Abstract
The effects of herbivores on plant production and fitness may not relate directly to the quantity of biomass removed because folivory may alter photosynthetic rates at a considerable distance from the damaged tissue [Welter, S. C. (1989) in Insect-Plant Interactions, ed. Bernays, E. A. (CRC, Boca Raton), pp. 135–151.]. An impediment to understanding the effects of leaf damage on photosynthesis has been an inability to map photosynthetic function within a single leaf. We developed an instrument for imaging chlorophyll fluorescence and used it to map the effects of caterpillar feeding on whole-leaf photosynthesis in wild parsnip. The adverse effects of caterpillar feeding on photosynthesis were found to extend well beyond the areas of the leaflet in which caterpillars removed tissue. These “indirectly” affected areas remained impaired for at least 3 days after the caterpillars were removed and were six times as large as the area directly damaged by the caterpillars. Although photosynthesis in indirectly affected areas was reduced and not eliminated, these areas accounted for three times as much of the overall reduction in photosynthesis as the area removed by the caterpillars. The size of the indirect effects was positively correlated with defense-related synthesis of furanocoumarins, suggesting that costs of chemical defense may be one factor that accounts for the indirect effects of herbivory on plants.
In average years, net primary productivity removed by herbivores ranges from 2 to 15% in forests and from 4 to 24% in oldfields and grasslands (1). However, impacts on individual plants and plant communities are not readily predicted by the amounts of tissue taken by herbivores. Such unpredictability results from a variety of phenomena, including distribution of the damage within a plant (concentrated in one area or dispersed; ref. 2), specific location (e.g., proximity to a vein; ref. 3), induced production of allelochemicals that can be costly to produce (4), and the potential for compensatory increases of photosynthetic rates in intact leaf tissue (5).
Many of the phenomena that mediate the effects of damage on photosynthesis have been undetectable to researchers because measurement of small areas of leaves by conventional gas exchange methods has been technically impossible. We developed an instrument that spatially maps chlorophyll fluorescence of intact leaves and extends the capabilities of previous instruments by providing a complete fluorescence-quenching analysis (6), imaging of larger areas of leaves (4–6 cm2), and simultaneous gas-exchange measurements. In this study, we produced high-resolution images of the quantum efficiency of photosystem II (F′q/F′m). The pixel values are directly related to the rate of electron transfer through the photosystems (7) and are highly correlated with the rate of carbon dioxide assimilation (8). We used this instrument to examine the effects of caterpillar damage in wild parsnip (Pastinaca sativa), a weedy species of European origin that occurs widely in the northeastern United States. When foliage of this species is damaged, furanocoumarins, compounds with biocidal activity against a wide range of organisms (9), accumulate rapidly and in abundance. The induction is localized and is often limited within the compound leaves to the leaflet that is damaged (10). Moreover, furanocoumarin production is costly, both in terms of seed production (11) and increased respiration (12).
Methods
In the vegetative state, wild parsnips are rosettes with several compound leaves. Previous studies have shown that sister leaflets—leaflets located across from each other along the midvein of the leaf—are physiologically and chemically equivalent to one another and are independent of one another in terms of responses to damage, i.e., damage to one leaflet does not affect its sister leaflet (10, 12). Plants were grown in a greenhouse under long days (16 hr light/8 hr dark) at 27°C from seeds collected in a wild population in Champaign County, IL. When each plant had produced several large compound leaves, a leaflet subtending the terminal leaflet of a recently expanded leaf was selected to be damaged by a caterpillar, and its “sister” leaflet was designated the undamaged control. Ten pairs of leaflets in all were included in this study. A small clip cage (internal diameter of 3 cm with nylon screening on top and bottom) was affixed to each control and damaged leaflet. A single ultimate instar cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae), which had been reared in the laboratory on wild parsnip plants, was placed inside the cage on the underside of each leaflet to be damaged. The larvae were permitted to feed for 24 h before the first photosynthesis measurement, at which time cages and larvae were removed.
