Abstract
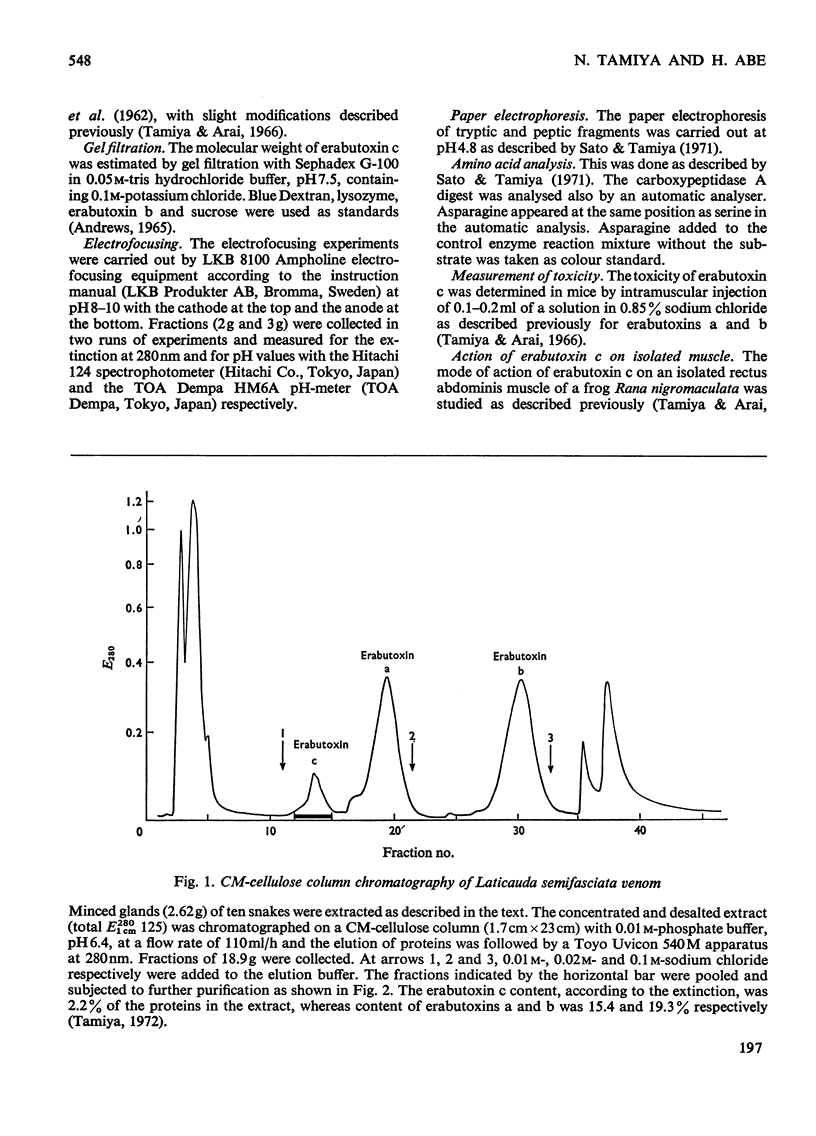
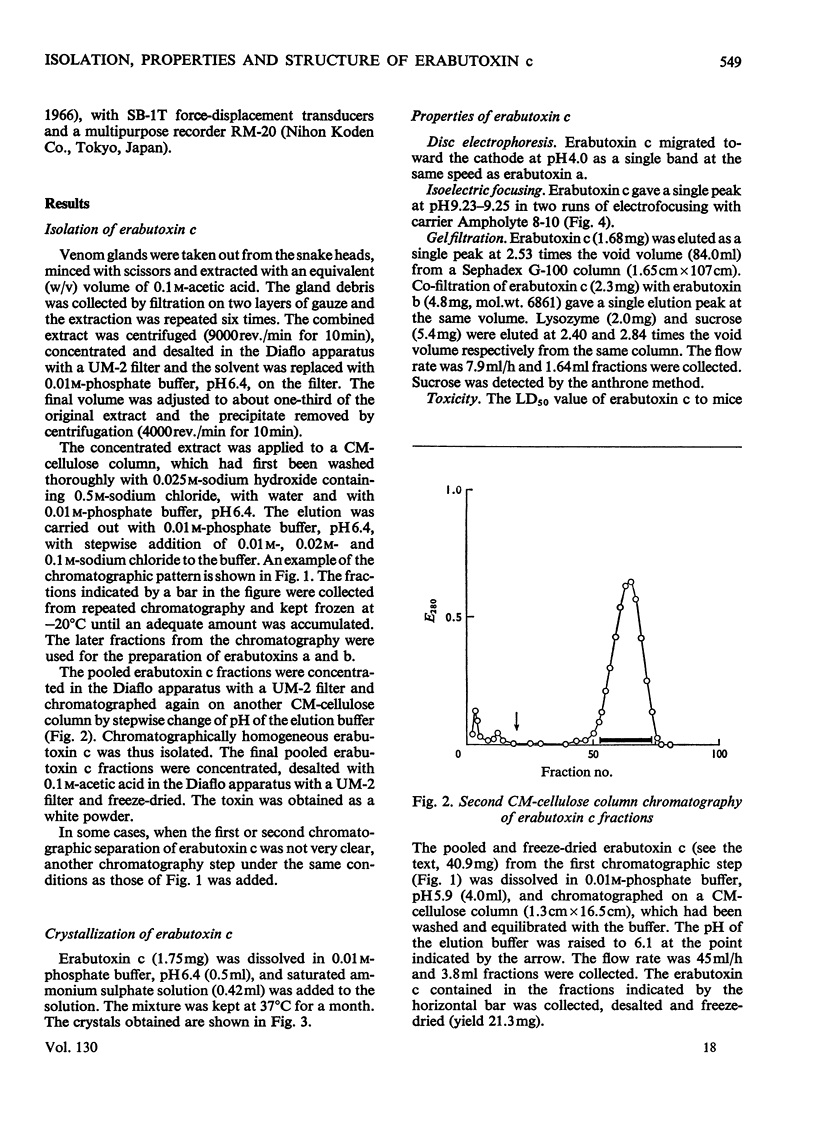
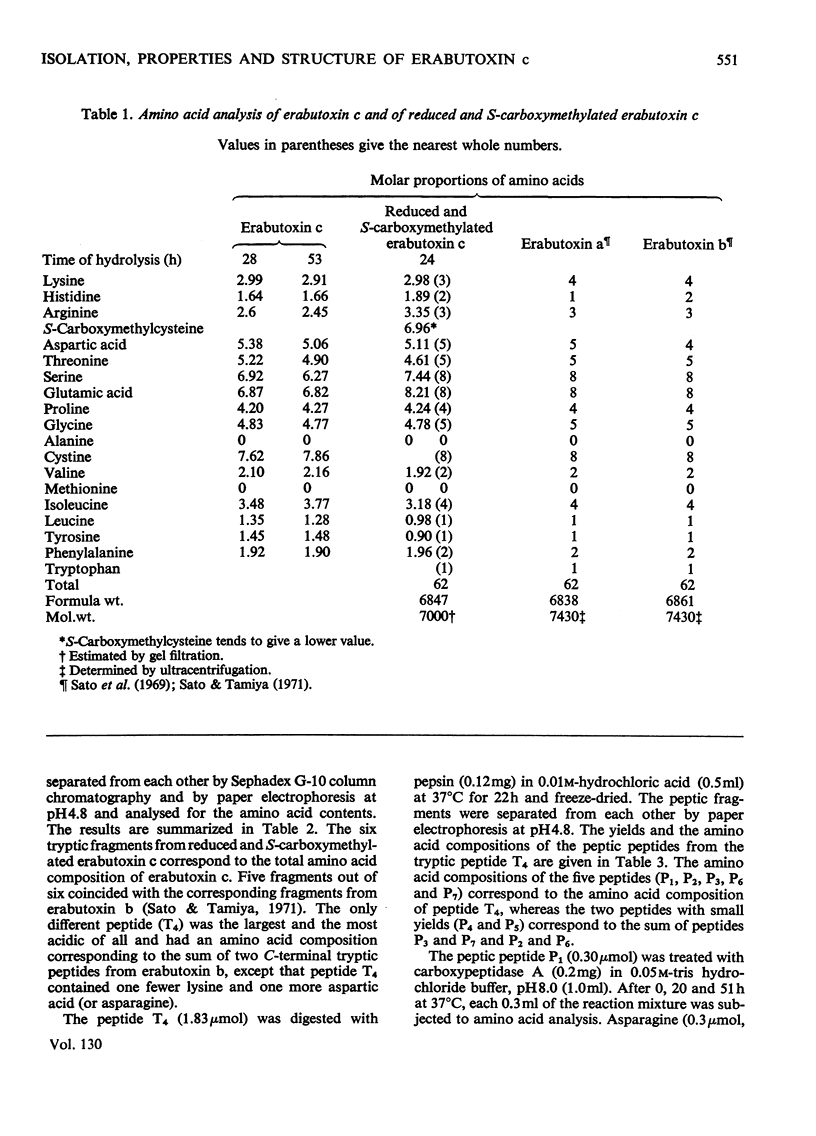
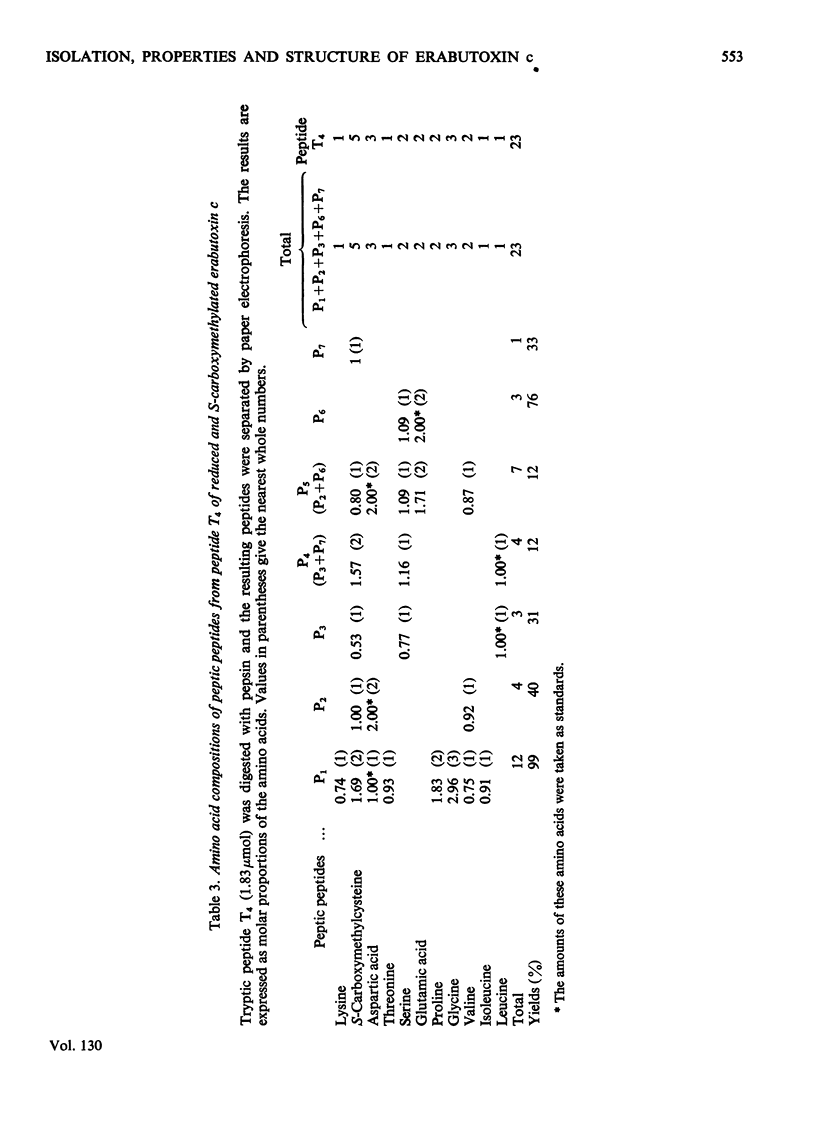
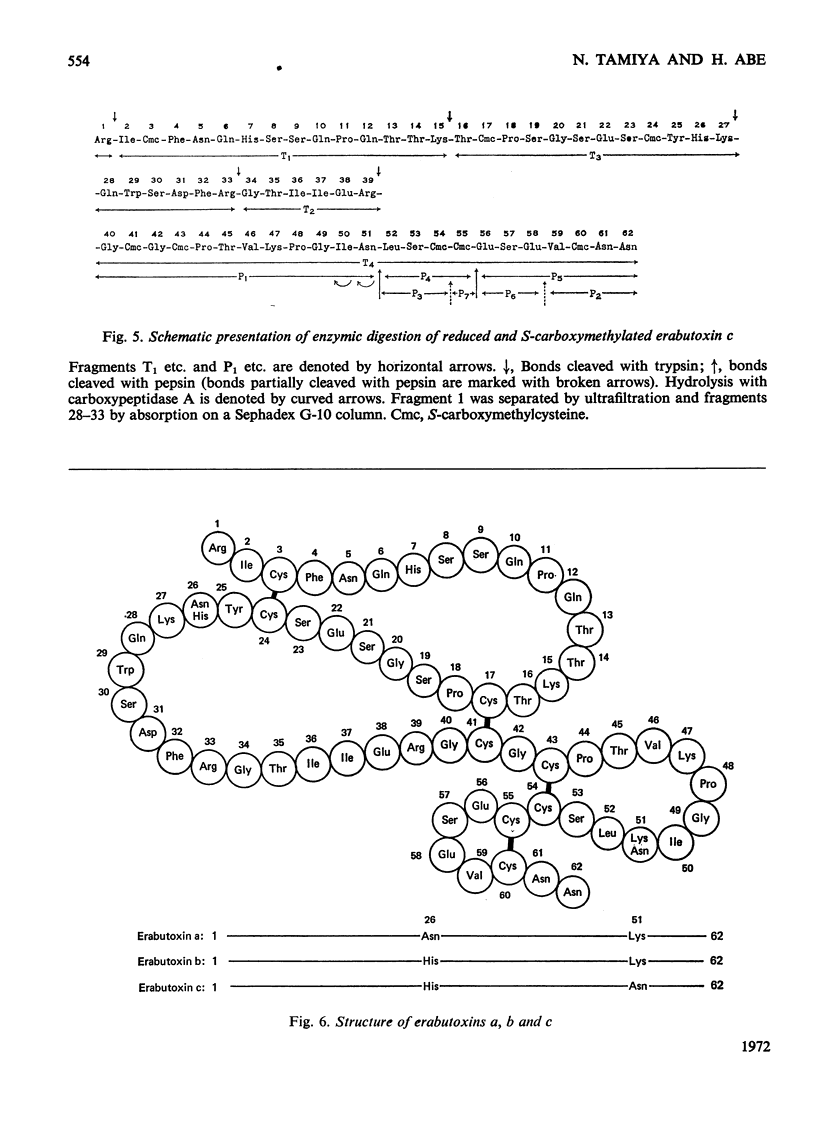
Erabutoxin c, a minor neurotoxic component of the venom of a sea snake Laticauda semifasciata, was isolated in pure form by repeated column chromatography on CM-cellulose columns. The toxin was crystallizable and monodisperse in rechromatography, disc electrophoresis and isoelectric focusing (isoelectric point, pH9.23–9.25). The molecular weight of the toxin, as estimated by gel filtration, was 7000. The toxin showed the same lethal activity to mice (0.13μg/g body wt., intramuscular injection) and the same effect on isolated frog muscle as erabutoxins a and b, the main toxic components of the venom. The toxin inhibited the acetylcholine contracture but not the potassium chloride contracture of muscle. Erabutoxin c consisted of 62 