Abstract
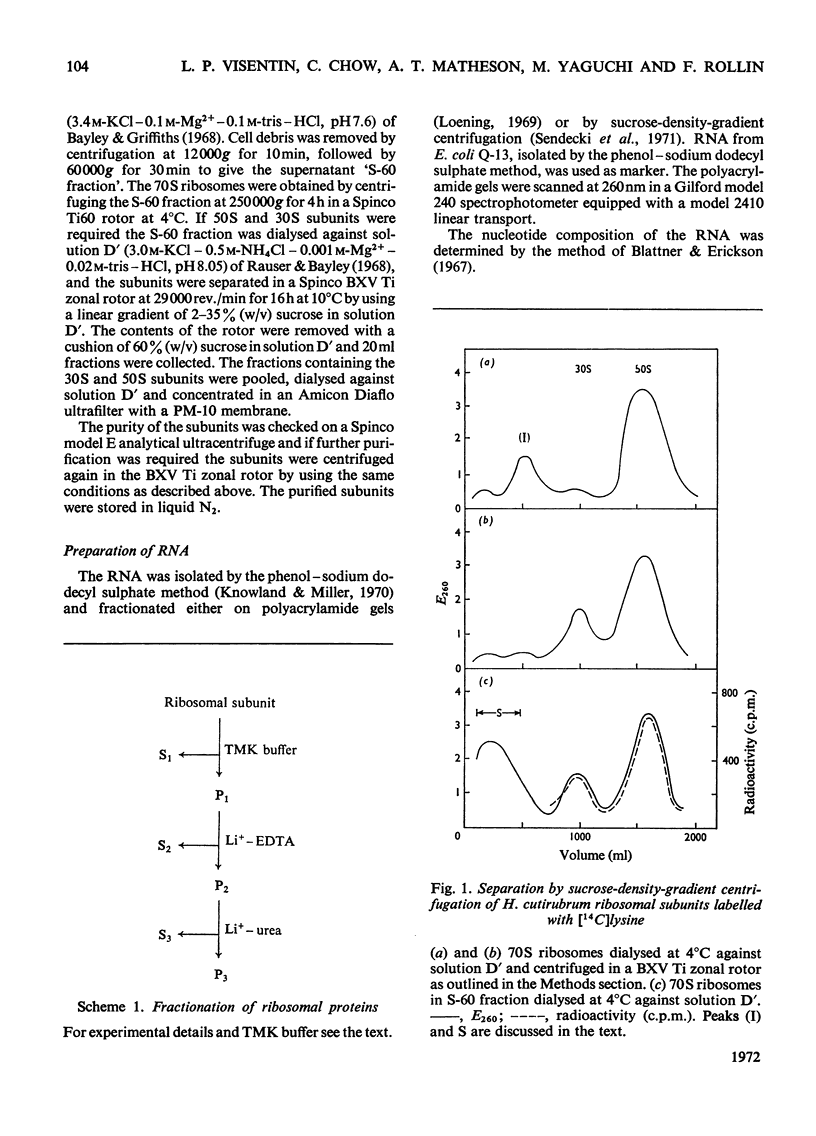
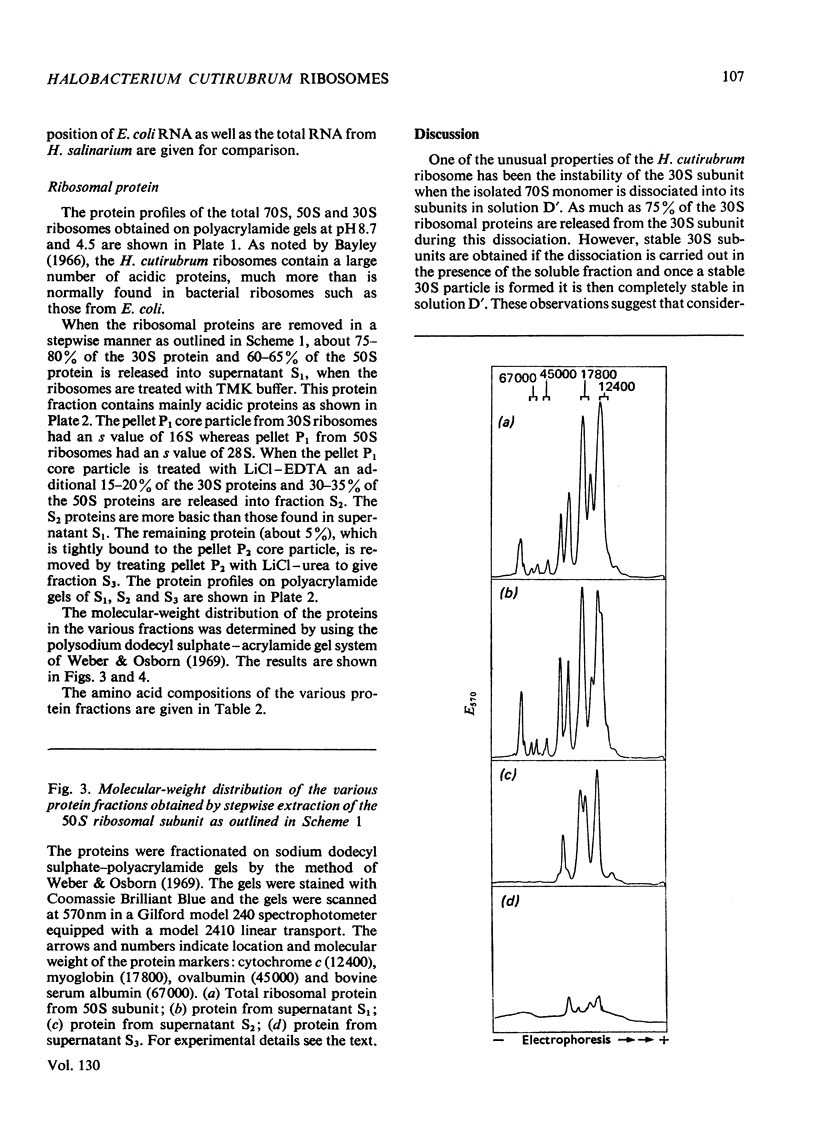
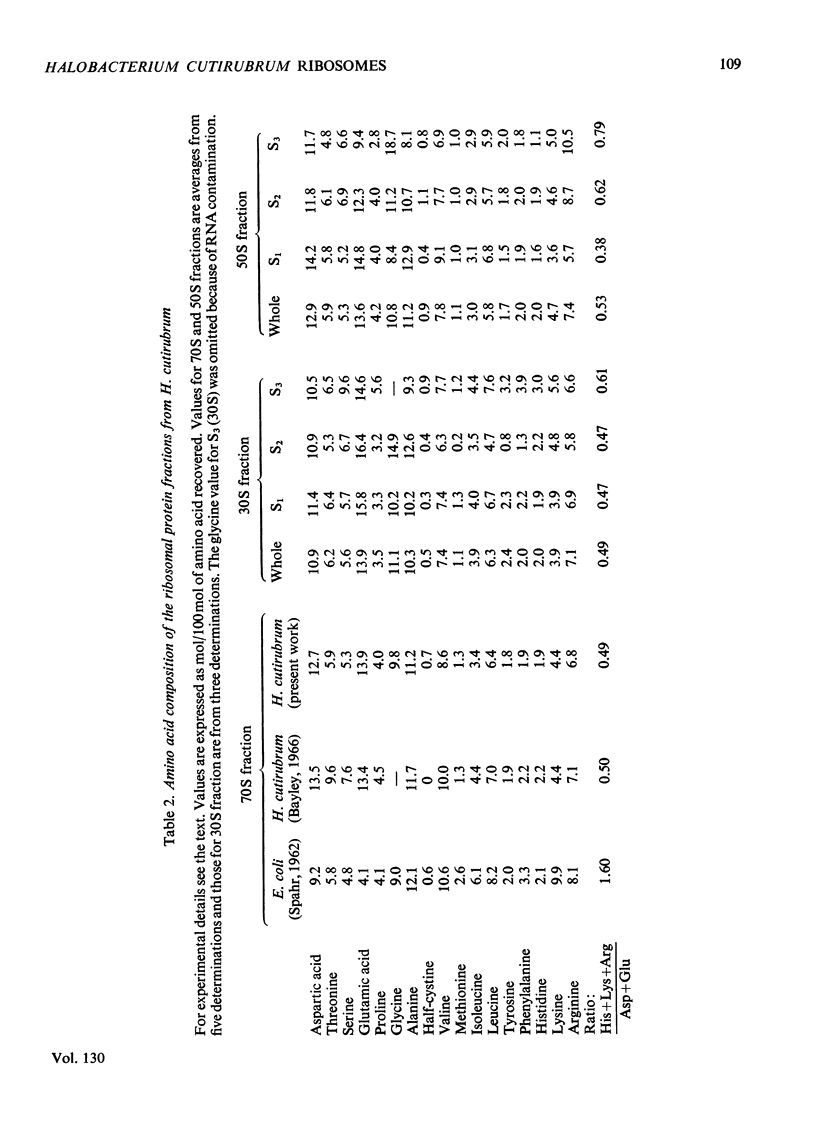
1. The 30S ribosomal subunit of the extreme halophile Halobacterium cutirubrum is unstable and loses 75% of its ribosomal protein when the 70S ribosome is dissociated into the two subunits. A stable 30S subunit is obtained if the dissociation of the 70S particle is carried out in the presence of the soluble fraction. 2. A fractionation procedure was developed for the selective removal of groups of proteins from the 30S and 50S subunits. When the ribosomes, which are stable in 4m-K+ and 0.1m-Mg2+, were extracted with low-ionic-strength buffer 75–80% of the 30S proteins and 60–65% of the 50S proteins as well as the 5S rRNA were released. The proteins in this fraction are the most acidic of the H. cutirubrum ribosomal proteins. Further extraction with Li+–EDTA releases additional protein, leaving a core particle containing either 16S rRNA or 23S rRNA and about 5% of the total ribosomal protein. The amino acid composition, mobility on polyacrylamide gels at pH4.5 and 8.7, and the molecular-weight distribution of the various protein fractions were determined. 3. The s values of the rRNA are 5S, 16S and 23S. The C+G contents of the 16S and 23S rRNA were 56.1 and 58.8% respectively and these are higher than C+G contents of the corresponding Escherichia coli rRNA (53.8 and 54.1%).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAXTER R. M., GIBBONS N. E. Effects of sodium and potassium chloride on certain enzymes of Micrococcus halodenitrificans and Pseudomonas salinaria. Can J Microbiol. 1956 Oct;2(6):599–606. doi: 10.1139/m56-072. [DOI] [PubMed] [Google Scholar]
