Abstract
Background
Prostaglandin E2 (PGE2) has multiple physiologic roles mediated by G protein coupled receptors designated E-prostanoid, or "EP" receptors. Evidence supports an important role for the EP2 receptor in regulating fertility, vascular tone and renal function.
Results
The full-length rabbit EP2 receptor cDNA was cloned. The encoded polypeptide contains 361 amino acid residues with seven hydrophobic domains. COS-1 cells expressing the cloned rabbit EP2 exhibited specific [3H]PGE2 binding with a Kd of 19.1± 1.7 nM. [3H]PGE2 was displaced by unlabeled ligands in the following order: PGE2>>PGD2=PGF2α=iloprost. Binding of [3H]PGE2 was also displaced by EP receptor subtype selective agonists with a rank order of affinity consistent with the EP2 receptor (butaprost>AH13205>misoprostol>sulprostone). Butaprost free acid produced a concentration-dependent increase in cAMP accumulation in rabbit EP2 transfected COS-1 cells with a half-maximal effective concentration of 480 nM. RNase protection assay revealed high expression in the ileum, spleen, and liver with lower expression in the kidney, lung, heart, uterus, adrenal gland and skeletal muscle. In situ hybridization localized EP2 mRNA to the uterine endometrium, but showed no distinct localization in the kidney. EP2 mRNA expression along the nephron was determined by RT-PCR and its expression was present in glomeruli, MCD, tDL and CCD. In cultured cells EP2 receptor was not detected in collecting ducts but was detected in renal interstitial cells and vascular smooth muscle cells. EP2 mRNA was also detected in arteries, veins, and preglomerular vessels of the kidney.
Conclusion
EP2 expression pattern is consistent with the known functional roles for cAMP coupled PGE2 effects in reproductive and vascular tissues and renal interstitial cells. It remains uncertain whether it is also expressed in renal tubules.
Background
Prostaglandin E2 is a major cyclooxygenase metabolite of arachidonic acid which exerts diverse effects on vascular smooth muscle tone and epithelial solute transport via G-protein coupled receptors [1,2]. At least four distinct G-protein coupled PGE2 receptors have been cloned: the EP1, EP2, EP3, and EP4[1]. Pharmacological studies suggest PGE2 relaxes vascular smooth muscle through two of these receptors, the EP2 and EP4 receptors, which couple to cyclic-AMP generation [3]. In contrast, PGE2 constricts other vascular smooth muscle through EP3 receptors which inhibit cAMP generation via Gi or EP1 receptors which increase Ca2+ mobilization [3]. Although both EP2 and EP4 receptors relax smooth muscle, EP2 receptors are uniquely sensitive to the agonists butaprost and AH13205 [3,4]. Previous studies have shown that PGE2 increases the cAMP concentration in many tissues including kidney, intestine and uterus, but the relative contribution of EP2 versus EP4 receptors to these effects is incompletely defined [5,6]. Recent studies in mice with targeted disruption of these receptors have suggested an important role for EP2 receptor in regulating systemic hemodynamics. Deficiency of the EP2 receptor is associated with a defect in counteracting vasopressor effect of PGE2 [7] and the development of salt sensitive hypertension [8], suggesting a predominant normal vasodepressor role of EP2 receptor in blood pressure regulation.
The vasodepressor action of PGE2 may also play an important role in the kidney. It has been previously shown that PGE2 not only directly dilates renal arteries [9-11] but also attenuates the AngII-induced increase in renal vascular resistance [12] through inhibition of AngII-elicited intracellular Ca++ increase in vascular smooth muscle cells of preglomerular vasculature [13]. Based on the fact that PGE2 stimulates cAMP generation in freshly isolated preglomerular renal arterioles [14], the cAMP-stimulating EP2 or EP4 receptor has been thought to mediate these effects of PGE2 on renal resistance vessels.
There is also evidence suggesting that intrarenal EP2 and EP4 receptor activates cAMP-stimulated salt and water transport along the nephron [15-18]. Although the localization of EP1, EP3 and EP4 has been determined in human kidney by in situ hybridization, segmental distribution of cAMP-coupled EP2 receptor along the nephron was not detected by this method [19]. To more clearly define the physiologic role of this cAMP coupled EP receptor, we cloned the rabbit EP2 receptor, characterized its pharmacology and signaling. These studies also defined the tissue distribution with a focus on vascular, intrarenal and reproductive localization of the rabbit EP2 receptor mRNA.
Results
Receptor isolation
Screening of 5 × 105 plaques from a rabbit genomic library using a full-length human cDNA for the EP2 receptor resulted in isolation of two independent clones designated 4B1 (13 kb) and 6A (11 kb). A 5' coding exon of EP2 receptor was contained in clone 4B1, while a 3' coding exon was contained in clone 6A. Sequence data indicated that the entire coding region was present in these two exons. A potential translation initiation codon in the NotI/BamHI fragment (3 kb) of clone 4B1 starts an open reading frame that encodes a polypeptide sequence highly homologous to the first six transmembrane domains of the human EP2 receptor [20]. The amino acid sequence of the open reading frame in the NotI fragment (6 kb) of clone 6A was homologous to the seventh transmembrane domain (TMD VII), followed by a short C-terminal tail and an in-frame stop codon. An intron with a donor and acceptor splice site was located following the coding region corresponding to the sixth transmembrane domain of the protein.
A functional cDNA clone was obtained by screening a rabbit uterus cDNA library using a 700 bp AvaII cDNA fragment from clone 4B1. Two types of positive overlapping clones (18A and REP2-2.7) were isolated. Of the 18 positive cDNA clones isolated, 8 belonged to the type designated REP2-18A, and 10 clones belonged to the type designated REP2-2.7. Sequence analysis indicated that these two cDNAs possess an identical open reading frame and 1,664 bp of down-stream 3' untranslated region (3'UTR), however clone REP2-18A possesses an 467 additional base pairs in the 3'UTR compared with REP2-2.7. It is also noted that there are 6 and 7 AUUUA motifs in 3' UTR of REP2-2.7 and REP2-18A, respectively, suggesting an instability of rabbit EP2 receptor mRNA in vivo.
Because both rabbit EP2 cDNA clones, 18A and REP2-2.7, lacked ~50 codons corresponding to the 5' end of the human EP2 receptor and which were present in the rabbit genomic sequence, a full-length sequence of the rabbit EP2 was constructed by ligating a SacII/XhoI cDNA fragment (nucleotide 340–1655) from the cDNA clone 18A with the upstream Hind III/SacII fragment (nucleotide 1–340) of the rabbit genomic clone. This created an open reading frame of 1,083 base pairs with an in-frame TGA stop codon. The existence of this 5' UTR sequence in native cDNA was confirmed by RT-PCR on RNA isolated from rabbit uterus (data not shown). The predicted polypeptide is comprised of 361 amino acids with a calculated molecular weight of ~40 kDa. Its hydrophobic profile reveals the presence of seven hydrophobic stretches characteristic of G-protein-coupled receptors [20] (Fig. 1). Comparison of the deduced amino acid sequence of rabbit EP2 with human and mouse EP2 shows 90% and 82% sequence identity, respectively (Fig. 1).
