [8, 132]. Under chronic stress, the neurotrophic factor GDNF alters the expression of the blood-brain barrier tight junction-associated protein Cldn5. The inflammatory environment impairs BBB structural integrity, promoting BBB hyperpermeability [133]. Additionally, under lipopolysaccharide (LPS) stimulation, the caspase-4/11-GSDMD signaling pathway in brain endothelium is activated, leading to inflammatory BBB damage [128].
Leakage of the BBB allows cytokines and chemokines to enter the brain [134] and interact with various receptors. They directly damage neurons and exacerbate depressive symptoms by decreasing serotonin, dopamine, and norepinephrine levels [135, 136]. Inflammatory mediators entering the brain through the damaged BBB also reduce synaptic plasticity in neurons and impede neurogenesis, affecting mood regulation [137]. Moreover, cytokines and chemokines further activate microglia and astrocytes, creating a feedback loop. Overactivation of microglia releases cytokines such as IL-1β, causing oxidative stress, apoptosis and impairment of neuronal function, all of which are crucial pathological bases for MDD [138]. The entry of IL-1β and TNF-α into the brain via the BBB, induces the production of CXCL1 and Ccl2 from astrocytes, which attracts immune cells to infiltrate brain tissue and damage neurons [139]. Immune cells also enter the brain by upregulating adhesion molecules and integrins, adhering to the vessel wall [140]. T cells and macrophages, enter the brain parenchyma, via the BBB, causing or exacerbating neurological damage and depressive symptoms.
Cytokine and neurotrophic factors
Within the central nervous system, cytokines contribute to neuroinflammation and disruption of neuronal functions underlying mood regulation in MDD [141, 142]. The balance between pro-inflammatory and anti-inflammatory cytokines plays crucial role in the pathogenesis of MDD. Once this balance is disrupted, a vicious cycle emerges wherein an enhanced inflammatory response further triggers depressive symptoms. These symptoms, in turn, may exacerbate the inflammatory response, creating a complex and prolonged pathological process [140].
Pro-inflammatory cytokine TNF-α directly acts on neurons in the brain, inducing oxidative stress and neurotoxicity, activating astrocytes, caus hippocampal atrophy [143–145]. Inhibition of the TNF-α/TNFR1/NF-κB signaling pathway reduces microglial overactivation and neuroinflammation, preventing the worsening of depressive symptoms [146]. Additionally, TNF-α also enhance neuronal excitability by modulating glutamate receptor function and promoting the transcription of Nav1.3 and Nav1.8 [147]. Administration of TNF-α inhibitors infliximab, reduces cortical neuronal activity and ameliorates depressive-like behavior in adults with bipolar depression reporting physical and/or sexual abuse [20, 148].
Clinical studies have shown patients with MDD experience elevated IL-1β concentrations in cerebrospinal fluid and increased hippocampal inflammation [149]. Unlike TNF-α, IL-1β inhibits neuronal excitability by modulating glutamate receptors and decreasing ionic fluxes through voltage-gated sodium channel(Nav), voltage-gated calcium channel(Cav), and voltage-gated potassium channel(Kv) channels [150], leading to depressive-like responses.
IL-6 inhibits excitatory neurotransmission and basal neural activity by promoting the upregulation of the adenosine A1 receptor and modulation of the Cav channel [151, 152]. In the cerebrospinal fluid, IL-6 activates the MAPK and IDO pathways, reducing monoamine availability and directly affecting synaptic neurotransmission. It also indirectly affects intracellular pathways through its G-protein-coupled receptor, negatively regulating neurotransmitter release and impacting neuronal activity and plasticity [153].
IFNγ exhibits presynaptic actions that increase the release of the inhibitory neurotransmitter GABA via nitric oxide [154], leading to learning and memory impairments and social behavioral deficits [155]. Blockade of the IFNγ receptor or specific deletion of the IFN-I receptor in microglial cells (IFNAR) reduces synaptic loss, reactive microglial cell proliferation, and learning deficits [9].
Interleukin-4 (IL-4), Interleukin-8 (IL-8), interleukin-10 (IL-10), and transforming growth factor-β (TGF-β) play protective roles in the development of MDD [156]. IL-4 regulating hippocampal neurogenesis and resilience to MDD through Arg1 + microglia [157]. Defects in IL-4Rα cause reduced neuronal inhibitory synaptic function and vesicles, increase cortical excitability [158], alleviating depressive symptoms, anxiety, and sleep disturbances [159]. In patients with MDD, IL-10 levels are reduced [160]. Exogenous IL-10 restores the density of dendritic spines in microglia in the hippocampus and alleviate depressive symptoms [161, 162]. Higher levels of IL-8 attenuate depressive responds caused by endotoxin response [163–166]. Lack of these anti-inflammatory cytokines may exacerbate neuroinflammation and mood dysregulation.
Neurotrophic factors are essential for the survival, maintenance, and regeneration of specific neuronal populations in the mature brain, and they significantly contribute to the development of MDD. Brain-derived neurotrophic factor (BDNF) levels are decreased in patients with MDD [167], particularly in the hippocampus [168]. This reduction leads to diminished synaptic plasticity, atrophy of dopaminergic neurons, and atrophy of the hippocampal and prefrontal cortex, primarily affecting brain homeostasis [169]. In vitro studies indicate that the BDNF-TrkB pathway is crucial for forming functional neural networks; blocking TrkB receptors impairs calcium activity, alters synaptic structure, and disrupts mitochondrial function, thus affecting neural network development [170]. In mouse model, knockdown of astrocytic glutamate transporters GLAST/GLT-1 with siRNA in the infralimbic cortex induced behavioral abnormalities characteristic of a depressive-like phenotype, accompanied by reduced serotonin release in the dorsal raphe nucleus and decreased BDNF expression [171]. Conversely, increased BDNF levels support neuronal survival and differentiation, improving the structure and function of multiple brain regions and influence MDD progression [172, 173]. Research on ketamine as a novel antidepressant suggests that BDNF release in the medial prefrontal cortex [174] activates the TrkB signaling pathway, promoting neurogenesis [175–177]. Blocking postsynaptic N-methyl-D-aspartate (NMDA) receptors can rapidly enhance BDNF protein translation [178, 179], coordinating CREB/mTOR activation and leading to synaptic potentiation [180].
Glial cell line-derived neurotrophic factor (GDNF) is also vital for neural function and signal transduction, supporting dopaminergic neuron survival in the substantia nigra and exhibiting protective effects [181]. Studies on conditional Gfra1 knockout mice demonstrate that GDNF and its receptor Gfra1 mediate dendritic growth and spine formation in hippocampal neurons via neural cell adhesion molecule signaling [182, 183]. Another study showed that administering exogenous GDNF increases dopamine receptor D1 protein levels, activating the protein kinase A pathway and producing a prolonged antidepressant response [184]. The rhesus monkey model confirmed that GDNF is widely expressed in hippocampal dendrites, associated with neuronal survival, nerve regeneration, and functional recovery [185]. In addition, clinical studies reveal an association between reduced gray matter volume in the basal frontal lobe in patients with MDD and GDNF [186]. Other neurotrophic factors, such as midbrain astrocyte-derived neurotrophic factor (MANF), nerve growth factor (NGF), neurotrophin-3 (NT-3) and neurotrophin-4 (NT-4), also play protective roles in hippocampal neuronal survival and growth, with decreases hindering the alleviation of depressive symptoms [187–190]. Endothelial growth factor (VEGF), in addition to its angiogenic role [191], possesses neurotrophic and neuroprotective potential [192, 193]. It can stimulate the genesis of hippocampal neurons and safeguard neurons associated with stress from damage [194, 195]. Erythropoietin (EPO) can modulate the JAK2/STAT5 signaling pathway, thereby exerting anti-inflammatory effects and preventing neuroinflammation [196].
Central nervous system inflammation
Compromised blood-brain barrier integrity, along with dysregulated cytokine and neurotrophic factor signaling, predisposes the central nervous system to an inflammatory state. Inflammation activates microglia and astrocytes, causes neuronal damage and apoptosis, impairs functional connectivity and significantly alters the structure and function of critical brain regions (including the hippocampus, frontal lobe, amygdala and striatum) [138, 197, 198].
The hippocampus is predominantly associated with learning, memory, and emotional regulation [199]. Hippocampal volume reduction represents a characteristic alteration in MDD. Reduced hippocampal gray matter volume has been closely linked to memory and emotional disturbances in MDD patients. MDD-related neuroinflammation has also been reported to affect cognitive and memory functions by affecting neurogenesis in the dentate gyrus (DG), leading to inattention, impaired working memory, and increased negative cognitive bias [200]. In addition, reduced serotonin levels lead to neuronal degeneration, swelling, and significant neuronal loss in the hippocampal CA1, CA3, and DG regions, further exacerbates the negative emotional state [201]. Effective antidepressant treatment and electroconvulsive therapy (ECT) has been shown to reverse structural changes in the hippocampus, increase hippocampal volume, and improve depressive symptoms [202, 203].
The amygdala, together with the hippocampus, constitutes key components of the cortico-limbic system, which is essential for emotion processing and regulation [204]. In MDD, amygdala dysfunction is characterized by hyper-reactivity, particularly in response to negative stimuli and decreased functional connectivity between the amygdala and other brain region [205]. Notably, inflammation within the central nervous system (CNS) has been shown to reduce functional connectivity between the amygdala and the ventromedial prefrontal cortex (vmPFC), a disruption that correlates with the severity of anxiety symptoms in patients with MDD [206]. Elevated levels of inflammatory biomarkers have also been associated with increased amygdala activation, which is linked to symptoms of anxious arousal [207]. Recent research demonstrates that peripheral inflammation and amygdala-specific neuroinflammation, as indicated by higher diffusion basis spectral imaging-based restricted fraction (DBSI-RF), are independently associated with depressive symptoms [208]. Clinical studies have shown that selective serotonin reuptake inhibitors (SSRIs) effectively reduce amygdala reactivity to negative stimuli [209].
Frontal lobe exhibits significant structural and functional abnormalities in patients with MDD, particularly in the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), and dorsolateral prefrontal cortex (DLPFC) [210]. These changes include reduced gray matter volume, cortical thinning, and decreased activity, which are associated with emotional dysregulation, lack of motivation, and cognitive impairments [211]. The ACC shows altered connectivity with the DLPFC and amygdala, acting as a bridge for attention and emotion regulation [212], while the OFC’s dysfunction reduce inhibition of negative stimuli [213]. Post-mortem brain tissue from Brodmann Area 10 exhibits upregulation of inflammatory cytokines, suggesting a link between local inflammation and pathological changes [214]. Antidepressants, ECT, and transcranial magnetic stimulation (TMS) can partially reverse these abnormalities, correlating with depressive symptom improvement.