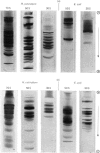
- BAYLEY S. T., KUSHNER D. J. THE RIBOSOMES OF THE EXTREMELY HALOPHILIC BACTERIUM, HALOBACTERIUM CUTIRUBRUM. J Mol Biol. 1964 Sep;9:654–669. doi: 10.1016/s0022-2836(64)80173-x. [DOI] [PubMed] [Google Scholar]
- Bayley S. T. Composition of ribosomes of an extremely halophilic bacterium. J Mol Biol. 1966 Feb;15(2):420–427. doi: 10.1016/s0022-2836(66)80117-1. [DOI] [PubMed] [Google Scholar]
- Bayley S. T., Griffiths E. A cell-free amino acid incorporating system from an extremely halophilic bacterium. Biochemistry. 1968 Jun;7(6):2249–2256. doi: 10.1021/bi00846a030. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN J. H., WALTHO J. A. Solute concentrations within cells of halophilic and non-halophilic bacteria. Biochim Biophys Acta. 1962 Dec 17;65:506–508. doi: 10.1016/0006-3002(62)90453-5. [DOI] [PubMed] [Google Scholar]
- Ducharme L., Matheson A. T., Yaguchi M., Visentin L. P. Utilization of amino acids by Halobacterium cutirubrum in chemically defined medium. Can J Microbiol. 1972 Aug;18(8):1349–1351. doi: 10.1139/m72-207. [DOI] [PubMed] [Google Scholar]
- Itoh T., Otaka E., Osawa S. Release of ribosomal proteins from Escherichia coli ribosomes with high concentrations of lithium chloride. J Mol Biol. 1968 Apr 14;33(1):109–122. doi: 10.1016/0022-2836(68)90284-2. [DOI] [PubMed] [Google Scholar]