Figure 1.
Alignment of the deduced amino acid sequence of rabbit EP2 (rabEP2) with human (hEP2) and mouse EP2 (mEP2). Underlined sequences denote transmembrane domains. Sequences were deduced from cDNA sequences and aligned using CLUSTALV program. (*) = identical sequence; (·) = conserved sequence. (-)= gaps in sequence alignment. (GenBank accession number (pending).
Ligand binding properties of the EP2 receptor
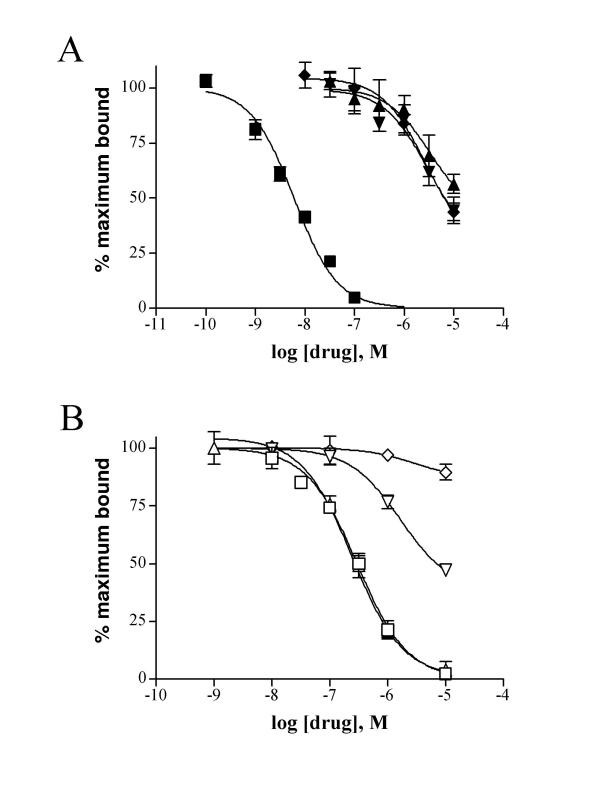
The putative rabbit EP2 receptor was pharmacologically characterized using membranes from COS-1 cells transiently transfected with a rabbit EP2 expression vector, pcDNA3/REP2. Membranes from EP2 receptor transfected COS-1 cells, exhibited saturable [3H]PGE2 binding with a dissociation constant of 19.1 ± 1.7 nM, and a maximum binding capacity (Bmax) of 2.8 pmol/mg of membrane protein. Radioligand binding studies were performed using various prostanoids and EP-receptor subtype-specific agonists to compete for PGE2 binding. Competition binding experiments revealed a rank order affinity consistent with an EP receptor: PGE2 >> iloprost = PGD2 = PGF2α (Fig. 2A). [3H]PGE2 binding was partially displaced by the EP2/3/4 agonist misoprostol and was not displaced by the EP1/3 agonist sulprostone (Fig. 2B). Furthermore, the EP2 selective agonists butaprost free acid and AH13205 competed for [3H]PGE2 binding with Ki values of 3–7 μM and 3–4 μM, respectively, suggesting this receptor is the rabbit EP2 receptor [20,21].
Figure 2.
Competition binding profile of the EP2 receptor expressed in COS-1 cells. Membranes were incubated with 1.5–2 nM [3H]PGE2 and varying concentrations of competitors. The data shown are from a single experiment performed in triplicate and are representative of three to four experiments. Panel A: ■ PGE2; ▼ PGF2a; ▲ PGD2; ◆ iloprost. Panel B: □ butaprost free acid; ?? AH13205; ▽ misoprostol; ◇ sulprostone.
Signal transduction properties of the EP2 receptor
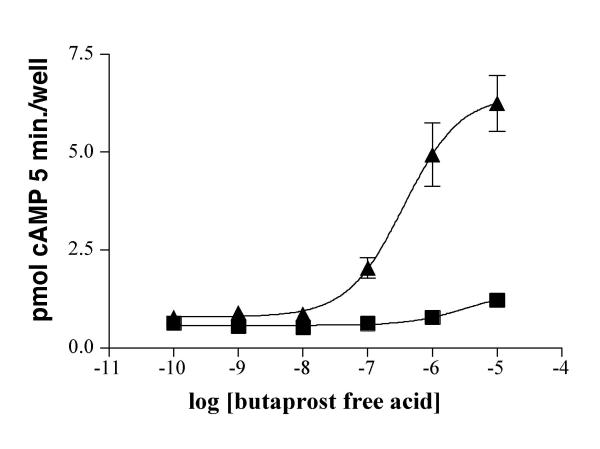
Incubation of EP2-transfected COS1 cells with butaprost free acid produced a concentration-dependent increase in cAMP accumulation with an EC50 of 480 nM (Fig. 3). Cells transfected with the vector alone demonstrated no significant increase in cAMP in response to butaprost free acid.
Figure 3.
Butaprost free acid mediated stimulation of intracellular cAMP production in COS-1 cells transfected with the rabbit EP2 receptor. Cells were incubated with various concentrations of PGE2 in the presence of IBMX for 5 minutes. The reactions were stopped with 10% TCA, and cell lysates were analyzed for cAMP content by a cAMP EIA kit. The data shown are from a single experiment and are representative of 3 independent experiments. ▲ rabbit EP2 transfected; ■ vector only transfected.
