Abstract
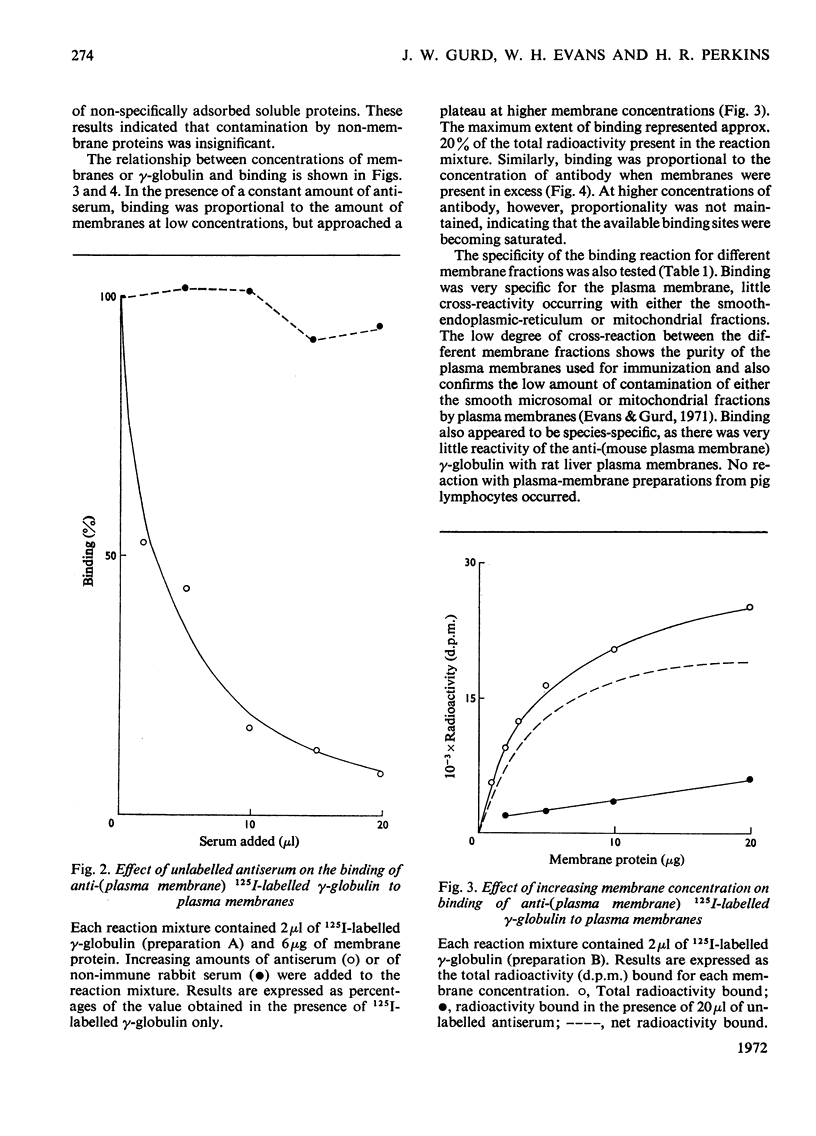
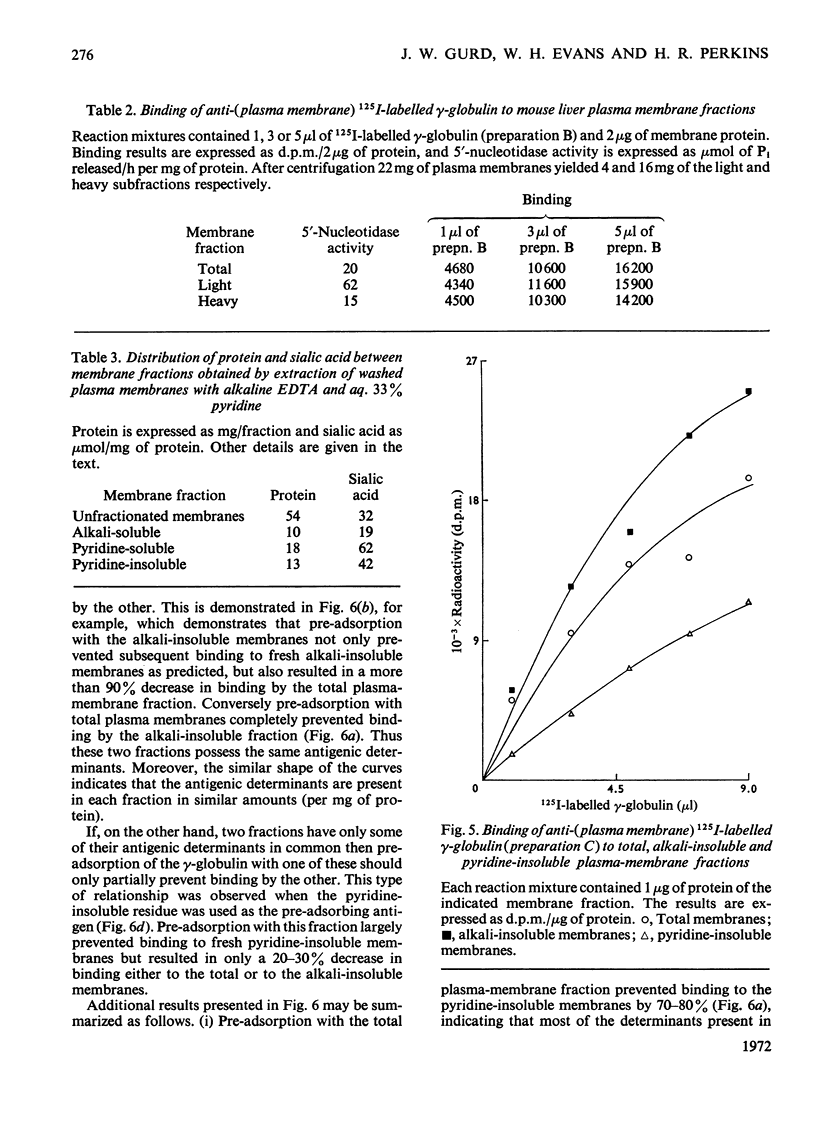
1. Antiserum to purified mouse liver plasma membranes was prepared and the partially purified γ-globulin antibody fraction was iodinated with 125I. The reaction of the 125I-labelled γ-globulin antibody with isolated mouse liver plasma membranes was studied. 2. The γglobulin antibody bound specifically to mouse liver plasma membranes and there was little reaction with mouse liver intracellular membranes or with surface-membrane fractions from either rat liver or pig lymphocytes. 3. `Light' and `heavy' mouse liver plasma-membrane subfractions bound similar amounts of γ-globulin antibody, and this is consistent with a surface origin for the light fraction. 5. Plasma membranes were fractionated by sequential extraction with 50mm-NaHCO3–Na2CO3 buffer, pH10.2, containing 10mm-EDTA and aq. 33% (v/v) pyridine. The alkali-soluble and -insoluble fractions and the pyridine-soluble and -insoluble fractions all reacted with the antiserum, and the cross-reactivity among the various fractions and with the total plasma membranes was investigated. 5. The results are discussed in terms of the arrangement of the antigenic determinants within the membrane.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan D., Crumpton M. J. Preparation and characterization of the plasma membrane of pig lymphocytes. Biochem J. 1970 Nov;120(1):133–143. doi: 10.1042/bj1200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C. Sulfanilic acid diazonium salt: a label for the outside of the human erythrocyte membrane. Biochim Biophys Acta. 1969 Jun 3;183(1):65–78. doi: 10.1016/0005-2736(69)90130-8. [DOI] [PubMed] [Google Scholar]
- Blomberg F., Perlmann P. Immunochemical characterization of enzyme activities in liver plasma membranes of the rat. Exp Cell Res. 1971 May;66(1):104–112. doi: 10.1016/s0014-4827(71)80017-4. [DOI] [PubMed] [Google Scholar]
- Boyse E. A., Old L. J., Stockert E. An approach to the mapping of antigens on the cell surface. Proc Natl Acad Sci U S A. 1968 Jul;60(3):886–893. doi: 10.1073/pnas.60.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. A major protein which spans the human erythrocyte membrane. J Mol Biol. 1971 Jul 28;59(2):351–357. doi: 10.1016/0022-2836(71)90055-6. [DOI] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton M. J., Parkhouse R. M.E. Comparison of the effects of various detergents on antigen-antibody interaction. FEBS Lett. 1972 May 1;22(2):210–212. doi: 10.1016/0014-5793(72)80047-4. [DOI] [PubMed] [Google Scholar]
