Abstract
Epithelial-mesenchymal transition (EMT) is a dynamic process of lineage plasticity in which epithelial cancer cells acquire mesenchymal traits, enabling them to metastasize to distant organs. This review explores the current understanding of how lineage plasticity and phenotypic reprogramming drive prostate cancer progression to lethal stages, contribute to therapeutic resistance, and highlight strategies to overcome the EMT phenotype within the prostate tumor microenvironment (TME). Emerging evidence reveals that prostate tumor cells can undergo lineage switching, adopting alternative growth pathways in response to anti-androgen therapies and taxane-based chemotherapy. These adaptive mechanisms support tumor survival and growth, underscoring the need for deeper insights into the processes driving prostate cancer differentiation, including neuroendocrine differentiation and lineage plasticity. A comprehensive understanding of these mechanisms will pave the way for innovative therapeutic strategies. Effectively targeting prostate cancer cells with heightened plasticity and therapeutic vulnerability holds promise for overcoming treatment resistance and preventing tumor recurrence. Such advancements are critical for developing effective approaches to prostate cancer treatment and improving patient survival outcomes.
Keywords: Prostate cancer, differentiation, lineage plasticity, phenotypic reprogramming
Introduction
Prostate cancer represents a significant health challenge, being the most commonly diagnosed cancer and the second leading cause of cancer-related deaths among American men. Approximately 1 in 7 men will be diagnosed with prostate cancer in their lifetime [1]. The primary challenge in treating lethal prostate cancer lies in tumor recurrence following androgen-deprivation therapy (ADT), antiandrogen treatments, and chemotherapy, which contribute to high mortality rates. Addressing this challenge requires an urgent understanding of the mechanisms driving castration-resistant prostate cancer (CRPC), including those that operate independently of androgen receptor (AR) signaling, to develop targeted therapies and improve patient outcomes.
The tumor microenvironment (TME) plays a critical role in supporting cancer cell survival and invasion by activating key signaling pathways that inhibit anoikis, a form of cell death triggered by insufficient cell-extracellular matrix (ECM) interactions. The suppression of anoikis facilitates tumor cell invasion and metastatic colonization, as cancer epithelial cells and adjacent stromal cells, including endothelial cells and fibroblasts, adapt to survive without firm ECM attachment [2,3]. Epithelial-mesenchymal transition (EMT), a process by which epithelial cells acquire mesenchymal, migratory, and invasive properties, is closely tied to anoikis resistance [4,5]. In prostate cancer, EMT enables tumor cells to de-differentiate and invade, while mesenchymal-epithelial transition (MET) facilitates metastatic colonization and tumor recurrence [6,7]. Loss of E-cadherin and disruption of adherens junctions during EMT further enhance metastatic progression and therapeutic resistance [6,8]. Current treatment strategies predominantly target the AR signaling pathway through ADT or second-generation antiandrogens, such as enzalutamide, which directly inhibit AR activity. While these approaches initially reduce tumor size and prostate-specific antigen (PSA) levels [9], resistance inevitably develops, leading to the emergence of CRPC [10]. Mechanisms of resistance include AR amplification, the generation of AR splice variants, androgen-independent AR activation, and alternative androgen production [11,12]. Furthermore, anti-androgen treatments can induce lineage plasticity in prostate cancer cells, reprogramming them toward stem-like or EMT-like states [13] and, in some cases, promoting neuroendocrine differentiation [14,15]. These changes allow tumor cells to grow independently of AR signaling, further compounding treatment resistance.