- Knowland J., Miller L. Reduction of ribosomal RNA synthesis and ribosomal RNA genes in a mutant of Xenopus laevis which organizes only a partial nucleolus. I. Ribosomal RNA synthesis in embryos of different nucleolar types. J Mol Biol. 1970 Nov 14;53(3):321–328. doi: 10.1016/0022-2836(70)90068-9. [DOI] [PubMed] [Google Scholar]
- LEBOY P. S., COX E. C., FLAKS J. G. THE CHROMOSOMAL SITE SPECIFYING A RIBOSOMAL PROTEIN IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Dec;52:1367–1374. doi: 10.1073/pnas.52.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ONISHI H., MCCANCE E., GIBBONS N. E. A SYNTHETIC MEDIUM FOR EXTREMELY HALOPHILIC BACTERIA. Can J Microbiol. 1965 Apr;11:365–373. doi: 10.1139/m65-044. [DOI] [PubMed] [Google Scholar]
- Rauser W. E., Bayley S. T. Ribosomal complexes from an extremely halophilic bacterium and the role of cations. J Bacteriol. 1968 Oct;96(4):1304–1313. doi: 10.1128/jb.96.4.1304-1313.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEHGAL S. N., GIBBONS N. E. Effect of some metal ions on the growth of Halobacterium cutirubrum. Can J Microbiol. 1960 Apr;6:165–169. doi: 10.1139/m60-018. [DOI] [PubMed] [Google Scholar]
- SPAHR P. F. Amino acid composition of ribosomes from Escherichia coli. J Mol Biol. 1962 May;4:395–406. doi: 10.1016/s0022-2836(62)80020-5. [DOI] [PubMed] [Google Scholar]
- Sendecki W., Mikulik K., Matheson A. T. Some properties of ribosomes from Myxobacter 495. Can J Biochem. 1971 Dec;49(12):1333–1339. doi: 10.1139/o71-193. [DOI] [PubMed] [Google Scholar]
- WALLER J. P. FRACTIONATION OF THE RIBOSOMAL PROTEIN FROM ESCHERICHIA COLI. J Mol Biol. 1964 Nov;10:319–336. doi: 10.1016/s0022-2836(64)80050-4. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]