The striatum contains the putamen, the caudate, and the ventral striatum. Decreased gray matter volume and activity in the ventral striatum are associated with impaired reward processing and suicidal behaviors in patients with MDD [215]. The putamen shows reduced volume in patients with MDD, its increased functional activity has been linked to emotional dyscontrol and heightened feelings of self-hatred [216]. The caudate nucleus, a key component of the brain’s reward system, also shows reduced volume and activity in MDD, correlating with disease severity and disrupted dopaminergic signaling [217]. Research in non-human primates demonstrated peripheral inflammatory induce decreases in striatal dopamine release, which occur in association with reduced effort-based sucrose consumption [218]. In patients with MDD, peripheral inflammation marked by elevated CRP and sICAM-1, is associated with reduced striatal activation during reward processing, particularly in response to intermediate reward magnitudes [219].
An increased density of microglia has been observed in the amygdala and prefrontal lobes of the brains of depressed patients [220]. Microglia differentiate into multiple phenotypes at different stages of CNS development, stress and disease [221], the polarization of the M1/M2 phenotype is associated with the development of MDD [222–224]. M1 type microglia secrete pro-inflammatory cytokines and neurotoxic mediators to exacerbate neuronal damage, while M2 type microglia promote a repairing anti-inflammatory response. Microglial polarization toward M1 phenotype disrupts neurogenesis in the hippocampus [225], inducing functional brain damage [226, 227]and exacerbating depressive symptoms [228]. Activated microglia release monocyte chemotactic protein-1 (MCP-1), which recruits monocytes to the brain. Subsequently, the brain secretes pro-inflammatory cytokines IL-1β and IL-6 [229, 230], disrupting hippocampal neurons and synaptic plasticity, thereby affecting brain function and inducing MDD [231, 232].
In MDD, the important effects of M1/M2 polarization dysregulation focus primarily on neurogenic inhibition. Although some degree of neurogenesis is also present in other psychiatric disorders like schizophrenia, the mechanism is more prominent in MDD. Unlike with MDD, M1/M2 polarization dysregulation in schizophrenia is mainly characterized by significant neuronal connectivity and neural circuitry abnormalities [233].
Microglia also acquire a neurotoxic phenotype through the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway, which produces reactive oxygen species, nitric oxide, proteases, and pro-inflammatory cytokines that accelerate neuronal damage [234]. This significantly affects the DGs and CA3 region of the hippocampus and leads to MDD. Inhibition of neural stem cell proliferation and differentiation in the DGs, a critical region for neurogenesis, affects pattern segregation in learning memory, and disruption of synaptic connectivity of neurons in the CA3 region, with a reduced density of dendritic spines, leads to cognitive dysfunction and emotion dysregulation [235, 236].
MDD and other psychiatric disorders, such as schizophrenia (SCZ), sahres pathological alterations in the brain, but the patterns and characteristics of these changes differ significantly. MDD is primarily associated with emotional dysregulation, neurocognitive impairments (such as attention and memory deficits), and alterations in reward processing, while schizophrenia cases experience both social cognitive deficits (difficulties in identifying emotions, feeling connected to others, inferring people’s thoughts and reacting emotionally to others [237, 238]) and neurocognitive impairments. For instances, hippocampal abnormalities are observed across both disorders, in MDD, the hippocampus shows reduced neurogenesis prominently in the DGs and CA3 [204]. In contrast, SCZ displays more diverse hippocampal abnormalities, including subfield-specific volume reductions (mainly in CA1 and CA2 regions), synaptic alterations, and disrupted connectivity with other brain regions [239]. Moreover, schizophrenia is associated with broader pathological abnormalities, extending beyond the hippocampus to include regions such as the prefrontal cortex, thalamus, posterior cingulate cortex, and medial temporal lobes [240–242]. These distinctions highlight the unique neurobiological profiles of MDD compared to other psychiatric disorders.
In conclusion, the central immune mechanisms in MDD reveal inflammation as a pivotal orchestrator in disease pathophysiology, driving complex interactions between BBB disruption, neuroinflammation, and neuronal dysfunction. BBB compromise allows peripheral inflammatory mediators to enter the brain, activating microglia and disrupting neural homeostasis. The balance of pro- and anti-inflammatory cytokines, along with neurotrophic factors, critically influences neuronal function, synaptic plasticity, and neurogenesis. This inflammatory cascade particularly affects key brain regions including the hippocampus, amygdala, frontal lobe, and striatum, which ultimately leads to the manifestation of characteristic clinical symptoms of MDD.
Therapeutic interventions targeting immune mechanisms
Pharmacological approaches
Regarding the immune mechanisms involved in the onset of MDD, drug strategies targeting the immune system have become a promising field of research. Clinical studies indicate that antidepressant drugs can reduce the levels of peripheral inflammatory factors and peripheral inflammatory responses [243].
A study by Casarotto et al.pointed out that all antidepressant, including tricyclic antidepressants (TCA), SSRI, monoamine oxidase inhibitors (MAOI), and ketamine are able to directly bind to TrkB and denature to increase BDNF signaling, directly linking the effects of antidepressants to neuronal plasticity, which has important implications for reducing neuroinflammation [244].
Non-steroidal anti-inflammatory drugs (NSAIDs) have shown antidepressant effects in several short-term clinical studies [245]. NSAIDs inhibit COX activity and reduce the synthesis of inflammatory mediators such as prostaglandins, thereby reducing chronic inflammation [246]. Celecoxib, a COX-2 selective inhibitor, protects neurons by inhibiting oxidative stress and in this way mediates its antidepressant effects [247].
Antibiotics also have potential antidepressant effects, with a notable decrease in Hamilton Depression Scale scores in patients with MDD who receive adjunctive treatment with minocycline [248]. Minocycline attenuates the effects of neuroinflammation on MDD by decreasing the expression of proinflammatory cytokines and reducing the release of neuroactive kynurenine metabolites [249].
Inhibitors targeting specific cytokines have also garnered attention. Monoclonal antibodies to TNF-α (e.g. infliximab), IL-6 receptor antagonists (e.g. tocilizumab), and antibodies to IL-4α receptors (e.g. dupilumab) have demonstrated direct and indirect ameliorative effects on depressive symptoms [250–253]. Additional clinical studies have noted positive effects on depressive symptoms with IL-12/23 antagonists [254, 255]. These cytokine antagonists inhibit inflammatory signaling by blocking the binding of cytokines to their receptors, reducing inflammation levels throughout the periphery and brain, and contributing to the recovery of neurological function. Furthermore, given IL-10’s anti-inflammatory role in MDD, reducing IDO expression through IL-10-dependent pathways promote the recovery of MDD [256].
Targeting microglia, particularly over the P2X7-NLRP3 axis, is currently a focus of drug development for neurological disorders, P2X7 activation promotes IL-6 release, stimulates free radical production, phospholipase activation, and apoptosis. In the adaptive immune response, P2X7 stimulation is involved in T-cell activation, and ATP-P2X7 signaling reduces the inhibitory activity and viability of regulatory T-cells (Treg cells) and favors T-cell polarization to T-helper cells (TH17 cells), disrupting the balance between the two and leading to MDD [257]. Brain-permeable P2X7 antagonists can reverse stress-induced depressive-like behaviors [258].
Research indicates that the efficacy of antidepressants varies by gender. Males generally respond better to TCAs, whereas females exhibit greater responsiveness to SSRIs [259]. This difference may result from estrogen’s ability to rapidly shorten the onset time of SSRIs by activating GPER receptors [260]. Additionally, estradiol (E2) enhances the mRNA levels of 5-HT2A and upregulates serotonin transporter expression, contributing to an antidepressant effect [261].
Studies also reveal that females are more sensitive to ketamine dosages, resulting in a more pronounced antidepressant response [262]. Estrogen increases BDNF mRNA and protein levels in the hippocampus, cerebral cortex, and spinal cord [263]. Combined with ovarian hormones’ role in facilitating BDNF signaling, BDNF may mediate the heightened sensitivity to ketamine in females. However, a study on Sprague-Dawley rats showed that ketamine was effective in adult males but ineffective in females [264]. The sexual dimorphism in antidepressant drugs and their mechanisms requires further exploration.
Hormones also significantly influence drug efficacy. Progesterone and estradiol are key estrogens that play vital roles in cellular proliferation, synaptic structure and function, and neuroprotection. They modulate neural activity through various receptors and impact neurotransmitter systems [265]. Additionally, gender differences in antidepressant response are linked to variations in CYP enzymes and the hypothalamic-pituitary-adrenal (HPA) axis [266, 267]. The exploration of gender differences provides critical insights into the use of antidepressants, underscoring the importance of considering patient gender in treatment planning.
Non-pharmacological approaches
Diets that modulate the immune response and inflammatory state are gaining attention in the treatment of MDD. Anti-inflammatory diets, such as the Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in omega-3 polyunsaturated fatty acids, antioxidants, and multivitamins, can reduce systemic levels of inflammation and indirectly ameliorate depressive symptoms [268, 269]. By modulating the intestinal microbiota, these diet can increase the production of anti-inflammatory short-chain fatty acids [270, 271]. Additionally, certain prebiotics can promote the growth of beneficial bacteria and maintain neurological stability [272].
Physical activity also provides an important non-pharmacological approach in the management of MDD. Exercise has been demonstrated to contribute to immune system maintenance and gut health, reducing the negative impact of inflammation on the brain by enhancing immune cell function, lowering cortisol levels [273], promoting neurotrophic factor production [274], and optimizing gut microbial diversity.
Psychological interventions serve as an important intervention method of MDD. Interpersonal Psychotherapy (IPT) has been shown to have a significant effect on improving social functioning and reducing depressive symptoms and anxiety [275]. And in children and adolescents with MDD, family therapy may be slightly more effective than individual psychotherapy [276]. Cognitive behavioral therapy (CBT), helps alleviate depressive symptoms by altering cognitive interpretations of stressful events, decreasing the stress response, and consequently reducing the release of inflammatory mediators. CBT also increases the expression of nerve growth factors, such as BDNF, and promotes neural repair and neuroplasticity [277, 278]. Moreover, CBT encourages physical activity, which complemented with exercise therapy, lowers inflammation levels and promotes mood recovery [279–282].
Combining pharmacological and non-pharmacological therapies provides a more comprehensive and personalized approach to MDD treatment. This integrative approach targets the immune system while adjusting the individual’s physiological and psychological state. Such approach considers the multidimensional effects of immunity, neurotransmitters, stress response, and lifestyle, potentially improving treatment efficacy, reduce side effects, and promote long-term recovery for patients.These therapeutic interventions are illustrated in Fig. 2.
Fig. 2.