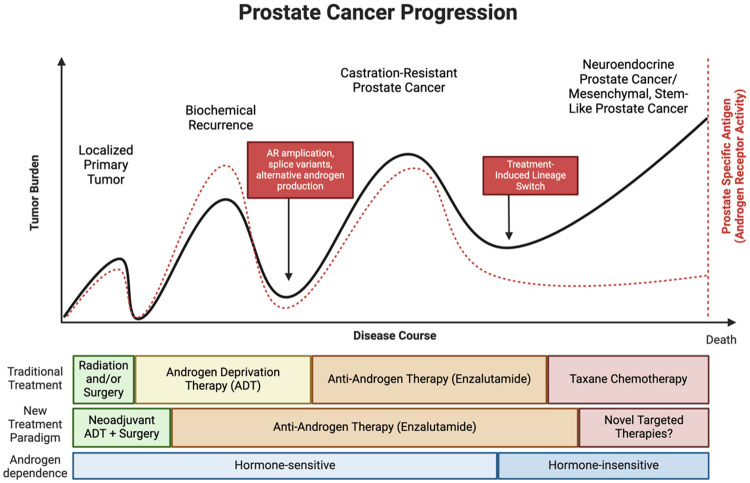
Hyperactivation of AR signaling remains the primary driver of prostate tumorigenesis [16]. Initial treatments typically involve surgery or radiation to ablate the primary tumor, followed by ADT, which causes tumor shrinkage and suppresses PSA levels [9]. However, CRPC eventually arises, fueled by mechanisms that bypass AR dependency (Figure 1). Next-generation antiandrogens have been developed to counteract these pathways [17-19], yet resistance to these therapies is inevitable. Studies reveal that treatment-induced lineage plasticity is driven by transcriptional and epigenetic reprogramming [20-22], though the precise mechanisms underlying these changes remain incompletely understood due to the complexity of prostate cancer biology.
Figure 1.
Natural history of prostate cancer progression. The progression of prostate adenocarcinoma can be clinically assessed by monitoring the levels of prostate-specific antigen (PSA) in the bloodstream. PSA is produced by the prostate epithelium and serves as a biomarker for androgen receptor (AR) activity and coincides with tumor burden/size as long as tumor express AR. As the disease advances and treatments are implemented, prostate tumors display reduced dependence on androgens due to abnormal reactivation of the AR or through lineage switch mechanisms. Novel anti-AR agent such as enzalutamide, apalutamide, or darolutamide, are now being implemented during hormone sensitive stages of prostate cancer. Loss of androgen dependency is associated with treatment failure and further disease progression.
Unraveling the molecular drivers of lineage plasticity and phenotypic reprogramming in prostate cancer is critical for addressing therapeutic resistance and disease progression. This knowledge will enable the development of novel strategies to overcome treatment resistance and improve survival outcomes for patients with advanced prostate cancer.
Signaling targets for EMT-directed therapy
Transcriptional activation pathways
Numerous epithelial-mesenchymal transition (EMT) transcription factors (EMT-TFs) have been identified as critical regulators of the EMT process. Key EMT-TFs include the zinc-finger homeobox (ZEB) family, SNAIL family, and TWIST family. These factors act in concert to decrease cell adhesion, disrupt cell polarity, and drive EMT and metastasis [23,24]. The ZEB family, particularly ZEB1 and ZEB2, binds to E-box regions of the CDH1 promoter to suppress E-cadherin expression [25]. ZEB1 also represses Syndecan-1, a proteoglycan essential for maintaining epithelial integrity [26]. Elevated ZEB expression correlates with higher tumor grade and therapeutic resistance in prostate, bladder, breast, and other cancers [27]. Additionally, ZEB1 represses genes such as HUGL2 and Crumbs3, which are crucial for cell polarity [28]. The expression of ZEB is regulated by several pathways, including receptor tyrosine kinases (RTKs), TGF-β via SMAD signaling, Wnt, PI3K/Akt, and NF-κB pathways [29-31]. The SNAIL family, including SNAI1, SNAI2, and SNAI3, also suppresses CDH1 transcription upon nuclear accumulation, leading to reduced E-cadherin levels. Elevated SNAIL expression has been linked to high tumor grade and metastatic potential in prostate [32,33], breast [34], ovarian [35], and hepatocellular cancers [36]. Notably, SNAIL promotes metastasis through both enhanced local invasion and immunosuppressive mechanisms [37].