Photosynthesis of control and damaged leaflets was measured while they were still attached to the plant. Leaflets were allowed to equilibrate for 30 min before measurement at an irradiance of 350 μmol m−2⋅s−1 (photosynthetic flux density, 400–700 nm). Fluorescence and gas exchange were measured simultaneously for a 6-cm2 area by inserting a portion of the leaflet into a cuvette that was situated in the imaging instrument and connected to a Li-Cor 6400 (Li-Cor, Lincoln, NE) gas-exchange system. Illumination of the leaf surface was provided by a combination of 1,200 red and blue LEDs that deliver measuring pulses, an over-saturation pulse, or continuous actinic light. Fluorescence yield was quantified during short (typically <100 μsec), weak (<1% actinic) pulses of blue light. The fluorescence image was captured by a JAI (JAI, Laguna Hills, CA) progressive scan charge-coupled device camera (659 × 494, 8-bit, 10 Hz). Instrument parameters (timing, intensities, LED color ratio, shuttering times) were adjusted independently to optimize measurements. After the measurement in the light, dark respiration was measured for a subset of plants by gas exchange. Three of these plants also were measured at 24 h and 72 h after removal of the larvae to assess the persistence of effects of damage on intact tissues. Control of the instrument, image capture, manipulation, analysis, and presentation were executed with customized software (13, 14). The underlying assumption of the fluorescence imaging method is that the flux of electrons passing through the photosystems results in carbon fixation. This assumption was confirmed for wild parsnip by comparing gas-exchange measurements with fluorescence-based measurements at several CO2 levels in a low O2 (2%) environment that suppresses photorespiration (correlation between measures was 0.979).
All images were analyzed with SCION IMAGE for Windows software (BETA VERSION 4.02, Scion, Frederick, MD). This software allows the user to select regions within an image that fall within a range of pixel values, and it calculates the average pixel value and area for the selection. Average pixel values were then converted to original F′q/F′m values by a linear calibration curve. For each plant, an average photosynthetic rate was determined from the image of the control leaflet. The damaged leaflet then was analyzed by using the software's density-slice tool to select and measure areas of the leaflet with average rates of photosynthesis that were the same as the undamaged control leaves; these areas were not affected by the damage. The same tool was used to isolate and measure the area of the leaflet directly damaged (areas in which tissue was removed and, hence, had no photosynthesis). The remaining area that was measured was intact but exhibited reduced rates of photosynthesis relative to the control leaflet; this area consisted of tissues that were indirectly affected by caterpillar damage.
We compared actual reductions in net photosynthesis measured by gas exchange with predicted reductions based on fluorescence imaging of directly and indirectly affected areas as well as unaffected areas. Average photosynthesis of the damaged leaflet was calculated by multiplying the areas of the leaf that were directly affected, indirectly affected, and unaffected by their respective assimilation rates, summing these products, and then dividing this sum by the total measured area.
Also, we quantified the effects of insect damage on furanocoumarin accumulation. The control and damaged leaflets of each of the remaining seven plants were excised and, as mirror images of one another, were placed one on top of the other with top surfaces facing. All of the tissues that seemed to be damaged, including a 1-mm margin of intact tissue, were carefully removed with a scalpel. Thus, we were able to compare furanocoumarin accumulation in the damaged area of the treatment leaflet with that in an identical area of the sister control leaflet. These samples, as well as the remaining intact areas of control and damaged leaflets, were oven-dried at 50°C for 24 h, ground to a fine powder with a glass rod inside of a 1.5-ml centrifuge tube, and extracted with 500 μl ethyl acetate for 2 h. Each extract (10 μl) was analyzed for furanocoumarin content by HPLC (12).
All statistical tests, except correlations, involved paired t tests between values in the control leaflet and the damaged leaflet. All tests were two-tailed, except for a single, one-tailed paired t test involving dark respiration, which we knew from previous work (12) would increase with damage.