Therapeutic interventions targeting immune mechanisms in MDD
The figure depicts various therapeutic approaches for MDD, including anti-inflammatory diets, exercise, psychological interventions, non-steroidal anti-inflammatory drugs, antibiotics, targeted therapies against cytokines and their receptors, strategies targeting microglia (P2X7-NLRP3 axis), and cell-derived biomimetic nanoparticles
Conclusion and future directions
The complex interplay between peripheral and central immune mechanisms has emerged as a fundamental pathway in the pathogenesis of major depressive disorder. This review has synthesized current evidence demonstrating how peripheral and central immune mechanisms converge to influence MDD pathogenesis. Multiple pathological processes, including BBB dysfunction, neuroinflammation, and cytokine dysregulation, contribute independently and synergistically to the development and progression of depressive symptoms. While these findings have significantly advanced our understanding, several critical challenges remain to be addressed.
The development of targeted immune-modulating therapies, particularly those addressing BBB dysfunction and neuroinflammation, represents an exciting therapeutic frontier. However, the current evidence for neuroinflammation in MDD, while compelling, has notable limitations. Most studies are correlational rather than causal, and animal models may not fully replicate human complexity. Longitudinal studies tracking inflammatory markers alongside clinical progression could better establish causation, and novel techniques like single-cell sequencing may help unravel cell-type specific contributions to neuroinflammation. By focusing on translational research with clinical applications, we can work toward more effective, personalized treatments for MDD based on immune mechanisms.
The current understanding of immune mechanisms in MDD has opened new avenues for therapeutic intervention and biomarker development, and the success of adoptive cell therapy (such as CAR-T cell therapy) in cancer treatment has inspired scientists to apply similar strategies to immune-related diseases, including MDD. However, there is still relatively little research in this area. Future research is still needed for developing more effective, personalized treatment strategies for this devastating disorder.
Author contributions
Jiao WL, Lin JY and Deng YF conceived the manuscript topic and structural design. Jing X, Ji YL and Jiao WL prepared the figures. Jiao WL, Lin JY and Deng YF drafted and finalised the manuscript with critical input and revisions from Yan FX, Liang CY, Wei SJ, and Jing X.
Funding
This work was supported by the National Natural Science Foundation of China (82204655, 82200616), Medical Research Foundation of Guangdong Province (A2022047), and the Traditional Chinese Medicine Bureau of Guangdong Provincial (20231082). The figures were created with Biorender.com.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenli Jiao, Jiayi Lin and Yanfang Deng contributed equally to this work.
Contributor Information
Xi Jing, Email: jingxi@jun.edu.cn.
Fengxia Yan, Email: fengxiayan@jnu.edu.cn.
References
- 1.Depressive disorder. (depression). [cited 2024 Jan 9]. https://www.who.int/news-room/fact-sheets/detail/depression
- 2.Gold SM, Köhler-Forsberg O, Moss-Morris R, Mehnert A, Miranda JJ, Bullinger M, et al. Comorbid depression in medical diseases. Nat Rev Dis Primers. 2020;6:69. [DOI] [PubMed] [Google Scholar]
- 3.Zhong X, Qiang Y, Wang L, Zhang Y, Li J, Feng J, et al. Peripheral immunity and risk of incident brain disorders: a prospective cohort study of 161,968 participants. Transl Psychiatry. 2023;13:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley ÉM, Parkinson JT, Mitchell RE, Turner L, Khandaker GM. Peripheral blood cellular immunophenotype in depression: a systematic review and meta-analysis. Mol Psychiatry. 2023;28:1004–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbull AV, Rivier C. Regulation of the HPA axis by cytokines. Brain Behav Immun. 1995;9:253–75. [DOI] [PubMed] [Google Scholar]
- 6.Huang X, Hussain B, Chang J. Peripheral inflammation and blood-brain barrier disruption: effects and mechanisms. CNS Neurosci Ther. 2021;27:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Koyama Y, Shimada S. Inflammation from Peripheral organs to the brain: how does systemic inflammation cause Neuroinflammation? Front Aging Neurosci. 2022;14:903455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agirman G, Yu KB, Hsiao EY. Signaling inflammation across the gut-brain axis. Science. 2021;374:1087–92. [DOI] [PubMed] [Google Scholar]
- 9.Garber C, Soung A, Vollmer LL, Kanmogne M, Last A, Brown J, et al. T cells promote microglia-mediated synaptic elimination and cognitive dysfunction during recovery from neuropathogenic flaviviruses. Nat Neurosci. 2019;22:1276–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Roub A, Al Madhoun A, Akhter N, Thomas R, Miranda L, Jacob T, et al. IL-1β and TNFα cooperativity in regulating IL-6 expression in Adipocytes depends on CREB binding and H3K14 acetylation. Cells. 2021;10:3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qing H, Desrouleaux R, Israni-Winger K, Mineur YS, Fogelman N, Zhang C, et al. Origin and function of stress-Induced IL-6 in murine models. Cell. 2020;182:372–e38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biltz RG, Sawicki CM, Sheridan JF, Godbout JP. The neuroimmunology of social-stress-induced sensitization. Nat Immunol. 2022;23:1527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui L, Li S, Wang S, Wu X, Liu Y, Yu W, et al. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Signal Transduct Target Ther. 2024;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan N, Luo Y, Ou Y, He H. Altered serum levels of TNF-α, IL-6, and IL-18 in depressive disorder patients. Hum Psychopharmacol. 2017;32. [DOI] [PubMed]
- 15.Xue C, Li G, Zheng Q, Gu X, Shi Q, Su Y, et al. Tryptophan metabolism in health and disease. Cell Metabol. 2023;35:1304–26. [DOI] [PubMed] [Google Scholar]
- 16.Wan J, Peng W, Li X, Qian T, Song K, Zeng J, et al. A genetically encoded sensor for measuring serotonin dynamics. Nat Neurosci. 2021;24:746–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:702–21. [DOI] [PubMed] [Google Scholar]
- 18.van Heesch F, Prins J, Korte-Bouws GAH, Westphal KGC, Lemstra S, Olivier B, et al. Systemic tumor necrosis factor-alpha decreases brain stimulation reward and increases metabolites of serotonin and dopamine in the nucleus accumbens of mice. Behav Brain Res. 2013;253:191–5. [DOI] [PubMed] [Google Scholar]
- 19.Kaster MP, Gadotti VM, Calixto JB, Santos ARS, Rodrigues ALS. Depressive-like behavior induced by tumor necrosis factor-α in mice. Neuropharmacology. 2012;62:419–26. [DOI] [PubMed] [Google Scholar]
- 20.McIntyre RS, Subramaniapillai M, Lee Y, Pan Z, Carmona NE, Shekotikhina M, et al. Efficacy of Adjunctive Infliximab vs Placebo in the treatment of adults with bipolar I/II depression: a Randomized Clinical Trial. JAMA Psychiatry. 2019;76:783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang D, Xing Q, Su Y, Zhao X, Xu W, Wang X, et al. The conserved non-coding sequences CNS6 and CNS9 control Cytokine-Induced Rorc transcription during T Helper 17 cell differentiation. Immunity. 2020;53:614–e6264. [DOI] [PubMed] [Google Scholar]
- 22.Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–4. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q-Y, Ye X-P, Zhou Z, Zhu C-F, Li R, Fang Y, et al. Lymphocyte infiltration and thyrocyte destruction are driven by stromal and immune cell components in Hashimoto’s thyroiditis. Nat Commun. 2022;13:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Yang H, Shi W, Wang T, Ruan Q. MicroRNA-mediated regulation of T helper type 17/regulatory T-cell balance in autoimmune disease. Immunology. 2018;155:427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westfall S, Caracci F, Zhao D, Wu Q, Frolinger T, Simon J, et al. Microbiota metabolites modulate the T helper 17 to regulatory T cell (Th17/Treg) imbalance promoting resilience to stress-induced anxiety- and depressive-like behaviors. Brain Behav Immun. 2021;91:350–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rush G, O’Donovan A, Nagle L, Conway C, McCrohan A, O’Farrelly C, et al. Alteration of immune markers in a group of melancholic depressed patients and their response to electroconvulsive therapy. J Affect Disord. 2016;205:60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Zhang Y, Gao Y, Zhang Z. Elevated levels of Hs-CRP and IL-6 after delivery are associated with depression during the 6 months post partum. Psychiatry Res. 2016;243:43–8. [DOI] [PubMed] [Google Scholar]
- 28.Ng A, Tam WW, Zhang MW, Ho CS, Husain SF, McIntyre RS, et al. IL-1β, IL-6, TNF- α and CRP in Elderly patients with Depression or Alzheimer’s disease: systematic review and Meta-analysis. Sci Rep. 2018;8:12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo Y, He H, Zhang M, Huang X, Fan N. Altered serum levels of TNF-α, IL-6 and IL-18 in manic, depressive, mixed state of bipolar disorder patients. Psychiatry Res. 2016;244:19–23. [DOI] [PubMed] [Google Scholar]
- 30.Buspavanich P, Adli M, Himmerich H, Berger M, Busche M, Schlattmann P, et al. Faster speed of onset of the depressive episode is associated with lower cytokine serum levels (IL-2, -4, -6, -10, TNF-α and IFN-γ) in patients with major depression. J Psychiatr Res. 2021;141:287–92. [DOI] [PubMed] [Google Scholar]
- 31.Huang C, Zhang F, Li P, Song C, Low-Dose. IL-2 attenuated Depression-like behaviors and pathological changes through restoring the balances between IL-6 and TGF-β and between Th17 and Treg in a chronic stress-Induced Mouse Model of Depression. Int J Mol Sci. 2022;23:13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poletti S, Zanardi R, Mandelli A, Aggio V, Finardi A, Lorenzi C, et al. Low-dose interleukin 2 antidepressant potentiation in unipolar and bipolar depression: safety, efficacy, and immunological biomarkers. Brain Behav Immun. 2024;118:52–68. [DOI] [PubMed] [Google Scholar]
- 33.Del Giudice M, Gangestad SW, Rethinking. IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. 2018;70:61–75. [DOI] [PubMed] [Google Scholar]
- 34.Felger JC, Haroon E, Patel TA, Goldsmith DR, Wommack EC, Woolwine BJ, et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. 2020;25:1301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kappelmann N, Arloth J, Georgakis MK, Czamara D, Rost N, Ligthart S, et al. Dissecting the Association between Inflammation, metabolic dysregulation, and specific depressive symptoms: a genetic correlation and 2-Sample mendelian randomization study. JAMA Psychiatry. 2021;78:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh D, Guest PC, Dobrowolny H, Vasilevska V, Meyer-Lotz G, Bernstein H-G, et al. Changes in leukocytes and CRP in different stages of major depression. J Neuroinflammation. 2022;19:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamers F, Milaneschi Y, Smit JH, Schoevers RA, Wittenberg G, Penninx BWJH. Longitudinal Association between Depression and inflammatory markers: results from the Netherlands study of depression and anxiety. Biol Psychiatry. 2019;85:829–37. [DOI] [PubMed] [Google Scholar]