- Crumpton M. J., Wilkinson J. M. The immunological activity of some of the chymotryptic peptides of sperm-whale myoglobin. Biochem J. 1965 Mar;94(3):545–556. doi: 10.1042/bj0940545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker K., Bischoff E. Purification and properties of nucleotide pyrophosphatase from rat liver plasma membranes. FEBS Lett. 1972 Mar;21(1):95–98. doi: 10.1016/0014-5793(72)80172-8. [DOI] [PubMed] [Google Scholar]
- Evans W. H., Bruning J. W. Studies on the distribution of some histocompatibility antigens in mouse liver plasma membrane and microsomal fractions. Immunology. 1970 Nov;19(5):735–741. [PMC free article] [PubMed] [Google Scholar]
- Evans W. H. Fractionation of liver plasma membranes prepared by zonal centrifugation. Biochem J. 1970 Mar;116(5):833–842. doi: 10.1042/bj1160833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H. Glycoproteins of mouse liver smooth microsomal and plasma membrane fractions. Biochim Biophys Acta. 1970 Sep 15;211(3):578–581. doi: 10.1016/0005-2736(70)90264-6. [DOI] [PubMed] [Google Scholar]
- Evans W. H., Gurd J. W. Biosynthesis of liver membranes. Incorporation of ( 3 H)leucine into proteins and of ( 14 C)glucosamine into proteins and lipids of liver microsomal and plasma-membrane fractions. Biochem J. 1971 Nov;125(2):615–624. doi: 10.1042/bj1250615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H. Subfractionation of rat liver plasma membranes. FEBS Lett. 1969 Jun;3(4):237–241. doi: 10.1016/0014-5793(69)80146-8. [DOI] [PubMed] [Google Scholar]
- Gurd J. W., Evans W. H., Perkins H. R. Chemical characterization of the proteins and glycoproteins of mouse liver plasma membranes solubilized by sequential extraction with aqueous and organic solvents. Biochem J. 1972 Feb;126(3):459–466. doi: 10.1042/bj1260459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. I., Teather C., Andrews H. Organizational difference of cell surface "hematoside" in normal and virally transformed cells. Biochem Biophys Res Commun. 1968 Nov 25;33(4):563–568. doi: 10.1016/0006-291x(68)90332-x. [DOI] [PubMed] [Google Scholar]
- Kahane I., Razin S. Immunological analysis of Mycoplasma membranes. J Bacteriol. 1969 Oct;100(1):187–194. doi: 10.1128/jb.100.1.187-194.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUCY J. A. GLOBULAR LIPID MICELLES AND CELL MEMBRANES. J Theor Biol. 1964 Sep;7:360–373. doi: 10.1016/0022-5193(64)90080-3. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Permeability of membrane junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):441–472. doi: 10.1111/j.1749-6632.1966.tb50175.x. [DOI] [PubMed] [Google Scholar]
- MADDY A. H. A FLUORESCENT LABEL FOR THE OUTER COMPONENTS OF THE PLASMA MEMBRANE. Biochim Biophys Acta. 1964 Sep 25;88:390–399. doi: 10.1016/0926-6577(64)90194-9. [DOI] [PubMed] [Google Scholar]