Tissue distribution of the rabbit EP2 receptor
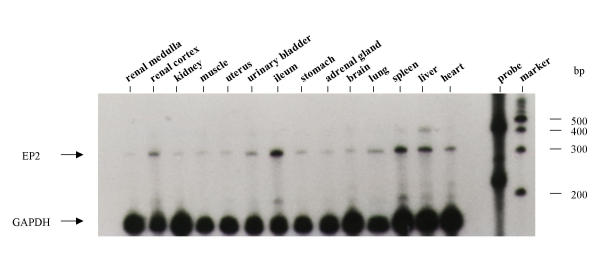
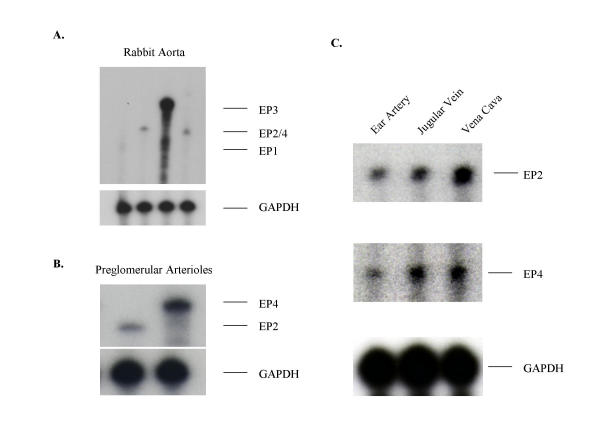
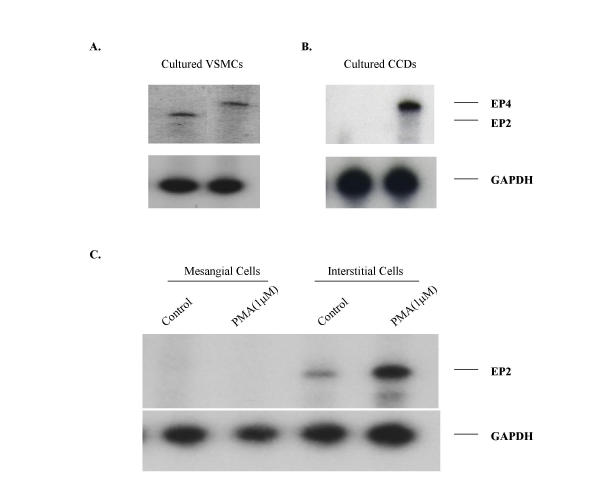
Solution hybridization/RNase protection assays with a radiolabeled antisense EP2 riboprobe resulted in a RNase-resistant fragment of the appropriate size (322 bp, Fig. 4). EP2 receptor mRNA was detected in several tissues including ileum, spleen, and liver. Lower expression was found in kidney, uterus, lung, heart, liver, adrenal gland, brain, urinary bladder and skeletal muscle. Furthermore, EP2 receptor mRNA was detected by RNase protection assay in all blood vessels examined (Fig. 5) including arteries and veins of large and small caliber (Fig. 5A &5C), and renal preglomerular vasculature (Fig. 5B). The expression level of the EP2 receptor mRNA in these vessels was comparable to that of the EP4 receptor (Fig. 5). EP2 receptor mRNA was also detected in vascular smooth muscle cells cultured from rabbit aorta (Fig. 6A).
Figure 4.
RNase protection assay demonstrating the distribution of EP2 and GAPDH mRNA in normal rabbit tissues. The protected fragments were electrophoretically separated on a 6% agarose/7 M urea gel. The film was exposed for 36 hours. A 100 bp ladder was used as a size marker. Densitometry was used to quantitate EP2 mRNA expression, RNA loading was corrected by normalizing to GAPDH mRNA expression.
Figure 5.
RNase protection assay demonstrating the distribution of EP1, EP2, EP3, EP4 and GAPDH mRNA in rabbit vascular tissues. The protected fragments were electrophoretically separated on a 6% agarose/7 M urea gel. The film was exposed for 36 hours. A 100 bp ladder was used as a size marker. Panel A: EP1, EP2, EP3, and EP4 expression in rabbit aorta. Panel B: EP2 and EP4 expression in preglomerular microvessels in the kidney. Panel C: EP2 expression in the rabbit ear artery, jugular vein and vena cava.
Figure 6.
RNase protection assay demonstrating EP2 and EP4 expression in cultured vascular smooth muscle cells isolated from the rabbit aorta (Panel A), cultured cortical collecting duct cells (CCD) (Panel B) and cultured glomerular mesangial cells (MCs) and medullary interstitial cells (MICs) (Panel C). To determine the regulation of EP2 expression in MCs and MICs, cells were treated with 1 μM phorbol 12-myristate 13-acetate (PMA) for 6 hours and EP2 mRNA level was examined by Nuclease protection assay (Panel C).
Intrarenal localization of the rabbit EP2 receptor
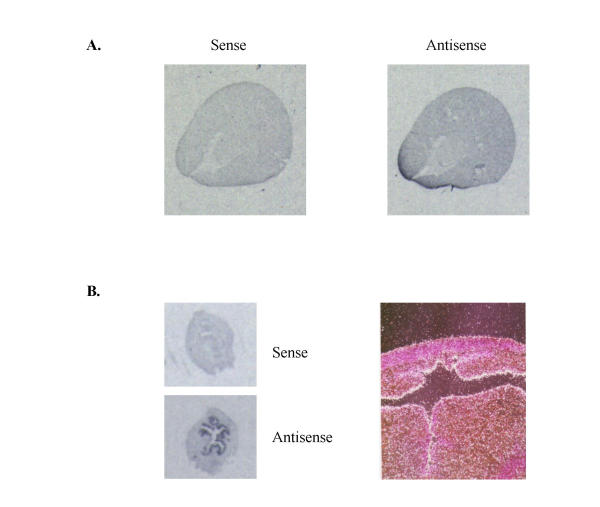
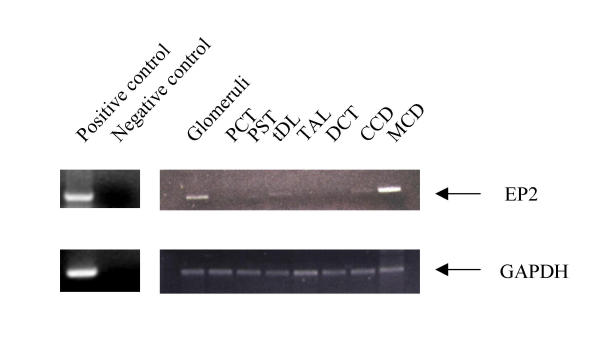
In situ hybridization was performed to examine the distribution of the EP2 receptor gene expression in the kidney and uterus. Sections of rabbit renal and uterine tissues were hybridized with the [35S]-labeled riboprobe from the same cDNA probe used in RNase-protection assays. As observed for the mouse EP2 in the uterus, a significant hybridization signal was detected mainly in the endometrial epithelium of the rabbit uterus with less expression in myometrium (Fig. 7). Consistent with previous observation in man [19], only a diffuse weak EP2 mRNA signal was detected in the rabbit kidney (Figure 7). To further elucidate the distribution of EP2 receptor along the nephron, nephron segments were dissected and expression of EP2 was determined using RT-PCR. As shown in figure 8, PCR products with expected sizes were obtained for both EP2 receptor (336 bp) and GAPDH (411 bp). Identity of the amplified products was confirmed by Southern hybridization (not shown) and sequencing. As positive control, RT-PCR was performed using 1 μg of total RNA isolated from rabbit spleen (figure 8). As negative control, PCR were also performed in the absence of reverse transcription without identifiable product, indicating the origination of PCR products were from mRNA other than genomic DNA (figure 8). EP2 receptor mRNA was easily detected in glomeruli and medullary collecting ducts (MCD). Detectable EP2 mRNA was also present in thin descending limbs of Henle (tDL) and cortical collecting ducts (CCD).