amino acid residues, containing one fewer lysine and one more histidine than erabutoxin a and one fewer lysine and one more aspartic acid (or asparagine) than erabutoxin b. Erabutoxin c was reduced, S-carboxymethylated and hydrolysed with trypsin. The only fragment different from the corresponding fragments from erabutoxin b was hydrolysed further with pepsin. One of the peptic fragments, which was assumed to have the aspartic acid (or asparagine) residue in question at the C-terminal end, was treated with carboxypeptidase A. The C-terminal residue was found to be an asparagine. It was therefore concluded that erabutoxin c was [51-asparagine]-erabutoxin b.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botes D. P. Snake venom toxins. The amino acid sequences of toxins and from Naja nivea venom and the disulfide bonds of toxin . J Biol Chem. 1971 Dec 10;246(23):7383–7391. [PubMed] [Google Scholar]
- Botes D. P., Strydom D. J. A neurotoxin, toxin alpha, from Egyptian cobra (Naja haje haje) venom. I. Purification, properties, and complete amino acid sequence. J Biol Chem. 1969 Aug 10;244(15):4147–4157. [PubMed] [Google Scholar]
- Botes D. P., Strydom D. J., Anderson C. G., Christensen P. A. Snake venom toxins. Purification and properties of three toxins from Naja nivea (Linnaeus) (Cape cobra) venom and the amino acid sequence of toxin delta. J Biol Chem. 1971 May 25;246(10):3132–3139. [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Endo Y., Sato S., Ishii S., Tamiya N. The disulphide bonds of erabutoxin a, a neurotoxic protein of a sea-snake (Laticauda semifasciata) venom. Biochem J. 1971 May;122(4):463–467. doi: 10.1042/bj1220463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlanger B. F., Cooper A. G., Cohen W. The inactivation of chymotrypsin by diphenylcarbamyl chloride and its reactivation by nucleophilic agents. Biochemistry. 1966 Jan;5(1):190–196. doi: 10.1021/bi00865a025. [DOI] [PubMed] [Google Scholar]