- Makita A., Seyama Y. Alterations of Forssman-antigenic reactivity and of monosaccharide composition in plasma membrane from polyoma-transformed hamster cells. Biochim Biophys Acta. 1971 Aug 13;241(2):403–411. doi: 10.1016/0005-2736(71)90040-x. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Steers E., Jr Selective solubilization of a protein component of the red cell membrane. Science. 1968 Jan 12;159(3811):203–204. doi: 10.1126/science.159.3811.203. [DOI] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Hawthorne J. N. The site of diphosphoinositide synthesis in rat liver. Biochem Biophys Res Commun. 1965 Nov 22;21(4):333–338. doi: 10.1016/0006-291x(65)90198-1. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Marchesi V. T., Singer S. J. The localization of spectrin on the inner surface of human red blood cell membranes by ferritin-conjugated antibodies. J Cell Biol. 1971 Oct;51(1):265–272. doi: 10.1083/jcb.51.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons D. F., Subjeck J. R. The morphology of the polysaccharide coat of mammalian cells. Biochim Biophys Acta. 1972 Feb 14;265(1):85–113. doi: 10.1016/0304-4157(72)90020-2. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. Exposed protein on the intact human erythrocyte. Biochemistry. 1971 May 11;10(10):1766–1771. doi: 10.1021/bi00786a006. [DOI] [PubMed] [Google Scholar]
- Schneider H., Smith I. C. A study of the structural integrity of spin-labelled proteins in some fractions of human erythrocyte ghosts. Biochim Biophys Acta. 1970;219(1):73–80. doi: 10.1016/0005-2736(70)90062-3. [DOI] [PubMed] [Google Scholar]
- Sheffield J. B., Emmelot P. Studies on plasma membranes. XVI. Tissue specific antigens in the liver cell surface. Exp Cell Res. 1972 Mar;71(1):97–105. doi: 10.1016/0014-4827(72)90268-6. [DOI] [PubMed] [Google Scholar]
- Simon F. R., Blumenfeld O. O., Arias I. M. WTwo protein fractions obtained from hepatic plasma membranes. Studies of their composition and differential turnover. Biochim Biophys Acta. 1970 Dec 1;219(2):349–360. doi: 10.1016/0005-2736(70)90212-9. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Wallach D. F. The dispositions of proteins in the plasma membranes of animal cells: analytical approaches using controlled peptidolysis and protein labels. Biochim Biophys Acta. 1972 Feb 14;265(1):61–83. doi: 10.1016/0304-4157(72)90019-6. [DOI] [PubMed] [Google Scholar]
- Yariv J., Kalb A. J., Katchalski E., Goldman R., Thomas E. W. Two locations of the lac permease sulphydryl in the membrane of E. coli. FEBS Lett. 1969 Nov 12;5(3):173–176. doi: 10.1016/0014-5793(69)80324-8. [DOI] [PubMed] [Google Scholar]
- Zahler W. L., Fleischer B., Fleischer S. Gel electrophoresis patterns of the proteins of organelles isolated from bovine liver. Biochim Biophys Acta. 1970 Apr 21;203(2):283–290. doi: 10.1016/0005-2736(70)90142-2. [DOI] [PubMed] [Google Scholar]