Figure 7.
In situ hybridization of rabbit EP2 showing mRNAdistribution in rabbit kidney (A) and uterus (B). A: autoradiographydemonstrating low and ubiquitous expression of EP2 mRNA expression inrabbit kidney. Dark black areas indicate regions of hybridization with the antisense riboprobe (right panel). In contrast, no hybridization was seen using a sense probe (left panel). B: In situ hybridization showing rabbit EP2 mRNA signals in the uterus. Autoradiography (left panels) and photomicrograph (right panel, 400 Xdarkfield, white grains) showing specific hybridization of the antisense probe to uterus endometrium.
Figure 8.
RT-PCR analysis demonstrating distribution of EP2 receptor (right upper panel) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (right lower panel) mRNA along rabbit nephron. PCR products were electrophoretically separated on a 1% agarose gel. RNA loading was corrected by normalizing to GAPDH mRNA expression. Data are from a single experiment and represent three independent experiments. Left panels showing positive and negative control for EP2 (upper) and GAPDH (lower). Note: PCT, proximal convoluted tubules; PST, proximal straight tubules; tDL, the thin descending limb of Henle; TAL, thick ascending limb; DCT, distal convoluted tubules; CCD and MCD, cortical and medullary collecting ducts.
In cultured cells RNase protection assay detected EP2 expression in renal medullary interstitial cells (MICs), while no signal was detected in cultured cortical collecting ducts or glomerular mesangial cells (Fig. 6B &6C). EP2 mRNA was also detected in cultured rabbit aortic vascular smooth muscle cells (Figure 6A).
Discussion
The present studies describe the cloning and characterization of the rabbit EP2 receptor. With this information, cDNA sequence for all four rabbit EP receptors is now available [22-24]. Several similarities between the rabbit and mouse EP2 receptors exist [5,21]. The rabbit EP2 genomic clone contains an intron with consensus splice donor and acceptor sites near the end of transmembrane domain (TM) VI. The relative position of the intron to the coding regions is also identical to that found in the human TP, the human and mouse DP, and the human EP1 gene [2]. This provides further evidence that the prostanoid receptor family arose from a common ancestral gene. The rabbit EP2 gene contains a 1,083 bp open reading frame coding for a 361 amino acid polypeptide, with a calculated molecular weight of 39,919 Daltons. Its amino acid profile reveals the presence of seven hydrophobic regions which likely represent transmembrane domains. Unlike the TP and EP3 receptors, no alternative splicing in the EP2 receptor was found in the coding region [21,25]. Diversity of EP2 mRNAs in the mouse was found in both the 5' UTR and the 3' UTR [21]. Similar diversity in the 3' sequence of the rabbit EP2 receptor cDNA clones isolated from the uterine library was observed in the present studies. The significance of this mRNA splicing is not yet known, but it might play a role in stability or translation efficiency of EP2 transcripts [21].
Ligand binding studies confirm that the rabbit EP2 receptor preferentially binds PGE2 with significantly higher affinity than other endogenous prostanoids. Its low affinity for PGD2, PGF2α, iloprost, misoprostol and sulprostone suggest it is not an EP1, EP3 or IP receptor [2,3]. As demonstrated for the EP2 receptor in other species [6,20,21], butaprost free acid and AH 13205, two synthetic PG agonists with selectivity for the EP2 subtype, efficiently and dose dependently compete for [3H] PGE2 binding to this newly cloned rabbit receptor. Inhibition of PGE2 binding to the rabbit EP2 receptor by AH13205 is about 100 times greater than that to rabbit EP4 in transfected COS-1 cells (unpublished data). Finally, the EP2 selective agonist butaprost free acid dose-dependently stimulated cAMP generation in COS-1 cells transfected with the rabbit EP2 receptor. These results are in agreement with observations with human, rat and mouse EP2 receptors [6,20,21], which also couple to cAMP generation. Taken together, these results clearly demonstrate that the pharmacology of this receptor corresponds to an EP2 receptor rather than an EP4 receptor.
The expression level of rabbit EP2 receptor mRNA is relatively low. Nuclease protection analysis demonstrated rabbit EP2 expression is highest in ileum, liver, and spleen, with a lower expression in kidney, uterus, brain, adrenal gland, lung, heart, bladder, stomach and skeletal muscle. This mRNA expression pattern is similar to that of the EP2 in the mouse [5,21]. PGE2 has been reported to stimulate cAMP formation in many tissues, including intestine, microdissected renal tubule segments, and uterus via butaprost sensitive and insensitive receptors [14,26-29]. The mRNA expression results are consistent with these functional studies and suggest that the EP2 receptor plays important roles in mediating PGE2 stimulated cAMP generation in these tissues [14,21,26-33].
The presence of the rabbit EP2 receptor mRNA in the uterus also support a role for this receptor as an important mediator of PGE2 action in this organ. This is in agreement with previous studies which demonstrate that EP2 is expressed and highly regulated in uterine luminal epithelium cells by ovarian steroids during the peri-implantation period [5,21,34,35]. The change in level of the EP2 receptor expression in the uterus may contribute to the increase in GS protein content and cAMP concentration in mid-gestation and the decrease at term [26,27]. Because the EP2 receptor is a GS-coupled cAMP-stimulating receptor, the EP2 receptor may participate in the physiological regulation of uterine relaxation during pregnancy and the transition into uterine contraction at the onset of the labor. Lack of normal uterine EP2 expression may also contribute to implantation defect reported in EP2-/- mice [8,36].
PGE2 also plays a critical role in regulating systemic blood pressure [37,38]. Although the receptor mediating the predominant vasodilator effect of PGE2 remains only partially defined, recent gene targeting studies suggest that the EP2 receptor may be an important vasodilator in vivo[7,8]. The rabbit ear artery has been used as a classic pharmacological model of an EP2 responsive tissue, while the rabbit jugular vein has been proposed to be an EP4 bearing target tissue [3,4,39], however in the present studies EP2 receptor mRNA was detected in both vessels. EP2 mRNA expression was also detected in the rabbit aorta and inferior vena cava thus providing further evidence that the EP2 receptor is functionally expressed in both arteries and veins. Expression of this GS-coupled cAMP-stimulating PGE2 receptor in cultured vascular smooth muscle cells isolated from the aorta supports a direct role of the EP2 receptor in mediating vascular relaxation.