- 38.Monaco C, Nanchahal J, Taylor P, Feldmann M. Anti-TNF therapy: past, present and future. Int Immunol. 2015;27:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mishra B, Ivashkiv L. Regulation of inflammatory NF-kB target gene activation by Jak-STAT signaling. J Immunol. 2023;210:16201. [Google Scholar]
- 40.Zhang K, Wang F, Zhai M, He M, Hu Y, Feng L, et al. Hyperactive neuronal autophagy depletes BDNF and impairs adult hippocampal neurogenesis in a corticosterone-induced mouse model of depression. Theranostics. 2023;13:1059–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Młynarska E, Gadzinowska J, Tokarek J, Forycka J, Szuman A, Franczyk B, et al. The role of the Microbiome-Brain-Gut Axis in the pathogenesis of depressive disorder. Nutrients. 2022;14:1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kronsten VT, Tranah TH, Pariante C, Shawcross DL. Gut-derived systemic inflammation as a driver of depression in chronic liver disease. J Hepatol. 2022;76:665–80. [DOI] [PubMed] [Google Scholar]
- 43.Parker A, Fonseca S, Carding SR. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 2019;11:135–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng Y, Zhou M, Wang J, Yao J, Yu J, Liu W et al. Involvement of the microbiota-gut-brain axis in chronic restraint stress: disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 13:1869501. [DOI] [PMC free article] [PubMed]
- 45.Cheung SG, Goldenthal AR, Uhlemann A-C, Mann JJ, Miller JM, Sublette ME. Systematic review of Gut Microbiota and Major Depression. Front Psychiatry. 2019;10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CSM. The gut microbiota in anxiety and depression – a systematic review. Clin Psychol Rev. 2021;83:101943. [DOI] [PubMed] [Google Scholar]
- 47.Zhu X, Sakamoto S, Ishii C, Smith MD, Ito K, Obayashi M, et al. Dectin-1 signaling on colonic γδ T cells promotes psychosocial stress responses. Nat Immunol. 2023;24:625–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barki N, Bolognini D, Börjesson U, Jenkins L, Riddell J, Hughes DI, et al. Chemogenetics defines a short-chain fatty acid receptor gut-brain axis. Elife. 2022;11:e73777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caetano-Silva ME, Rund L, Hutchinson NT, Woods JA, Steelman AJ, Johnson RW. Inhibition of inflammatory microglia by dietary fiber and short-chain fatty acids. Sci Rep. 2023;13:2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamilton JA, Johnson RA, Corkey B, Kamp F. Fatty acid transport: the diffusion mechanism in model and biological membranes. J Mol Neurosci. 2001;16:99–108. discussion 151–157. [DOI] [PubMed] [Google Scholar]
- 51.Ge T, Yao X, Zhao H, Yang W, Zou X, Peng F, et al. Gut microbiota and neuropsychiatric disorders: implications for neuroendocrine-immune regulation. Pharmacol Res. 2021;173:105909. [DOI] [PubMed] [Google Scholar]
- 52.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–78. [DOI] [PubMed] [Google Scholar]
- 53.Zhu P, Lu T, Wu J, Fan D, Liu B, Zhu X, et al. Gut microbiota drives macrophage-dependent self-renewal of intestinal stem cells via niche enteric serotonergic neurons. Cell Res. 2022;32:555–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aaldijk E, Vermeiren Y. The role of serotonin within the microbiota-gut-brain axis in the development of Alzheimer’s disease: a narrative review. Ageing Res Rev. 2022;75:101556. [DOI] [PubMed] [Google Scholar]
- 55.Huang S, Xu P, Shen D-D, Simon IA, Mao C, Tan Y, et al. GPCRs steer Gi and Gs selectivity via TM5-TM6 switches as revealed by structures of serotonin receptors. Mol Cell. 2022;82:2681–e26956. [DOI] [PubMed] [Google Scholar]
- 56.Heli Z, Hongyu C, Dapeng B, Yee Shin T, Yejun Z, Xi Z, et al. Recent advances of γ-aminobutyric acid: physiological and immunity function, enrichment, and metabolic pathway. Front Nutr. 2022;9:1076223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fogaça MV, Duman RS. Cortical GABAergic Dysfunction in Stress and Depression: New Insights for Therapeutic Interventions. Front Cell Neurosci. 2019;13:87. [DOI] [PMC free article] [PubMed]
- 58.Dang G, Wu W, Zhang H, Everaert N. A new paradigm for a new simple chemical: butyrate & immune regulation. Food Funct. 2021;12:12181–93. [DOI] [PubMed] [Google Scholar]
- 59.Wong CK, McLean BA, Baggio LL, Koehler JA, Hammoud R, Rittig N, et al. Central glucagon-like peptide 1 receptor activation inhibits toll-like receptor agonist-induced inflammation. Cell Metab. 2024;36:130–e1435. [DOI] [PubMed] [Google Scholar]
- 60.Chen X, Zhao P, Wang W, Guo L, Pan Q. The antidepressant effects of GLP-1 receptor agonists: a systematic review and Meta-analysis. Am J Geriatric Psychiatry. 2024;32:117–27. [DOI] [PubMed] [Google Scholar]
- 61.Bodor AL, Katona I, Nyíri G, Mackie K, Ledent C, Hájos N, et al. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen C-J, Zheng D, Li K-X, Yang J-M, Pan H-Q, Yu X-D, et al. Cannabinoid CB1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat Med. 2019;25:337–49. [DOI] [PubMed] [Google Scholar]
- 63.Liu L, Wang H, Chen X, Zhang Y, Zhang H, Xie P. Gut microbiota and its metabolites in depression: from pathogenesis to treatment. eBioMedicine. 2023;90:104527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang J, Zheng P, Li Y, Wu J, Tan X, Zhou J, et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci Adv. 2020;6:eaba8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie Z, Huang J, Sun G, He S, Luo Z, Zhang L, et al. Integrated multi-omics analysis reveals gut microbiota dysbiosis and systemic disturbance in major depressive disorder. Psychiatry Res. 2024;334:115804. [DOI] [PubMed] [Google Scholar]
- 66.Guo X, Tang R, Yang S, Lu Y, Luo J, Liu Z. Rutin and its Combination with Inulin Attenuate Gut Dysbiosis, the inflammatory status and endoplasmic reticulum stress in paneth cells of obese mice Induced by High-Fat Diet. Front Microbiol. 2018;9:2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoffmann M, Messlik A, Kim SC, Sartor RB, Haller D. Impact of a probiotic Enterococcus faecalis in a gnotobiotic mouse model of experimental colitis. Mol Nutr Food Res. 2011;55:703–13. [DOI] [PubMed] [Google Scholar]
- 68.Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385–96. [DOI] [PubMed] [Google Scholar]
- 69.Rose CF, Amodio P, Bajaj JS, Dhiman RK, Montagnese S, Taylor-Robinson SD, et al. Hepatic encephalopathy: novel insights into classification, pathophysiology and therapy. J Hepatol. 2020;73:1526–47. [DOI] [PubMed] [Google Scholar]
- 70.Swain MG, Jones DEJ. Fatigue in chronic liver disease: new insights and therapeutic approaches. Liver Int. 2019;39:6–19. [DOI] [PubMed] [Google Scholar]
- 71.Brandebura AN, Paumier A, Onur TS, Allen NJ. Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat Rev Neurosci. 2023;24:23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–7. [DOI] [PubMed] [Google Scholar]
- 73.Zhang X, Wang D, Zhang B, Zhu J, Zhou Z, Cui L. Regulation of microglia by glutamate and its signal pathway in neurodegenerative diseases. Drug Discov Today. 2020;25:1074–85. [DOI] [PubMed] [Google Scholar]
- 74.Galea I. The blood-brain barrier in systemic infection and inflammation. Cell Mol Immunol. 2021;18:2489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giuffrè M, Moretti R. The gut-liver-brain Axis: from the Head to the feet. Int J Mol Sci. 2023;24:15662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith ML, Wade JB, Wolstenholme J, Bajaj JS. Gut microbiome-brain-cirrhosis axis. Hepatology. 2024;80:465–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fülling C, Dinan TG, Cryan JF. Gut microbe to Brain Signaling: what happens in Vagus…. Neuron. 2019;101:998–1002. [DOI] [PubMed] [Google Scholar]
- 78.Palafox-Sánchez V, Ying Z, Royes LFF, Gomez-Pinilla F. The Interaction between brain and liver regulates lipid metabolism in the TBI pathology. Biochim et Biophys acta Mol Basis Disease. 2021;1867:166078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haleem DJ, Gul S. Circulating leptin, cortisol and gender differences associated with anorexia or obesity in depression. World J Biol Psychiatry. 2020;21:195–202. [DOI] [PubMed] [Google Scholar]
- 80.Hackl MT, Fürnsinn C, Schuh CM, Krssak M, Carli F, Guerra S, et al. Brain leptin reduces liver lipids by increasing hepatic triglyceride secretion and lowering lipogenesis. Nat Commun. 2019;10:2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peiseler M, Schwabe R, Hampe J, Kubes P, Heikenwälder M, Tacke F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J Hepatol. 2022;77:1136–60. [DOI] [PubMed] [Google Scholar]
- 82.Lewis SM, Williams A, Eisenbarth SC. Structure and function of the immune system in the spleen. Sci Immunol. 2019;4:eaau6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang X, Lei B, Yuan Y, Zhang L, Hu L, Jin S, et al. Brain control of humoral immune responses amenable to behavioural modulation. Nature. 2020;581:204–8. [DOI] [PubMed] [Google Scholar]
- 84.Lynall M-E, Kigar SL, Lehmann ML, DePuyt AE, Tuong ZK, Listwak SJ, et al. B-cells are abnormal in psychosocial stress and regulate meningeal myeloid cell activation. Brain Behav Immun. 2021;97:226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Williams DW, Flores BR, Xu Y, Wang Y, Yu D, Peters BA, et al. T-cell activation state differentially contributes to neuropsychiatric complications in women with HIV. Brain Behav Immun Health. 2022;25:100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sato D, Hamada Y, Narita M, Mori T, Tezuka H, Suda Y, et al. Tumor suppression and improvement in immune systems by specific activation of dopamine D1-receptor-expressing neurons in the nucleus accumbens. Mol Brain. 2022;15:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhan H, Huang F, Yan F, Zhao Z, Zhang J, Cui T, et al. Alterations in splenic function and gene expression in mice with depressive-like behavior induced by exposure to corticosterone. Int J Mol Med. 2017;39:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cathomas F, Russo SJ. Brain–spleen connection aids antibody production. Nature. 2020;581:142–3. [DOI] [PubMed] [Google Scholar]
- 89.Ma Y, Kroemer G. The cancer-immune dialogue in the context of stress. Nat Rev Immunol. 2024;24:264–81. [DOI] [PubMed] [Google Scholar]