Results
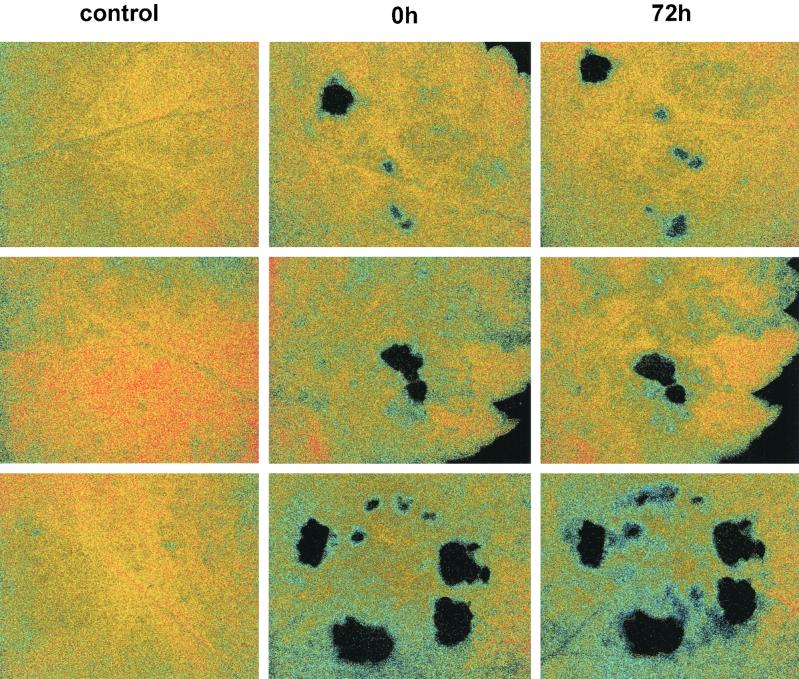
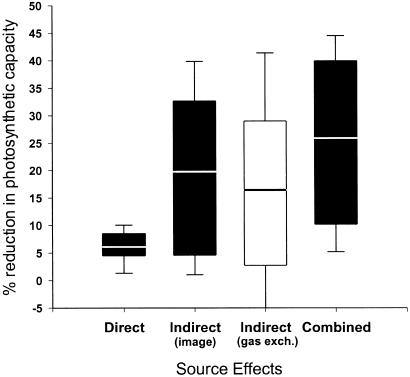
A single caterpillar feeding for 24 h significantly decreased the photosynthetic activity of remaining intact tissue as measured both by conventional gas exchange (paired t test, t = 3.26, d.f. = 9, P = 0.010) and fluorescence imaging (paired t test, t = 2.58, d.f. = 9, P = 0.029). Additionally, the imaging method revealed the existence of large patches of photosynthetically depressed cells extending well beyond the area consumed by the caterpillar (Fig. 1). The size of these affected areas varied among the 10 plants analyzed, with some plants exhibiting little or no appreciable indirect effects and others exhibiting effects extending throughout the leaflet. These indirect effects were manifested within 24 h after placement of the larvae on the leaflets and, in some of the plants, persisted unchanged for 3 days after removal of the caterpillars. On average, indirectly affected area (mean = 242 mm2) was six times that of the area directly affected by tissue removal (mean = 41 mm2, paired t = 3.02, d.f. = 9, P = 0.015). Because the indirectly affected area remained photosynthetically active, but at a reduced rate, its contribution to the observed reductions in photosynthesis was three, not six, times that of the direct effect (Fig. 2, paired t = 2.536, d.f. = 9, P = 0.032). Thus, estimates of herbivore effects on photosynthesis based solely on leaf area removed could underestimate these impacts by as much as 3-fold.
Figure 1.
Patterns of photosynthesis in control leaflets and paired leaflets that were damaged by caterpillars. The three pairs of control and damaged leaflets are arranged from top to bottom. The measurements at times 0 and 72 h postfeeding by the caterpillar are shown only for the damaged leaflet. The false coloring in this image corresponds to electron fluxes through photosystem II (Genty efficiency, F′q/F′m) as follows: red = 0.55, yellow = 0.35, blue = 0.15, black = 0.
Figure 2.
Magnitudes of direct, indirect, and total effects of caterpillar damage on suppression of photosynthesis in wild parsnip foliage. The black bars depict data obtained by fluorescence imaging, and the white bar depicts data from gas exchange. The area of leaflet measured in all cases was 6 cm2. The line inside each bar is the mean, the ends of the bars show the 10th and 90th percentiles, and the whiskers show the 5th and 95th percentiles. (n = 10.)
The induction of furanocoumarin synthesis may have contributed to the indirect suppression of photosynthesis. Furanocoumarin content increased nearly 8-fold in the damaged tissues (paired t = 3.181, d.f. = 6, P = 0.019) and, consistent with a previous study (12), was accompanied by 45% higher respiration rates (paired one-tailed t = 2.222, d.f. = 5, P = 0.0385). Moreover, the area indirectly affected by damage was positively correlated (r = 0.813, P = 0.026) with the increase in furanocoumarin content of the entire damaged leaflet (Fig. 3).