PGE2 has also been shown to stimulate cAMP formation in several renal tissues, notably preglomerular microvasculature [14], renal glomeruli [40], thin descending limb [41], medullary and cortical thick ascending limb, and collecting duct [29,42]. Although EP2 receptor mRNA expression was detected in the kidney and this was more pronounced in the renal cortex than in the medulla, no specific regions of EP2 receptor mRNA expression could be detected in rabbit kidney by in situ hybridization [19]. Nonetheless, low levels of EP2 receptor mRNA expression were detected by RT-PCR or RNase protection assay in nephron segments within the kidney including preglomerular vasculature, glomeruli, thin limbs of Henle's loop and collecting ducts. The expression of EP2 receptor mRNA in medullary collecting duct is distinct from functional studies in the cortical collecting duct where butaprost had no functional effect [17,18]. These data suggest a possible unique role for the EP2 receptor in the medullary collecting duct as opposed to cortical collecting duct. Alternatively the PCR detection of EP2 receptor expression in these microdissected segments might result from the adherent renal interstitial cells other than collecting duct itself, since nuclease protection analysis revealed a relatively high EP2 level in cultured intersitial cells but failed to detect EP2 expression in cultured collecting duct cells. It is notable that glomeruli and collecting ducts are distinguished from other segments by high number of adherent cells. Selective expression of the EP2 receptor in interstitial cells is consistent with a lack of segmental localization by in situ hybridization. Whether the EP2 receptor participates in PGE2 stimulated cAMP generation in epithelial cells along the nephron awaits further investigation [14,42].
Conclusions
In summary, we have cloned and expressed a butaprost sensitive rabbit PGE2 receptor that stimulates cAMP formation and is present in many rabbit tissues. Physiological effects of PGE2 through the EP2 receptor may mediate important physiological actions in these tissues. EP2 expression in uterus, vasculature and the kidney suggests that this receptor may play important roles in the functions of reproductive system, vascular tissues and interstitium of the kidney.
Materials and Methods
Ligands
Unlabeled PGE2, PGD2, and PGF2α were purchased from Cayman Chemical (Ann Arbor). Butaprost free acid was a gift from Dr. Jilly Evans, Merck-Frosst. Sulprostone, misoprostol, iloprost and AH13205 were kindly provided by Dr. Harold Kluender of Miles Laboratories, P.J. Gardiner of Bayer UK, Dr. Paul Collins of G.D. Searle, Dr. Rubanie of Berlex Laboratories and Dr. R.A. Coleman of Glaxo, UK, respectively. [3H] PGE2 was purchased from Dupont NEN.
Rabbit genomic library screening
A rabbit genomic library in Lamda DASH® II vector (Stratagene) was screened with [32P]-labeled full-length human EP2 (gift from Dr. D. Woodward, Allergan Pharmaceuticals) [20]. The filters were fixed by ultraviolet light exposure (Stratalinker, Stratagene) and hybridized 18 hours at 56°C in a solution containing 6X SSC, 5X Denhardt's, 0.1% SDS, 150 μg/ml denatured herring sperm DNA and 3 × 106 cpm/ml labeled probe. Two representative positive clones, 4B and 6A, were isolated from 5 × 105 recombinant plaques and shown to exhibit unique restriction patterns. A XbaI/NotI fragment from clone 4B1 and a NotI fragment from 6A1 hybridized to the probe and were subcloned in pBluesript II SK(-) (Stratagene) for further analysis.
cDNA cloning
A rabbit uterus cDNA library in ZAP II (Stratagene) (a gift from Dr. J. Lytton, Harvard Medical School) was screened using an AvaII cDNA fragment of 750 bp from the genomic clone 4B1. 106 plaques were probed under high stringency, and 18 positive plaques were purified. The isolated cDNA-bearing phages were rescued to plasmids according to the manufacturer's instruction. Clones were analyzed by restriction digestion, and nucleotide sequencing was carried out on both strands by the dideoxy chain termination method.
Construction of an open reading frame of EP2 and its expression in COS-1 cells
One 5' truncated cDNA clone, REP2-18A, was highly homologous to the human EP2 receptor but lacked the 5' 150 bp encoding the first 50 amino acids (see results). REP2-18A was digested by Sac II/Xho I, and the 3' restriction fragment (1,312 bp) was ligated to the Sac II/Hind III fragment (350 bp) of the 4B1 genomic clone at the Sac II site. This resulted in a 1.7 kb fragment, YZ4, containing a putative open reading frame. YZ4 was then subcloned into the eukaryotic expression vector pcDNA3 (Invitrogen) and the resultant plasmid, pcDNA3/REP2, was transfected into COS-1 using lipofectamine according to the manufacturer's directions (Life Science Technologies) with 12 μg plasmid DNA and 45 μL lipofectamine solution. Cells were cultured for 72 hours, and 5 mM sodium butyrate was added to culture medium 16 hours before lysis. Total cell membranes were prepared as described previously [23].
Ligand binding analysis
Saturation isotherm ligand binding experiments were performed using 15–20 μg of membrane protein incubated with increasing concentrations of [3H] PGE2 at 30°C for 2 hours, in binding buffer (25 mM KPO4, pH6.2, 10 mM MgCl2, and 1 mM EDTA). The reaction was stopped by adding 1 ml of ice-cold binding buffer followed by rapid filtration on Inotech IH-201-A21 glass fiber filters employing an Inotech 96 well filtration manifold (Inotech Biosystems, Lansing, MI). Filters then were washed 3 times with binding buffer, dried briefly and counted in a Beckman LS 6500 multi-purpose scintillation counter. Nonspecific binding was defined as the radioactivity remaining bound to the filter in the presence of 50 μM unlabelled PGE2. For competition binding assays, 20 μg of membrane protein was incubated with 1 nM [3H]PGE2 and varying concentrations of unlabeled competitors. Reactions were carried out as above.
cAMP measurements
COS1 cells were transiently transfected with the EP2 receptor expression plasmid were distributed onto 24-well plates. The medium was replaced 24 hours later and incubated one additional hour at 37°C in 450 μL DMEM plus 0.25 mM 3-isobutyl-1-methylxanthine (IBMX), and 40 mM indomethacin. Following this medium containing varying amounts of butaprost free acid was added to each well for 5 minutes. The reactions were stopped by addition of 500 μL of 10% trichloroacetic acid. cAMP measurement of the cell lysates were performed by an EIA according to manufacturer's instructions (Stratagene).