- 90.Aboud R, Shafii M, Docherty JR. Investigation of the subtypes of alpha 1-adrenoceptor mediating contractions of rat aorta, vas deferens and spleen. Br J Pharmacol. 1993;109:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Durand M, Hagimont E, Louis H, Asfar P, Frippiat J-P, Singer M, et al. The β 1 -Adrenergic receptor contributes to Sepsis-Induced Immunosuppression through Modulation of Regulatory T-Cell inhibitory function. Crit Care Med. 2022;50:e707–18. [DOI] [PubMed] [Google Scholar]
- 92.Shelton RC, Miller AH. Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression. Prog Neurobiol. 2010;91:275–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tan BL, Norhaizan ME. Effect of High-Fat diets on oxidative stress, Cellular Inflammatory response and cognitive function. Nutrients. 2019;11:2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dutheil S, Ota KT, Wohleb ES, Rasmussen K, Duman RS. High-Fat Diet Induced anxiety and Anhedonia: impact on brain homeostasis and inflammation. Neuropsychopharmacology. 2016;41:1874–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chan KL, Poller WC, Swirski FK, Russo SJ. Central regulation of stress-evoked peripheral immune responses. Nat Rev Neurosci. 2023;24:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu C-H, Yang M-H, Zhang G-Z, Wang X-X, Li B, Li M, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. 2020;17:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sohn R, Jenei-Lanzl Z. Role of the sympathetic nervous system in mild chronic inflammatory diseases: Focus on Osteoarthritis. Neuroimmunomodulation. 2023;30:143–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cosentino M, Marino F, Maestroni GJM. Sympathoadrenergic modulation of hematopoiesis: a review of available evidence and of therapeutic perspectives. Front Cell Neurosci. 2015;9:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jung H, Mithal DS, Park JE, Miller RJ. Localized CCR2 activation in the bone marrow niche mobilizes monocytes by desensitizing CXCR4. PLoS ONE. 2015;10:e0128387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Monteiro S, Pinho AG, Macieira M, Serre-Miranda C, Cibrão JR, Lima R, et al. Splenic sympathetic signaling contributes to acute neutrophil infiltration of the injured spinal cord. J Neuroinflammation. 2020;17:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abe C, Inoue T, Inglis MA, Viar KE, Huang L, Ye H, et al. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat Neurosci. 2017;20:700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kreier F, Kap YS, Mettenleiter TC, van Heijningen C, van der Vliet J, Kalsbeek A, et al. Tracing from Fat Tissue, liver, and pancreas: a neuroanatomical Framework for the role of the brain in type 2 diabetes. Endocrinology. 2006;147:1140–7. [DOI] [PubMed] [Google Scholar]
- 103.Stanley S, Pinto S, Segal J, Pérez CA, Viale A, DeFalco J, et al. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc Natl Acad Sci U S A. 2010;107:7024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morinaga H, Mayoral R, Heinrichsdorff J, Osborn O, Franck N, Hah N, et al. Characterization of distinct subpopulations of hepatic macrophages in HFD/Obese mice. Diabetes. 2015;64:1120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tsuneki H, Maeda T, Takata S, Sugiyama M, Otsuka K, Ishizuka H, et al. Hypothalamic orexin prevents non-alcoholic steatohepatitis and hepatocellular carcinoma in obesity. Cell Rep. 2022;41:111497. [DOI] [PubMed] [Google Scholar]
- 106.Kim JG, Ea JY, Yoon B-J. Orexinergic neurons modulate stress coping responses in mice. Front Mol Neurosci. 2023;16:1140672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koren T, Yifa R, Amer M, Krot M, Boshnak N, Ben-Shaanan TL, et al. Insular cortex neurons encode and retrieve specific immune responses. Cell. 2021;184:5902–e591517. [DOI] [PubMed] [Google Scholar]
- 108.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–94. [DOI] [PubMed] [Google Scholar]
- 109.Fyfe I. Enteric nervous system transfers stress to the gut. Nat Rev Gastroenterol Hepatol. 2023;20:484–484. [DOI] [PubMed] [Google Scholar]
- 110.Schneider KM, Blank N, Alvarez Y, Thum K, Lundgren P, Litichevskiy L, et al. The enteric nervous system relays psychological stress to intestinal inflammation. Cell. 2023;186:2823–e283820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huffman WJ, Subramaniyan S, Rodriguiz RM, Wetsel WC, Grill WM, Terrando N. Modulation of Neuroinflammation and Memory Dysfunction using Percutaneous Vagus nerve stimulation in mice. Brain Stimul. 2019;12:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Herman JP, Nawreen N, Smail MA, Cotella EM. Brain mechanisms of HPA axis regulation: neurocircuitry and feedback in context Richard Kvetnansky lecture. Stress. 2020;23:617–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.ó Hartaigh B, Loerbroks A, Thomas GN, Engeland CG, Hollands MA, Fischer JE, et al. Age-dependent and -independent associations between depression, anxiety, DHEAS, and cortisol: from the MIPH Industrial Cohort studies (MICS). Psychoneuroendocrinology. 2012;37:929–36. [DOI] [PubMed] [Google Scholar]
- 114.Horowitz MA, Cattaneo A, Cattane N, Lopizzo N, Tojo L, Bakunina N, et al. Glucocorticoids prime the inflammatory response of human hippocampal cells through up-regulation of inflammatory pathways. Brain Behav Immun. 2020;87:777–94. [DOI] [PubMed] [Google Scholar]
- 115.Cheiran Pereira G, Piton E, Moreira Dos Santos B, Ramanzini LG, Muniz Camargo LF, Menezes, da Silva R et al. Microglia and HPA axis in depression: An overview of participation and relationship. World J Biol Psychiatry. 2022;23:165–82. [DOI] [PubMed]
- 116.Clark RD. Glucocorticoid receptor antagonists. Curr Top Med Chem. 2008;8:813–38. [DOI] [PubMed] [Google Scholar]
- 117.Flores BH, Kenna H, Keller J, Solvason HB, Schatzberg AF. Clinical and biological effects of mifepristone treatment for psychotic depression. Neuropsychopharmacology. 2006;31:628–36. [DOI] [PubMed] [Google Scholar]
- 118.Liu W-Z, Zhang W-H, Zheng Z-H, Zou J-X, Liu X-X, Huang S-H, et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat Commun. 2020;11:2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 2017;60:1–12. [DOI] [PubMed] [Google Scholar]
- 120.Belarbi K, Cuvelier E, Bonte M-A, Desplanque M, Gressier B, Devos D, et al. Glycosphingolipids and neuroinflammation in Parkinson’s disease. Mol Neurodegener. 2020;15:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang M, Zhang H, Liang J, Huang J, Chen N. Exercise suppresses neuroinflammation for alleviating Alzheimer’s disease. J Neuroinflammation. 2023;20:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Niu J, Tsai H-H, Hoi KK, Huang N, Yu G, Kim K, et al. Aberrant oligodendroglial-vascular interactions disrupt the blood brain barrier triggering CNS inflammation. Nat Neurosci. 2019;22:709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Han Y-M, Kim MS, Jo J, Shin D, Kwon S-H, Seo JB, et al. Decoding the temporal nature of brain GR activity in the NFκB signal transition leading to depressive-like behavior. Mol Psychiatry. 2021;26:5087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang H, Wu X, Xu J, Zhu R, Zhang S, Xu Z, et al. Acute stress during witnessing injustice shifts third-party interventions from punishing the perpetrator to helping the victim. PLoS Biol. 2024;22:e3002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hue CD, Cho FS, Cao S, Dale Bass CR, Meaney DF, Morrison B. Dexamethasone potentiates in vitro blood-brain barrier recovery after primary blast injury by glucocorticoid receptor-mediated upregulation of ZO-1 tight junction protein. J Cereb Blood Flow Metab. 2015;35:1191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Y, Sabbagh MF, Gu X, Rattner A, Williams J, Nathans J. Beta-catenin signaling regulates barrier-specific gene expression in circumventricular organ and ocular vasculatures. eLife 8:e43257. [DOI] [PMC free article] [PubMed]
- 127.McMillin M, Frampton G, Seiwell A, Patel N, Jacobs A, DeMorrow S. TGFβ1 exacerbates blood-brain barrier permeability in a mouse model of hepatic encephalopathy via upregulation of MMP9 and downregulation of claudin-5. Lab Invest. 2015;95:903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wei C, Jiang W, Wang R, Zhong H, He H, Gao X, et al. Brain endothelial GSDMD activation mediates inflammatory BBB breakdown. Nature. 2024;629:893–900. [DOI] [PubMed] [Google Scholar]
- 129.Peng Z, Peng S, Lin K, Zhao B, Wei L, Tuo Q, et al. Chronic stress-induced depression requires the recruitment of peripheral Th17 cells into the brain. J Neuroinflammation. 2022;19:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Matsuno H, Tsuchimine S, O’Hashi K, Sakai K, Hattori K, Hidese S, et al. Association between vascular endothelial growth factor-mediated blood-brain barrier dysfunction and stress-induced depression. Mol Psychiatry. 2022;27:3822–32. [DOI] [PubMed] [Google Scholar]
- 131.Dudek KA, Dion-Albert L, Lebel M, LeClair K, Labrecque S, Tuck E, et al. Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc Natl Acad Sci U S A. 2020;117:3326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yanagida K, Liu CH, Faraco G, Galvani S, Smith HK, Burg N, et al. Size-selective opening of the blood-brain barrier by targeting endothelial sphingosine 1-phosphate receptor 1. Proc Natl Acad Sci U S A. 2017;114:4531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dudek KA, Dion-Albert L, Kaufmann FN, Tuck E, Lebel M, Menard C. Neurobiology of resilience in depression: immune and vascular insights from human and animal studies. Eur J Neurosci. 2021;53:183–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Capogna E, Watne LO, Sørensen Ø, Guichelaar CJ, Idland AV, Halaas NB, et al. Associations of neuroinflammatory IL-6 and IL-8 with brain atrophy, memory decline, and core AD biomarkers - in cognitively unimpaired older adults. Brain Behav Immun. 2023;113:56–65. [DOI] [PubMed] [Google Scholar]
- 135.Klein RS, Hunter CA. Protective and pathological immunity during Central Nervous System infections. Immunity. 2017;46:891–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nutt DJ. Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatry. 2008;69(Suppl E):4–7. [PubMed] [Google Scholar]
- 137.Lin R, Zhou Y, Yan T, Wang R, Li H, Wu Z, et al. Directed evolution of adeno-associated virus for efficient gene delivery to microglia. Nat Methods. 2022;19:976–85. [DOI] [PubMed] [Google Scholar]