- Mebs D., Narita K., Iwanaga S., Samejima Y., Lee C. Y. Amino acid sequence of -bungarotoxin from the venom of Bungarus multicinctus. Biochem Biophys Res Commun. 1971 Aug 6;44(3):711–716. doi: 10.1016/s0006-291x(71)80141-9. [DOI] [PubMed] [Google Scholar]
- Nakai K., Sasaki T., Hayashi K. Amino acid sequence of toxin A from the venom of the Indian cobra (Naja naja). Biochem Biophys Res Commun. 1971 Aug 20;44(4):893–897. doi: 10.1016/0006-291x(71)90795-9. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Sato S., Tamiya N. The amino acid sequences of erabutoxins, neurotoxic proteins of sea-snake (Laticauda semifasciata) venom. Biochem J. 1971 May;122(4):453–461. doi: 10.1042/bj1220453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Yoshida H., Abe H., Tamiya N. Properties and biosynthesis of a neurotoxic protein of the venoms of s snakes Laticauda laticaudata and Laticauda colubrina. Biochem J. 1969 Oct;115(1):85–90. doi: 10.1042/bj1150085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strydom A. J., Botes D. P. Snake venom toxins. Purification, properties, and complete amino acid sequence of two toxins from Ringhals (Hemachatus haemachatus) venom. J Biol Chem. 1971 Mar 10;246(5):1341–1349. [PubMed] [Google Scholar]
- Tamiya N., Arai H. Studies on sea-snake venoms. Crystallization of erabutoxins a and b from Laticauda semifasciata venom. Biochem J. 1966 Jun;99(3):624–630. doi: 10.1042/bj0990624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. C., Yang H. J., Chiu R. H. The position of disulfide bonds in cobrotoxin. Biochim Biophys Acta. 1970 Aug 21;214(2):355–363. doi: 10.1016/0005-2795(70)90013-9. [DOI] [PubMed] [Google Scholar]
- Yang C. C., Yang H. J., Huang J. S. The amino acid sequence of cobrotoxin. Biochim Biophys Acta. 1969 Aug 12;188(1):65–77. doi: 10.1016/0005-2795(69)90046-4. [DOI] [PubMed] [Google Scholar]