Isolation of renal microvessels and dissection of nephron segments
Renal preglomerular microvessels were isolated according to a protocol from Chaudhari and Kirschenbaum [43]. Briefly, in anesthetized animals kidneys were exposed and the renal artery cannulated. The kidneys were perfused with 10 ml of ice cold normal saline, followed by 10 ml of a 1% suspension of magnetized iron oxide (Fe3O4, Aldrich) in normal saline. The cortex of the kidneys was minced with a tissue press and homogenized using a Polytron homogenizer at moderate speed for 15 s ×2. Microvessels were separated from nonvascular tissue in several washing steps (in 1× PBS) utilizing a strong magnet field. Washing and separation were repeated after passing the homogenate through 20-, 21- and 23-gauge needles, respectively, until the suspension was mostly free of glomeruli and other nonvascular tissue. This technique provided a large quantity of relatively pure preglomerular microvessels, with approximately 10–15 % of the suspension consisting of attached glomeruli and early segments of proximal tubules. Microdissection of nephron segments was performed in the kidney of New Zealand White rabbit as previously described [44]. The following segments were dissected: glomeruli, proximal convoluted tubules (PCT), proximal straight tubules (PST), thin limbs of Henle's loop (tDL/tAL), thick ascending limb (TAL), distal convoluted tubules (DCT), cortical collecting ducts (CCD) and medullary collecting duct (MCD) cells.
PCR ampification of Rabbit EP2 receptor Fragment
Total RNA was purified from dissected nephron segments using Tri Reagent (Molecular Research Center) and reverse-transcribed to single-stranded cDNA using Moloney murine leukemia virus reverse transcriptase and 2.5 μM of random hexamers according to the manufacturer's protocol (GeneAmp RNA PCR kit, Perkin Elmer Cetus, Norwalk, CT). The cDNAs were then amplified using EP2 and GAPDH selective primers. Primers were selected from rabbit cDNA sequences. For rabbit EP2: 5'-TCC TCC CGA AAA GAA AAG TGG-3' for sense and 5'-TGT TTA CCC CGT TTT ATC AGG-3' for antisense; for rabbit GAPDH: 5'-CGG AGC CAA AAG GGT CAT CAT-3' for sense and 5'-TTT CTC CAG GCG GCA GGT CAG-3' for antisense. These primers were used to amplify a 336 bp of EP2 cDNA fragment and 411 bp of GAPDH cDNA fragment. PCR reactions were carried out in 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, 0.2 mM dNTPs and 1 μM primers at 94°C for 0.5 min, 60°C for 0.5 min, and 72°C for 1.0 min for 35 cycles in a Perkin Elmer Cetus 9600 thermal cycler. PCR products were separated by 1% agarose gel and further confirmed by Southern hybridization and sequencing.
Culture of rabbit aortic smooth muscle cells (VSMCs), cortical collecting duct cells (CCD), glomerular mesangial cells (MCs) and renal medullary interstitial cells (MICs)
The aorta was cut open in a longitudinal section and the thin layer of endothelial cells was removed using a cotton-tipped applicator. The aorta was cut into small pieces and suspended in DMEM medium containing 20 % FCS and grown in a culture dish in standard incubation at 37°C. One week later, the VSMCs had outgrown from the tissue suspension, and were transferred to a new flask. The medium was changed every other day, the identity of the VSMCs was confirmed by α-actin fluorescence staining as described [45]. Glomerular MCs, CCD cells and MICs were cultured as we previously reported [22,44,46].
Solution hybridization/RNase protection assays
RNase protection assays were performed as described previously [22]. A Hind III fragment of the rabbit EP2 receptor located in the the 3' untranslated region (3'UTR) was subcloned into pBluescript SK(-) plasmid (Stratagene). Plasmids containing rabbit EP1 (250 bp) [22] EP2 (322 bp), EP3 (466 bp) [24] and EP4 (328 bp) [23] were used to synthesize cRNA probes for solution hybridization of total RNA from various tissues. Briefly, the fragments of EP1, EP2, EP3 and EP4 cDNA were subcloned in pBluscript SK(-) vector, and the antisense orientation was determined by dideoxy-DNA sequencing. Radiolabeled riboprobes were synthesized from linearized plasmid DNA (1 μg) transcribed in vitro with 10 U of T3 RNA polymerase by using MAXIscript™ kit (Ambion) for 1 hour at 37°C in a total reaction volume of 20 μl. The reaction buffer contained 10 mM dithiothreitol (DTT), 0.5 μM of ATP, CTP, and GTP, 2.5 μM of UTP, and 5 μl of 800 Ci/mmol [α-32P] UTP at 10 mCi/ml (Dupont NEN). Hybridization buffer included 80% deionized formamide, 100 mM sodium citrate, pH6.4, 300 mM sodium acetate, pH6.4 and 1 mM EDTA (RPAII, Ambion). Twenty micrograms of total RNAs from rabbit tissues, preglomerular vessels, cultured cells were incubated at 45°C overnight in hybridization buffer with 1 × 105 cpm labeled riboprobe. After hybridization, ribonuclease digestion (20 mg/ml) was carried out at 37°C for 30 minutes. Precipitated protected fragments were separated on a 4% polyacrylamide gel at 200 V for 3.5 hours. End-labeled 100 bp DNA markers were used as molecular weight standards (Promega). The gel was exposed to Kodak XAR-5 film overnight at -80°C, with intensifying screens.
In situ hybridization
In situ hybridization was performed as previously described [22]. Rabbit uterus and kidney were fixed in 4% paraformaldehyde, and embedded in paraffin. Seven μm sections were cut and hybridized with [35S]-labeled riboprobe used in the RNase protection assays at 55°C for 18 hours. Following hybridization, sections were washed at 50°C in 50% formamide, 2X SSC, and 100 mM β-mercaptoethanol for 60 minutes. Sections were treated with RNase A (10 μg/ml) at 37°C for 30 minutes, followed by washes in 10 mM Tris, 5 mM EDTA, 500 mM NaCl at 37°C; 2X SSC at 50°C; and 0.1X SSC at 50°C. Slides were dehydrated with ethanol containing 300 mM ammonium acetate. Photomicrographs were taken from slides dipped in emulsion (Ilford K5, Knutsford, Cheshire, England) diluted 1:1 with 2% glycerol and exposed for 7 days at 4°C. After developing in Kodak D-19, slides were counterstained with hematoxylin and eosin. Photomicrographs were taken by a Zeiss Axioskop microscope using dark-field optics.
Data analysis
All binding assays and cAMP measurements were plotted using PRISM (GraphPad, San Diego, CA).
Authors' contributions
YG carried out the molecular cloning studies, participated in the sequence alignment and drafted the manuscript. BAS carried out the cAMP assays. YZ participated in examining tissue distribution of EP2 receptor. AS participated in preparation of preglomeruli, OS carried out the nephron dissection. LSD did the in situ hybridization. RR participated in dissecting nephron segments. RMB and MDB supervised the design of the study.