- 138.Fornari Laurindo L, Aparecido Dias J, Cressoni Araújo A, Torres Pomini K, Machado Galhardi C, Rucco Penteado Detregiachi C, et al. Immunological dimensions of neuroinflammation and microglial activation: exploring innovative immunomodulatory approaches to mitigate neuroinflammatory progression. Front Immunol. 2023;14:1305933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hennessy E, Griffin ÉW, Cunningham C. Astrocytes are primed by Chronic Neurodegeneration to produce exaggerated chemokine and cell infiltration responses to Acute Stimulation with the cytokines IL-1β and TNF-α. J Neurosci. 2015;35:8411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107:234–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chang J, Jiang T, Shan X, Zhang M, Li Y, Qi X, et al. Pro-inflammatory cytokines in stress-induced depression: novel insights into mechanisms and promising therapeutic strategies. Prog Neuropsychopharmacol Biol Psychiatry. 2024;131:110931. [DOI] [PubMed] [Google Scholar]
- 142.Petralia MC, Mazzon E, Fagone P, Basile MS, Lenzo V, Quattropani MC, et al. The cytokine network in the pathogenesis of major depressive disorder. Close to translation? Autoimmun rev. 2020;19:102504. [DOI] [PubMed] [Google Scholar]
- 143.Lopez-Rodriguez AB, Hennessy E, Murray C, Nazmi A, Delaney HJ, Healy D, et al. Acute systemic inflammation exacerbates neuroinflammation in Alzheimer’s Disease: IL-1β drives amplified responses in primed astrocytes and neuronal network dysfunction. Alzheimers Dement. 2021;17:1735–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Berger T, Lee H, Young AH, Aarsland D, Thuret S. Adult hippocampal neurogenesis in major depressive disorder and Alzheimer’s Disease. Trends Mol Med. 2020;26:803–18. [DOI] [PubMed] [Google Scholar]
- 145.Ellwardt E, Pramanik G, Luchtman D, Novkovic T, Jubal ER, Vogt J, et al. Maladaptive cortical hyperactivity upon recovery from experimental autoimmune encephalomyelitis. Nat Neurosci. 2018;21:1392–403. [DOI] [PubMed] [Google Scholar]
- 146.Xu X, Piao H-N, Aosai F, Zeng X-Y, Cheng J-H, Cui Y-X, et al. Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways. Br J Pharmacol. 2020;177:5224–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.He X-H, Zang Y, Chen X, Pang R-P, Xu J-T, Zhou X, et al. TNF-α contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain. 2010;151:266–79. [DOI] [PubMed] [Google Scholar]
- 148.Zipp F, Bittner S, Schafer DP. Cytokines as emerging regulators of central nervous system synapses. Immunity. 2023;56:914–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Konsman JP. Inflammation and depression: a nervous Plea for Psychiatry to not become Immune to Interpretation. Pharmaceuticals (Basel). 2019;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 2015;96:70–82. [DOI] [PubMed] [Google Scholar]
- 151.Zyrianova T, Zou K, Lopez B, Liao A, Gu C, Olcese R, et al. Activation of endothelial large conductance potassium channels protects against TNF-α-Induced inflammation. Int J Mol Sci. 2023;24:4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alter C, Henseler A-S, Owenier C, Hesse J, Ding Z, Lautwein T, et al. IL-6 in the infarcted heart is preferentially formed by fibroblasts and modulated by purinergic signaling. J Clin Invest. 2023;133:e163799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, et al. Activation of CNS inflammatory pathways by Interferon-alpha: relationship to monoamines and Depression. Biol Psychiatry. 2009;65:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Döhne N, Falck A, Janach GMS, Byvaltcev E, Strauss U. Interferon-γ augments GABA release in the developing neocortex via nitric oxide synthase/soluble guanylate cyclase and constrains network activity. Front Cell Neurosci. 2022;16:913299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, Smirnov I, et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature. 2016;535:425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang L, Liu C, Li W, Ma Y, Huo S, Ozathaley A, et al. Depression-like behavior associated with E/I imbalance of mPFC and amygdala without TRPC channels in mice of knockout IL-10 from microglia. Brain Behav Immun. 2021;97:68–78. [DOI] [PubMed] [Google Scholar]
- 157.Zhang J, Rong P, Zhang L, He H, Zhou T, Fan Y, et al. IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. Sci Adv. 2021;7:eabb9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hanuscheck N, Thalman C, Domingues M, Schmaul S, Muthuraman M, Hetsch F, et al. Interleukin-4 receptor signaling modulates neuronal network activity. J Exp Med. 2022;219:e20211887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Han S, Li X-X, Wei S, Zhao D, Ding J, Xu Y, et al. Orbitofrontal cortex-hippocampus potentiation mediates relief for depression: a randomized double-blind trial and TMS-EEG study. Cell Rep Med. 2023;4:101060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Blume J, Douglas SD, Evans DL. Immune suppression and immune activation in depression. Brain Behav Immun. 2011;25:221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hoprekstad GE, Kjelby E, Gjestad R, Fathian F, Larsen TK, Reitan SK, et al. Depression trajectories and cytokines in schizophrenia spectrum disorders - a longitudinal observational study. Schizophr Res. 2023;252:77–87. [DOI] [PubMed] [Google Scholar]
- 162.Worthen RJ, Garzon Zighelboim SS, Torres Jaramillo CS, Beurel E. Anti-inflammatory IL-10 administration rescues depression-associated learning and memory deficits in mice. J Neuroinflammation. 2020;17:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kruse JL, Vasavada MM, Olmstead R, Hellemann G, Wade B, Breen EC, et al. Depression treatment response to ketamine: sex-specific role of interleukin-8, but not other inflammatory markers. Transl Psychiatry. 2021;11:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kruse JL, Olmstead R, Hellemann G, Breen EC, Tye SJ, Brooks JO, et al. Interleukin-8 and lower severity of depression in females, but not males, with treatment-resistant depression. J Psychiatr Res. 2021;140:350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kruse JL, Olmstead R, Hellemann G, Wade B, Jiang J, Vasavada MM, et al. Inflammation and depression treatment response to electroconvulsive therapy: sex-specific role of interleukin-8. Brain Behav Immun. 2020;89:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kruse JL, Boyle CC, Olmstead R, Breen EC, Tye SJ, Eisenberger NI, et al. Interleukin-8 and depressive responses to an inflammatory challenge: secondary analysis of a randomized controlled trial. Sci Rep. 2022;12:12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shi Y, Luan D, Song R, Zhang Z. Value of peripheral neurotrophin levels for the diagnosis of depression and response to treatment: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2020;41:40–51. [DOI] [PubMed] [Google Scholar]
- 168.Castrén E, Monteggia LM. Brain-derived neurotrophic factor signaling in Depression and Antidepressant Action. Biol Psychiatry. 2021;90:128–36. [DOI] [PubMed] [Google Scholar]
- 169.Notaras M, van den Buuse M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol Psychiatry. 2020;25:2251–74. [DOI] [PubMed] [Google Scholar]
- 170.Mishchenko TA, Mitroshina EV, Usenko AV, Voronova NV, Astrakhanova TA, Shirokova OM, et al. Features of neural network formation and their functions in primary hippocampal cultures in the context of chronic TrkB receptor system influence. Front Physiol. 2018;9:1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fullana MN, Ruiz-Bronchal E, Ferrés-Coy A, Juárez-Escoto E, Artigas F, Bortolozzi A. Regionally selective knockdown of astroglial glutamate transporters in infralimbic cortex induces a depressive phenotype in mice. Glia. 2019;67:1122–37. [DOI] [PubMed] [Google Scholar]
- 172.Cheng P-L, Song A-H, Wong Y-H, Wang S, Zhang X, Poo M-M. Self-amplifying autocrine actions of BDNF in axon development. Proc Natl Acad Sci U S A. 2011;108:18430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Carniel BP, da Rocha NS. Brain-derived neurotrophic factor (BDNF) and inflammatory markers: perspectives for the management of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2021;108:110151. [DOI] [PubMed] [Google Scholar]
- 174.Tian F, Lewis LD, Zhou DW, Balanza GA, Paulk AC, Zelmann R, et al. Characterizing brain dynamics during ketamine-induced dissociation and subsequent interactions with propofol using human intracranial neurophysiology. Nat Commun. 2023;14:1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Deyama S, Kondo M, Shimada S, Kaneda K. IGF-1 release in the medial prefrontal cortex mediates the rapid and sustained antidepressant-like actions of ketamine. Translational Psychiatry. 2022;12:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Abdallah CG, Adams TG, Kelmendi B, Esterlis I, Sanacora G, Krystal JH. Ketamine’s mechanism of action: a path to Rapid-Acting antidepressants. Depress Anxiety. 2016;33:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu S, Yao X, Li B, Cui R, Zhu C, Wang Y, et al. Uncovering the underlying mechanisms of ketamine as a Novel antidepressant. Front Pharmacol. 2022;12:740996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lituma PJ, Kwon H-B, Alviña K, Luján R, Castillo PE. Presynaptic NMDA receptors facilitate short-term plasticity and BDNF release at hippocampal mossy fiber synapses. Elife. 2021;10:e66612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pothula S, Kato T, Liu R-J, Wu M, Gerhard D, Shinohara R, et al. Cell-type specific modulation of NMDA receptors triggers antidepressant actions. Mol Psychiatry. 2021;26:5097–111. [DOI] [PubMed] [Google Scholar]
- 180.Moya-Alvarado G, Tiburcio-Felix R, Ibáñez MR, Aguirre-Soto AA, Guerra MV, Wu C, et al. BDNF/TrkB signaling endosomes in axons coordinate CREB/mTOR activation and protein synthesis in the cell body to induce dendritic growth in cortical neurons. Elife. 2023;12:e77455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gash DM, Zhang Z, Gerhardt G. Neuroprotective and neurorestorative properties of GDNF. Ann Neurol. 1998;44:S121–125. [DOI] [PubMed] [Google Scholar]
- 182.Irala D, Bonafina A, Fontanet PA, Alsina FC, Paratcha G, Ledda F. The GDNF-GFRα1 complex promotes the development of hippocampal dendritic arbors and spines via NCAM. Development. 2016;143:4224–35. [DOI] [PubMed] [Google Scholar]
- 183.Bonafina A, Trinchero MF, Ríos AS, Bekinschtein P, Schinder AF, Paratcha G, et al. GDNF and GFRα1 are required for proper integration of adult-born hippocampal neurons. Cell Rep. 2019;29:4308–e43194. [DOI] [PubMed] [Google Scholar]
- 184.Liu Y, Zhou X, Xue K, Sun R, Tang Y, Tang C. Reviving: restoring depression-like behaviour through glial cell-derived neurotrophic factor treatment in the medial prefrontal cortex. J Psychiatry Neurosci. 2024;49:E23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li H, Dan Q-Q, Chen Y-J, Chen L, Zhang H-T, Mu D-Z, et al. Cellular localization and distribution of TGF-β1, GDNF and PDGF-BB in the adult Primate Central Nervous System. Neurochem Res. 2023;48:2406–23. [DOI] [PubMed] [Google Scholar]