Figure 3.
Relationship between the area of wild parsnip leaflets that was indirectly affected by caterpillar damage and the increase in defense-related furanocoumarins in those leaflets.
Fluorescence imaging holds great potential for the study of the effects of herbivores on plant photosynthesis. Although the estimates of the indirect effects of damage obtained by fluorescence imaging can be obtained by gas exchange (Fig. 2), fluorescence imaging provides detailed spatial information that is unattainable by conventional methods. Such fine-scale mapping (in the case of wild parsnips, resolution was 70 cells per pixel) allows visualization of the topography of indirect effects with reference to anatomical landmarks that may provide insights into the biochemical mechanisms underlying these effects.
Interactions between plants and herbivores involve over half of all terrestrial species, and, by affecting plant growth and fitness, herbivory plays a critical role in determining community structure and ecosystem function. Traditional methods of assessing folivore damage as leaf area removed at any given time have fallen under sharp criticism because they may underestimate total leaf loss over a season by as much 2.6-fold (15, 16). In species such as wild parsnip, a similar magnitude of error will result if the indirect effects of tissue removal on remaining tissue are ignored. The method that we have developed thus can be of great value in generating more accurate estimates of the total impact of herbivory. These in turn are needed to make more robust predictions about the effects of nutrient enrichment on populations, to anticipate consequences of global climate change on community structure and composition, to devise appropriate pest management strategies, and to provide a greater mechanistic understanding of the basic ecology of interactions between plants and the insects that consume them (17).
Acknowledgments
We thank Dr. Gabriel Cornic for technical advice on the procedure for verifying the equivalence gas exchange and fluorescence measures and Drs. Daniel Herms and Anurag Agrawal for thoughtful comments on the manuscript. This project was funded as a Critical Research Initiative by the University of Illinois at Urbana-Champaign and by the National Science Foundation (DEB 9903867).
References
- 1.Cyr H, Pace M L. Nature (London) 1993;361:148–150. [Google Scholar]
- 2.Marquis R J. Ecology. 1992;73:143–152. [Google Scholar]
- 3.Oleksyn J, Karolewsdi P, Giertych M J, Zytkowiak R, Reich P B, Tjoelker M G. New Phytol. 1998;140:239–249. doi: 10.1046/j.1469-8137.1998.00270.x. [DOI] [PubMed] [Google Scholar]
- 4.Karban R, Baldwin I T. Induced Responses to Herbivory. Chicago: Univ. of Chicago Press; 1997. [Google Scholar]
- 5.Welter S C. In: Insect-Plant Interactions. Bernays E A, editor. Boca Raton: CRC; 1989. pp. 135–151. [Google Scholar]
- 6.Krause G H, Weis E. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:313–349. [Google Scholar]
- 7.Genty B, Briantais J-M, Baker W R. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- 8.Krall P J, Edwards B E. Plant Physiol. 1992;86:180–187. [Google Scholar]
- 9.Berenbaum M R, Zangerl A R. In: Phytochemical Redundancy in Ecological Interactions. Romeo J T, Saunders J A, Barbosa P, editors. New York: Plenum; 1996. pp. 1–24. [Google Scholar]
- 10.Zangerl A R, Berenbaum M R. Chemoecology. 1994/1995;5/6:37–42. [Google Scholar]
- 11.Zangerl A R, Berenbaum M R. Am Nat. 1997;150:491–504. doi: 10.1086/286077. [DOI] [PubMed] [Google Scholar]
- 12.Zangerl A R, Arntz M A, Berenbaum M R. Oecologia. 1997;109:433–441. doi: 10.1007/s004420050103. [DOI] [PubMed] [Google Scholar]
- 13.Oxborough K, Baker N R. Plant Cell Environ. 1997;20:1473–1483. [Google Scholar]
- 14.Baker N R, Oxborough K, Lawson T, Morison J I L. J Exp Bot. 2001;52:615–621. [PubMed] [Google Scholar]
- 15.Lowman M D. Biotropica. 1984;16:264–268. [Google Scholar]
- 16.Sand-Jensen K, Jacobsen D. Oikos. 1994;69:545–549. [Google Scholar]
- 17.Trlica M J, Rittenhouse L R. Ecol Appl. 1993;3:21–23. doi: 10.2307/1941783. [DOI] [PubMed] [Google Scholar]