Abbreviations
Prostaglandin E2 (PGE2); cortical thick ascending limb (cTAL); thin descending limb of Henle's loop (tDL); cortical and outer medullary collecting ducts (CCD, OMCD); reverse transcription-polymerase chain reaction (RT-PCR)
Acknowledgments
Acknowledgments
We thank Dr. David Woodward and John Regan for providing the human EP2 receptor cDNA. Support for this project was provided by NIH DK-37097 (to MDB) and a Veterans Administration Merit Award (MDB). Support was also provided by NIH grants DK46205, and GM-15431 (RMB). Dr. M. Breyer is the recipient of a VA Clinical Investigator Career Development Award. Support for this project was also provided by an American Heart Association Beginning Grant-in-Aid 0160200B (to YG), NIH DRTC grant P60-DK-20593 (to YG) and an Atorvastatin Research Award from Pfizer Pharmaceutics (to YG).
Contributor Information
Youfei Guan, Email: youfei.guan@mcmail.vanderbilt.edu.
Brett A Stillman, Email: bstillman@monad.net.
Yahua Zhang, Email: yahua.zhang@mcmail.vanderbilt.edu.
André Schneider, Email: schneide@uke.uni-hamburg.de.
Osamu Saito, Email: shu-sait@ga3.so-net.ne.jp.
Linda S Davis, Email: linda.s.davis@mcmail.vanderbilt.edu.
Reyadh Redha, Email: reyadh.redha@mcmail.vanderbilt.edu.
Richard M Breyer, Email: rich.breyer@vanderbilt.edu.
Matthew D Breyer, Email: matthew.breyer@mcmail.vanderbilt.edu.
References
- Breyer MD, Zhang Y, Guan YF, et al. Regulation of renal function by prostaglandin E receptors. Kidney Int Suppl. 1998;67:S88–94. doi: 10.1046/j.1523-1755.1998.06718.x. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Coleman RA, Smith WL, Narumiya S. VIII. International union of pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- Lydford SJ, McKechnie KC, Dougall IG. Pharmacolgical studies on prostanoid receptors in the rabbit isolated saphenous vein: a comparison with the rabbit isolated ear artery. Br J Pharmacol. 1996;117:13–20. doi: 10.1111/j.1476-5381.1996.tb15148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuyama M, Nishigaki N, Sugimoto Y, et al. The mouse prostaglandin E receptor EP2 subtype: cloning, expression, and Northern blot analysis. FEBS Letters. 1995;372:151–156. doi: 10.1016/0014-5793(95)00966-D. [DOI] [PubMed] [Google Scholar]
- Boie Y, Stocco R, Sawyer N, et al. Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur J Pharmacol. 1997. [DOI] [PubMed]
- Zhang Y, Guan Y, Schneider A, et al. Characterization of murine vasopressor and vasodepressor prostaglandin E(2) receptors. Hypertension. 2000;35:1129–1134. doi: 10.1161/01.hyp.35.5.1129. [DOI] [PubMed] [Google Scholar]
- Kennedy CR, Zhang Y, Brandon S, et al. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5:217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- Jackson EK, Heidemann HT, Branch RA, et al. Low dose intrarenal infusions of PGE2, PGI2, and 6-keto-PGE1 vasodilate the in vivo rat kidney. Circ Res. 1982;51:67–72. doi: 10.1161/01.res.51.1.67. [DOI] [PubMed] [Google Scholar]
- Chatziantoniou C, Arendshorst WJ. Prostaglandin interactions with angiotensin, norepinephrine, and thromboxane in rat renal vasculature. Am J Physiol. 1992;262:F68–76. doi: 10.1152/ajprenal.1992.262.1.F68. [DOI] [PubMed] [Google Scholar]
- Chatziantoniou C, Arendshorst WJ. Impaired ability of prostaglandins to buffer renal vasoconstriction in genetically hypertensive rats. Am J Physiol. 1992;263:F573–580. doi: 10.1152/ajprenal.1992.263.4.F573. [DOI] [PubMed] [Google Scholar]
- Edwards RM. Effects of prostaglandins on vasoconstrictor action in isolated renal arterioles. Am J Physiol. 1985;248:F779–784. doi: 10.1152/ajprenal.1985.248.6.F779. [DOI] [PubMed] [Google Scholar]
- Purdy KE, Arendshorst WJ. Prostaglandins buffer ANG II-mediated increases in cytosolic calcium in preglomerular VSMC. Am J Physiol. 1999;277:F850–858. doi: 10.1152/ajprenal.1999.277.6.F850. [DOI] [PubMed] [Google Scholar]
- Chaudhari A, Gupta S, Kirschenbaum MA. Biochemical evidence for PGI2 and PGE2 receptors in the rabbit renal preglomerular microvasculature. Biochim Biophys Acta. 1990;1053:156–161. doi: 10.1016/0167-4889(90)90008-2. [DOI] [PubMed] [Google Scholar]
- Ando Y, Asano Y. Luminal prostaglandin E2 modulates sodium and water transport in rabbit cortical collecting ducts. Am J Physiol. 1995;268:F1093–1101. doi: 10.1152/ajprenal.1995.268.6.F1093. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum MA, Stein JH. The effect of inhibition of prostaglandin synthesis on urinary sodium excretion in the conscious dog. J Clin Invest. 1976;57:517–521. doi: 10.1172/JCI108304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakairi Y, Jacobson HR, Noland TD, et al. Luminal prostaglandin E receptors regulate salt and water transport in rabbit cortical collecting duct. Am J Physiol. 1995;269:F257–265. doi: 10.1152/ajprenal.1995.269.2.F257. [DOI] [PubMed] [Google Scholar]
- Breyer MD, Breyer RM. Prostaglandin E receptors and the kidney. Am J Physiol Renal Physiol. 2000;279:F12–23. doi: 10.1152/ajprenal.2000.279.1.F12. [DOI] [PubMed] [Google Scholar]
- Breyer MD, Davis L, Jacobson HR, et al. Differential localization of prostaglandin E receptor subtypes in human kidney. Am J Physiol (Renal Electrolyte Physiol 39) 1996;270:F912–F918. doi: 10.1152/ajprenal.1996.270.5.F912. [DOI] [PubMed] [Google Scholar]
- Regan JW, Bailey TJ, Pepperl DJ, et al. Cloning of a novel human prostaglandin receptor with characteristics of the pharmacologically defined EP2 subtype. Mol Pharmacol. 1994;46:213–220. [PubMed] [Google Scholar]