- 186.Wu Y, Kong L, Yang A, Xin K, Lu Y, Yan X, et al. Gray matter volume reduction in orbitofrontal cortex correlated with plasma glial cell line-derived neurotrophic factor (GDNF) levels within major depressive disorder. Neuroimage Clin. 2023;37:103341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jin Y, Yu L, Li Y. Paroxetine Effect on Nerve Growth Factor, Human Neurotrophin-4, Brain-Derived Neurotrophic Factor Levels in Post-stroke Depression. Mol Neurobiol. 2024;61:7890–7. [DOI] [PubMed]
- 188.Zhu Y, Liu M, Cao C, Qu S, Zheng J, Zhu Z, et al. Dendrobium officinale flos increases neurotrophic factor expression in the hippocampus of chronic unpredictable mild stress-exposed mice and in astrocyte primary culture and potentiates NGF-induced neuronal differentiation in PC12 cells. Phytother Res. 2021;35:2665–77. [DOI] [PubMed] [Google Scholar]
- 189.Koshkina A, Dudnichenko T, Baranenko D, Fedotova J, Drago F. Effects of vitamin D3 in long-term ovariectomized rats subjected to chronic unpredictable mild stress: BDNF, NT-3, and NT-4 implications. Nutrients. 2019;11:1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xu K, Zheng P, Zhao S, Wang M, Tu D, Wei Q, et al. MANF/EWSR1/ANXA6 pathway might as the bridge between hypolipidemia and major depressive disorder. Transl Psychiatry. 2022;12:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019;176:1248–64. [DOI] [PMC free article] [PubMed]
- 192.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sene A, Chin-Yee D, Apte RS. Seeing through VEGF: innate and adaptive immunity in pathological angiogenesis in the eye. Trends Mol Med. 2015;21:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–35. [DOI] [PubMed] [Google Scholar]
- 195.Kirby ED, Kuwahara AA, Messer RL, Wyss-Coray T. Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proc Natl Acad Sci U S A. 2015;112:4128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Luo Y, Ali T, Liu Z, Gao R, Li A, Yang C, et al. EPO prevents neuroinflammation and relieves depression via JAK/STAT signaling. Life Sci. 2023;333:122102. [DOI] [PubMed] [Google Scholar]
- 197.Zhang F-F, Peng W, Sweeney JA, Jia Z-Y, Gong Q-Y. Brain structure alterations in depression: psychoradiological evidence. CNS Neurosci Ther. 2018;24:994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gallo S, El-Gazzar A, Zhutovsky P, Thomas RM, Javaheripour N, Li M, et al. Functional connectivity signatures of major depressive disorder: machine learning analysis of two multicenter neuroimaging studies. Mol Psychiatry. 2023;28:3013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Trifu SC, Trifu AC, Aluaş E, Tătaru MA, Costea RV. Brain changes in depression. Rom J Morphol Embryol. 2020;61:361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu A, Zhang J. Neuroinflammation, memory, and depression: new approaches to hippocampal neurogenesis. J Neuroinflammation. 2023;20:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ghaheri S, Niapour A, Sakhaie N, Sadegzadeh F, Saadati H. Postnatal depletion of serotonin affects the morphology of neurons and the function of the hippocampus in male rats. Int J Dev Neurosci. 2022;82:222–30. [DOI] [PubMed] [Google Scholar]
- 202.Frodl T, Jäger M, Smajstrlova I, Born C, Bottlender R, Palladino T, et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33:423–30. [PMC free article] [PubMed] [Google Scholar]
- 203.Joshi SH, Espinoza RT, Pirnia T, Shi J, Wang Y, Ayers B, et al. Structural plasticity of the Hippocampus and Amygdala Induced by Electroconvulsive Therapy in Major Depression. Biol Psychiatry. 2016;79:282–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jacob Y, Morris LS, Verma G, Rutter SB, Balchandani P, Murrough JW. Altered hippocampus and amygdala subregion connectome hierarchy in major depressive disorder. Transl Psychiatry. 2022;12:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Grogans SE, Fox AS, Shackman AJ. The Amygdala and Depression: a Sober reconsideration. Am J Psychiatry. 2022;179:454–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mehta ND, Haroon E, Xu X, Woolwine BJ, Li Z, Felger JC. Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: preliminary results. Brain Behav Immun. 2018;73:725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Boukezzi S, Costi S, Shin LM, Kim-Schulze S, Cathomas F, Collins A, et al. Exaggerated amygdala response to threat and association with immune hyperactivity in depression. Brain Behav Immun. 2022;104:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhang W, Rutlin J, Eisenstein SA, Wang Y, Barch DM, Hershey T, et al. Neuroinflammation in the amygdala is Associated with recent depressive symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging. 2023;8:967–75. [DOI] [PubMed] [Google Scholar]
- 209.Young KD, Friedman ES, Collier A, Berman SR, Feldmiller J, Haggerty AE, et al. Response to SSRI intervention and amygdala activity during self-referential processing in major depressive disorder. Neuroimage Clin. 2020;28:102388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Serra-Blasco M, Portella MJ, Gómez-Ansón B, de Diego-Adeliño J, Vives-Gilabert Y, Puigdemont D, et al. Effects of illness duration and treatment resistance on grey matter abnormalities in major depression. Br J Psychiatry. 2013;202:434–40. [DOI] [PubMed] [Google Scholar]
- 211.Bora E, Fornito A, Pantelis C, Yücel M. Gray Matter abnormalities in major depressive disorder: a meta-analysis of Voxel based morphometry studies. J Affect Disord. 2012;138:9–18. [DOI] [PubMed] [Google Scholar]
- 212.Lu Q, Li H, Luo G, Wang Y, Tang H, Han L, et al. Impaired prefrontal-amygdala effective connectivity is responsible for the dysfunction of emotion process in major depressive disorder: a dynamic causal modeling study on MEG. Neurosci Lett. 2012;523:125–30. [DOI] [PubMed] [Google Scholar]
- 213.Zhang X, Di X, Lei H, Yang J, Xiao J, Wang X, et al. Imbalanced spontaneous brain activity in orbitofrontal-insular circuits in individuals with cognitive vulnerability to depression. J Affect Disord. 2016;198:56–63. [DOI] [PubMed] [Google Scholar]
- 214.Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, et al. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry. 2011;16:751–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jacobs RH, Barba A, Gowins JR, Klumpp H, Jenkins LM, Mickey BJ, et al. Decoupling of the amygdala to other salience network regions in adolescent-onset recurrent major depressive disorder. Psychol Med. 2016;46:1055–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhong X, Pu W, Yao S. Functional alterations of fronto-limbic circuit and default mode network systems in first-episode, drug-naïve patients with major depressive disorder: a meta-analysis of resting-state fMRI data. J Affect Disord. 2016;206:280–6. [DOI] [PubMed] [Google Scholar]
- 217.Wagner G, Koch K, Schachtzabel C, Schultz CC, Sauer H, Schlösser RG. Structural brain alterations in patients with major depressive disorder and high risk for suicide: evidence for a distinct neurobiological entity? NeuroImage. 2011;54:1607–14. [DOI] [PubMed] [Google Scholar]
- 218.Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR, et al. Chronic interferon-α decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology. 2013;38:2179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Burrows K, Stewart JL, Kuplicki R, Figueroa-Hall L, Spechler PA, Zheng H, et al. Elevated peripheral inflammation is associated with attenuated striatal reward anticipation in major depressive disorder. Brain Behav Immun. 2021;93:214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, et al. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun. 2010;24:1058–68. [DOI] [PubMed] [Google Scholar]
- 221.Masuda T, Sankowski R, Staszewski O, Böttcher C, Amann L, Sagar null, et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;566:388–92. [DOI] [PubMed] [Google Scholar]
- 222.Liu L, Tang J, Liang X, Li Y, Zhu P, Zhou M, et al. Running exercise alleviates hippocampal neuroinflammation and shifts the balance of microglial M1/M2 polarization through adiponectin/AdipoR1 pathway activation in mice exposed to chronic unpredictable stress. Mol Psychiatry. 2024;29:2031–42. [DOI] [PubMed] [Google Scholar]
- 223.Zhang Y, Wang D, Liu J, Bai Y, Fan B, Lu C, et al. Structural characterization and antidepressant-like effects of Polygonum Sibiricum Polysaccharides on regulating Microglial polarization in chronic unpredictable mild stress-Induced zebrafish. Int J Mol Sci. 2024;25:2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lu R, Zhang L, Wang H, Li M, Feng W, Zheng X. Echinacoside exerts antidepressant-like effects through enhancing BDNF-CREB pathway and inhibiting neuroinflammation via regulating microglia M1/M2 polarization and JAK1/STAT3 pathway. Front Pharmacol. 2022;13:993483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nikolopoulos D, Manolakou T, Polissidis A, Filia A, Bertsias G, Koutmani Y, et al. Microglia activation in the presence of intact blood-brain barrier and disruption of hippocampal neurogenesis via IL-6 and IL-18 mediate early diffuse neuropsychiatric lupus. Ann Rheum Dis. 2023;82:646–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kalkman HO, Feuerbach D. Antidepressant therapies inhibit inflammation and microglial M1-polarization. Pharmacol Ther. 2016;163:82–93. [DOI] [PubMed] [Google Scholar]
- 227.Burke NN, Kerr DM, Moriarty O, Finn DP, Roche M. Minocycline modulates neuropathic pain behaviour and cortical M1-M2 microglial gene expression in a rat model of depression. Brain Behav Immun. 2014;42:147–56. [DOI] [PubMed] [Google Scholar]
- 228.Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR. Chronic stress Induced Remodeling of the Prefrontal cortex: structural re-organization of Microglia and the Inhibitory Effect of Minocycline. Cereb Cortex. 2013;23:1784–97. [DOI] [PubMed] [Google Scholar]
- 229.Sukoff Rizzo SJ, Neal SJ, Hughes ZA, Beyna M, Rosenzweig-Lipson S, Moss SJ, et al. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl Psychiatry. 2012;2:e199–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Koo JW, Duman RS. IL-1 is an essential mediator of the antineurogenic and anhedonic effects of stress. [DOI] [PMC free article] [PubMed]
- 231.Lee YJ, Kim Y-K. Neuron-to-microglia crosstalk in Psychiatric disorders. Curr Neuropharmacol. 2020;18:84–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Guo L-T, Wang S-Q, Su J, Xu L-X, Ji Z-Y, Zhang R-Y, et al. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J Neuroinflammation. 2019;16:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Laskaris LE, Di Biase MA, Everall I, Chana G, Christopoulos A, Skafidas E, et al. Microglial activation and progressive brain changes in schizophrenia. Br J Pharmacol. 2016;173:666–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cianciulli A, Porro C, Calvello R, Trotta T, Lofrumento DD, Panaro MA. Microglia mediated Neuroinflammation: focus on PI3K modulation. Biomolecules. 2020;10:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Su W-J, Li J-M, Zhang T, Cao Z-Y, Hu T, Zhong S-Y, et al. Microglial NLRP3 inflammasome activation mediates diabetes-induced depression-like behavior via triggering neuroinflammation. Prog Neuropsychopharmacol Biol Psychiatry. 2023;126:110796. [DOI] [PubMed] [Google Scholar]