- Katsuyama M, Sugimoto Y, Okano K, Segi E, Ikegami R, Negishi M, Ichikawa A. Characterization of the gene for the mouse prostaglandin E receptor subtype EP2: tissue-specific initiation of transcription in the macrophage and the uterus. Biochem J. 1998;15:1115–1121. doi: 10.1042/bj3301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Zhang Y, Breyer R, et al. PGE2 inhibits renal collecting duct Na+ absorption by activating the EP1 receptor. J Clin Invest. 1998;102:194–201. doi: 10.1172/JCI2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyer RM, Davis L, Jacobson H, et al. Cloning and Expression of the rabbit prostaglandin EP4 receptor. Am J Physiol. 1996;270:F485–F493. doi: 10.1152/ajprenal.1996.270.3.F485. [DOI] [PubMed] [Google Scholar]
- Breyer RM, Emeson RB, Tarng JL, Breyer MD, Davis LS, Abrosom RM, Ferrenbach SM. Alternative Splicing Generates Multiple isoforms of a Rabbit Prostaglandin E2 receptor. J Biol Chem. 1994;298:6163–6169. [PubMed] [Google Scholar]
- Nusing RM, Hirata M, Kakizuka A, et al. Characterization and chromosomal mapping of the human thromboxane A2 receptor gene. J Biol Chem. 1993;268:25253–25259. [PubMed] [Google Scholar]
- Senior J, Marshall K, Sangha R, et al. In vitro characterization of prostanoid EP-receptors in the non-pregnant human myometrium. Br J Pharmacol. 1991;102:747–753. doi: 10.1111/j.1476-5381.1991.tb12244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior J, Marshall K, Sangha R, et al. In vitro characterization of prostanoid receptors on human myometrium at term pregnancy. Br J Pharmacol. 1993;108:501–506. doi: 10.1111/j.1476-5381.1993.tb12832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simson JNL, Merhav A, Silen W. Alkaline secretion by amphibian duodenum. II. Effects of dBcAMP, theophyllin, and prostaglandins. Am J Physiol. 1981;241:G528–G536. doi: 10.1152/ajpgi.1981.241.6.G528. [DOI] [PubMed] [Google Scholar]
- Sonnenburg WK, Smith WL. Regulation of cyclic AMP metabolism in rabbit cortical collecting tubule cells by prostaglandins. J Biol Chem. 1988;263:6155–6160. [PubMed] [Google Scholar]
- Smock SL, Pan LC, Castleberry TA, et al. Cloning, structural characterization, and chromosomal localization of the gene encoding the human prostaglandin E(2) receptor EP2 subtype. Gene. 1999;237:393–402. doi: 10.1016/S0378-1119(99)00323-6. [DOI] [PubMed] [Google Scholar]
- Botella A, Delvaux M, Fioramonti J, et al. Stimulatory (EP1 and EP3) and inhibitory (EP2) prostaglandin E2 receptors in ileal smooth muscle cells. Eur J Pharmacol. 1993;237:131–137. doi: 10.1016/0014-2999(93)90102-N. [DOI] [PubMed] [Google Scholar]
- Pallone TL. Vasoconstriction of outer medullary vasa recta by angiotensin II is modulated by prostaglandin E2. Am J Physiol. 1994;266:F850–857. doi: 10.1152/ajprenal.1994.266.6.F850. [DOI] [PubMed] [Google Scholar]
- Ohno T, Katori M, Majima M, et al. Dilatation and constriction of rat gastric mucosal microvessels through prostaglandin EP2 and EP3 receptors. Aliment Pharmacol Ther. 1999;13:1243–1250. doi: 10.1046/j.1365-2036.1999.00577.x. [DOI] [PubMed] [Google Scholar]
- Lim H, Dey SK. Prostaglandin E2 receptor subtype EP2 gene expression in the mouse uterus coincides with differentiation of the luminal epithelium for implantation. Endocrinology. 1997;138:4599–4606. doi: 10.1210/en.138.11.4599. [DOI] [PubMed] [Google Scholar]
- Katsuyama M, Sugimoto Y, Morimoto K, et al. Distinct cellular localization of the messenger ribonucleic acid for prostaglandin E receptor subtypes in the mouse uterus during pseudopregnancy. Endocrinology. 1997;138:344–350. doi: 10.1210/en.138.1.344. [DOI] [PubMed] [Google Scholar]
- Tilley SL, Audoly LP, Hicks EH, et al. Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor [see comments]. J Clin Invest. 1999;103:1539–1545. doi: 10.1172/JCI6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siragy HM, Senbonmatsu T, Ichiki T, et al. Increased renal vasodilator prostanoids prevent hypertension in mice lacking the angiotensin subtype-2 receptor. J Clin Invest. 1999;104:181–188. doi: 10.1172/JCI6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels EG, Hinman JW, Leach BE, et al. Identification of prostaglandin E2 as the principle vasodepressor lipid of rabbit renal medulla. Nature. 1967;215:1298–1299. doi: 10.1038/2151298a0. [DOI] [PubMed] [Google Scholar]
- Lawrence RA, Jones RL. Investigation of the prostaglandin E (EP-) receptor subtype mediating relaxation of the rabbit jugular vein. Br J Pharmacol. 1992;105:817–824. doi: 10.1111/j.1476-5381.1992.tb09063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura S, Smyth DD, Pettinger WA. Regulation of renal cellular cAMP levels by prostaglandins and alpha 2-adrenoceptors: microdissection studies. Kidney Int. 1986;29:703–707. doi: 10.1038/ki.1986.55. [DOI] [PubMed] [Google Scholar]
- Torikai S, Kurokawa K. Effect of PGE2 on the cell cyclic AMP content in the thin descending limb of henle of the rat. Mineral Electrolyte Metab. 1984;10:21–25. [PubMed] [Google Scholar]
- Torikai S, Kurokawa K. Distribution of prostaglandin E2-sensitive adenylate cyclase along the rat nephron. Prostaglandins. 1981;21:427–438. doi: 10.1016/0090-6980(81)90088-5. [DOI] [PubMed] [Google Scholar]
- Chaudhari A, Kirschenbaum M. A rapid method for isolating rabbit renal microvessels. Am J Physiol. 1988;254:F291–F296. doi: 10.1152/ajprenal.1988.254.2.F291. [DOI] [PubMed] [Google Scholar]
- Yang T, Singh I, Pham H, et al. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol. 1998;274:F481–489. doi: 10.1152/ajprenal.1998.274.3.F481. [DOI] [PubMed] [Google Scholar]
- Thyberg J, Blomgren K. Effects of proteasome and calpain inhibitors on the structural reorganization and proliferation of vascular smooth muscle cells in primary culture. Lab Invest. 1999;79:1077–1088. [PubMed] [Google Scholar]
- Guan Y, Chang M, Cho WY, et al. Cloning, expression, and regulation of rabbit cyclooxygenase-2 in renal medullary interstitial cells. Am J Physiol. 1997;273:F18–F26. doi: 10.1152/ajprenal.1997.273.1.F18. [DOI] [PubMed] [Google Scholar]