- 236.Li S, Fang Y, Zhang Y, Song M, Zhang X, Ding X, et al. Microglial NLRP3 inflammasome activates neurotoxic astrocytes in depression-like mice. Cell Rep. 2022;41:111532. [DOI] [PubMed] [Google Scholar]
- 237.Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. 2015;16:620–31. [DOI] [PubMed] [Google Scholar]
- 238.Anticevic A, Repovs G, Barch DM. Emotion effects on attention, amygdala activation, and functional connectivity in schizophrenia. Schizophr Bull. 2012;38:967–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wegrzyn D, Juckel G, Faissner A. Structural and functional deviations of the Hippocampus in Schizophrenia and Schizophrenia Animal models. Int J Mol Sci. 2022;23:5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Shen C-L, Tsai S-J, Lin C-P, Yang AC. Progressive brain abnormalities in schizophrenia across different illness periods: a structural and functional MRI study. Schizophrenia (Heidelb). 2023;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pergola G, Selvaggi P, Trizio S, Bertolino A, Blasi G. The role of the thalamus in schizophrenia from a neuroimaging perspective. Neurosci Biobehav Rev. 2015;54:57–75. [DOI] [PubMed] [Google Scholar]
- 242.Gao W-J, Yang S-S, Mack NR, Chamberlin LA. Aberrant maturation and connectivity of prefrontal cortex in schizophrenia-contribution of NMDA receptor development and hypofunction. Mol Psychiatry. 2022;27:731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Köhler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, et al. Peripheral alterations in Cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and Meta-analysis. Mol Neurobiol. 2018;55:4195–206. [DOI] [PubMed] [Google Scholar]
- 244.Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 2021;184:1299–e131319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Rosoff DB, Smith GD, Lohoff FW. Prescription opioid use and risk for major depressive disorder and anxiety and stress-related disorders: a multivariable mendelian randomization analysis. JAMA Psychiatry. 2021;78:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Modi KK, Sendtner M, Pahan K. Up-regulation of ciliary neurotrophic factor in astrocytes by aspirin: implications for remyelination in multiple sclerosis. J Biol Chem. 2013;288:18533–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Song Q, Feng Y-B, Wang L, Shen J, Li Y, Fan C, et al. COX-2 inhibition rescues depression-like behaviors via suppressing glial activation, oxidative stress and neuronal apoptosis in rats. Neuropharmacology. 2019;160:107779. [DOI] [PubMed] [Google Scholar]
- 248.Husain MI, Cullen C, Umer M, Carvalho AF, Kloiber S, Meyer JH, et al. Minocycline as adjunctive treatment for treatment-resistant depression: study protocol for a double blind, placebo-controlled, randomized trial (MINDEP2). BMC Psychiatry. 2020;20:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Roman M, Irwin MR. Novel neuroimmunologic therapeutics in depression: a clinical perspective on what we know so far. Brain Behav Immun. 2020;83:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of Dupilumab versus Placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–48. [DOI] [PubMed] [Google Scholar]
- 251.Bekhbat M, Chu K, Le N-A, Woolwine BJ, Haroon E, Miller AH, et al. Glucose and lipid-related biomarkers and the antidepressant response to infliximab in patients with treatment-resistant depression. Psychoneuroendocrinology. 2018;98:222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Knight JM, Costanzo ES, Singh S, Yin Z, Szabo A, Pawar DS, et al. The IL-6 antagonist tocilizumab is associated with worse depression and related symptoms in the medically ill. Transl Psychiatry. 2021;11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lu J-J, Wu P-F, He J-G, Li Y-K, Long L-H, Yao X-P, et al. BNIP3L/NIX-mediated mitophagy alleviates passive stress-coping behaviors induced by tumor necrosis factor-α. Mol Psychiatry. 2023;28:5062–76. [DOI] [PubMed] [Google Scholar]
- 254.MRC ImmunoPsychiatry Consortium, Wittenberg GM, Stylianou A, Zhang Y, Sun Y, Gupta A, et al. Effects of immunomodulatory drugs on depressive symptoms: a mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol Psychiatry. 2020;25:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Langley RG, Feldman SR, Han C, Schenkel B, Szapary P, Hsu M-C, et al. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: results from a randomized, double-blind, placebo-controlled phase III trial. J Am Acad Dermatol. 2010;63:457–65. [DOI] [PubMed] [Google Scholar]
- 256.Laumet G, Edralin JD, Chiang AC-A, Dantzer R, Heijnen CJ, Kavelaars A. Resolution of inflammation-induced depression requires T lymphocytes and endogenous brain interleukin-10 signaling. Neuropsychopharmacology. 2018;43:2597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Savio LEB, de Andrade Mello P, da Silva CG, Coutinho-Silva R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front Pharmacol. 2018;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Bhattacharya A, Biber K. The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia. 2016;64:1772–87. [DOI] [PubMed] [Google Scholar]
- 259.LeGates TA, Kvarta MD, Thompson SM. Sex differences in antidepressant efficacy. Neuropsychopharmacology. 2018;44:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lu C-L, Herndon C. New roles for neuronal estrogen receptors. Neurogastroenterol Motil. 2017;29. [DOI] [PubMed]
- 261.Fink G, Sumner BE. Oestrogen and mental state. Nature. 1996;383:306. [DOI] [PubMed] [Google Scholar]
- 262.Benitah K, Siegel AN, Lipsitz O, Rodrigues NB, Meshkat S, Lee Y, et al. Sex differences in ketamine’s therapeutic effects for mood disorders: a systematic review. Psychiatry Res. 2022;312:114579. [DOI] [PubMed] [Google Scholar]
- 263.Allen AL, McCarson KE. Estrogen increases nociception-evoked brain-derived neurotrophic factor gene expression in the female rat. Neuroendocrinology. 2005;81:193–9. [DOI] [PubMed] [Google Scholar]
- 264.Ledesma-Corvi S, Jornet-Plaza J, García-Fuster MJ. Aromatase inhibition and ketamine in rats: sex-differences in antidepressant-like efficacy. Biology Sex Differences. 2023;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Barth C, Crestol A, de Lange A-MG, Galea LAM. Sex steroids and the female brain across the lifespan: insights into risk of depression and Alzheimer’s disease. Lancet Diabetes Endocrinol. 2023;11:926–41. [DOI] [PubMed] [Google Scholar]
- 266.Ho M-F, Correia C, Ingle JN, Kaddurah-Daouk R, Wang L, Kaufmann SH, et al. Ketamine and ketamine metabolites as novel estrogen receptor ligands: induction of cytochrome P450 and AMPA glutamate receptor gene expression. Biochem Pharmacol. 2018;152:279–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kokras N, Hodes GE, Bangasser DA, Dalla C. Sex differences in the hypothalamic–pituitary–adrenal axis: an obstacle to antidepressant drug development? Br J Pharmacol. 2019;176:4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zhou L, Xiong J-Y, Chai Y-Q, Huang L, Tang Z-Y, Zhang X-F, et al. Possible antidepressant mechanisms of omega-3 polyunsaturated fatty acids acting on the central nervous system. Front Psychiatry. 2022;13:933704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Yin W, Löf M, Chen R, Hultman CM, Fang F, Sandin S. Mediterranean diet and depression: a population-based cohort study. Int J Behav Nutr Phys Act. 2021;18:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Swann OG, Kilpatrick M, Breslin M, Oddy WH. Dietary fiber and its associations with depression and inflammation. Nutr Rev. 2020;78:394–411. [DOI] [PubMed] [Google Scholar]
- 271.Reigstad CS, Salmonson CE, Rainey JF, Szurszewski JH, Linden DR, Sonnenburg JL, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Bosscher D, Van Loo J, Franck A. Inulin and oligofructose as prebiotics in the prevention of intestinal infections and diseases. Nutr Res Rev. 2006;19:216–26. [DOI] [PubMed] [Google Scholar]
- 273.Liu W, Zhai X, Li H, Ji L. Depression-like behaviors in mice subjected to co-treatment of high-fat diet and corticosterone are ameliorated by AICAR and exercise. J Affect Disord. 2014;156:171–7. [DOI] [PubMed] [Google Scholar]
- 274.Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–72. [DOI] [PubMed] [Google Scholar]
- 275.Bian C, Zhao W-W, Yan S-R, Chen S-Y, Cheng Y, Zhang Y-H. Effect of interpersonal psychotherapy on social functioning, overall functioning and negative emotions for depression: a meta-analysis. J Affect Disord. 2023;320:230–40. [DOI] [PubMed] [Google Scholar]
- 276.Hazell P. Updates in treatment of depression in children and adolescents. Curr Opin Psychiatry. 2021;34:593–9. [DOI] [PubMed] [Google Scholar]
- 277.Black DS, Slavich GM. Mindfulness meditation and the immune system: a systematic review of randomized controlled trials. Ann N Y Acad Sci. 2016;1373:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gold SM, Friede T, Meyer B, Moss-Morris R, Hudson J, Asseyer S, et al. Internet-delivered cognitive behavioural therapy programme to reduce depressive symptoms in patients with multiple sclerosis: a multicentre, randomised, controlled, phase 3 trial. Lancet Digit Health. 2023;5:e668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Shields GS, Spahr CM, Slavich GM. Psychosocial interventions and Immune System function: a systematic review and Meta-analysis of Randomized clinical trials. JAMA Psychiatry. 2020;77:1031–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Chandran V, Bermúdez M-L, Koka M, Chandran B, Pawale D, Vishnubhotla R, et al. Large-scale genomic study reveals robust activation of the immune system following advanced Inner Engineering meditation retreat. Proc Natl Acad Sci U S A. 2021;118:e2110455118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ruiz-González D, Hernández-Martínez A, Valenzuela PL, Morales JS, Soriano-Maldonado A. Effects of physical exercise on plasma brain-derived neurotrophic factor in neurodegenerative disorders: a systematic review and meta-analysis of randomized controlled trials. Neurosci Biobehav Rev. 2021;128:394–405. [DOI] [PubMed] [Google Scholar]
- 282.Ross RE, VanDerwerker CJ, Saladin ME, Gregory CM. The role of exercise in the treatment of depression: biological underpinnings and clinical outcomes. Mol Psychiatry. 2023;28:298–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.