TWIST1 and TWIST2, members of the helix-loop-helix family, drive EMT by directly suppressing CDH1 expression and increasing N-cadherin expression [38]. In prostate cancer, TGF-β1, BMP2, and BMP4 serve as key EMT drivers [39-41]. These ligands activate the TGF-β receptor complex, leading to SMAD-dependent transcription of EMT-TFs such as SNAIL, TWIST, and ZEB [42]. Additionally, TGF-β signaling activates alternative pathways, including PI3K/Akt, Ras, FAK, and Src, further promoting EMT [43,44]. Growth factors such as HGF and IGF-1 utilize ERK signaling to enhance ZEB1 expression in prostate cancer cells [45], while PDGF has been shown to augment N-cadherin expression by suppressing E-cadherin during gastrulation [46]. Increased Src expression is associated with metastasis and castration-resistant prostate cancer (CRPC) [47,48]. FGF and FGFR are also markedly overexpressed during the transition from androgen dependence to CRPC [49]. IL-6 secreted by prostate cancer cells induces cancer-associated fibroblasts to disrupt cell polarity in cancer cells, promoting EMT [50].
The Wnt pathway is another major EMT regulator. Binding of the Wnt ligand to lipoprotein receptor-related protein (LRP) phosphorylates LRP, recruiting Dishevelled and Axin, which allows β-catenin to translocate to the nucleus. Nuclear β-catenin binds to LEF-1, suppressing CDH1 and reducing E-cadherin expression [51,52]. Wnt signaling also indirectly promotes SNAIL and TWIST expression via β-catenin nuclear activity [53,54]. The Gli family of transcription factors, particularly Gli1, enhances SNAIL transcription [55] and has been implicated in EMT-mediated invasion of neuroendocrine tumors [56]. Hedgehog signaling activates Gli1 through the binding of the Hh ligand to PTCH receptors, leading to Smoothened-mediated Gli1 expression [57]. Epigenetic reprogramming further modulates EMT through repression of polycomb function, promoting tumor growth and tissue invasion [58]. Extracellular heat-shock protein (Hsp) 90, secreted by tumor cells, interacts with EZH2 to drive EMT and neuroendocrine differentiation via histone methylation (H3K27) [58].
Mechanisms of EMT navigate prostate cancer progression
Epithelial-mesenchymal transition (EMT) is marked by the loss of epithelial markers such as E-cadherin and β-catenin, and the gain of mesenchymal markers like N-cadherin, vimentin, and fibronectin [59]. These markers are not merely identifiers but play pivotal roles in maintaining epithelial integrity and driving mesenchymal phenotypes. E-cadherin and β-catenin, essential for adherens junctions, ensure cell polarity and epithelial cohesion [60]. EMT transcription factors (EMT-TFs), including ZEB, SNAIL, and TWIST families, orchestrate EMT by suppressing epithelial markers and promoting mesenchymal ones [61]. For instance, ZEB and SNAIL bind E-box sequences in the CDH1 promoter to repress E-cadherin [62], while TWIST recruits nucleosome remodeling complexes to suppress epithelial markers [63], and upregulate mesenchymal markers like vimentin through Cullin2 circular RNAs [64]. Hypoxia-inducible factor 1α (HIF1α) and cytokines such as transforming growth factor β (TGF-β) are significant EMT initiators. HIF1α directly binds hypoxia response elements in the SNAIL1 promoter, driving its transcription [65], while hypoxia also upregulates ZEB1 in prostate cancer models [66].
Transforming growth factor β (TGF-β) signaling also regulates EMT. In healthy tissues, TGF-β can function as a tumor suppressor by phosphorylating SMAD proteins to regulate tissue differentiation and maintain tissue identity [67]. In its canonical pathway, TGF-β phosphorylates Smad proteins, which form complexes that translocate to the nucleus to regulate tissue differentiation genes, often acting as a tumor suppressor [68]. Loss of TGF-β receptor II (TGFBR2) is associated with higher Gleason scores, increased metastasis, and stemness-related genes like Sox2 and Nanog, highlighting the connection between EMT and lineage plasticity [69,70]. Non-canonical TGF-β signaling activates PI3K/AKT and MAPK/ERK pathways, downregulating tight junction proteins and upregulating EMT TFs like SNAIL and SLUG, thereby promoting cell migration and invasion [71-74].
Receptor tyrosine kinases (RTKs) further propel EMT by activating key transcription factors and signaling pathways (Figure 2). Among RTKs, fibroblast growth factor receptor 1 (FGFR1) is particularly implicated in prostate cancer progression, EMT, and treatment resistance. FGFR1 is minimally expressed in normal prostate tissue but becomes prominent in adenocarcinoma [75]. Mouse models demonstrate that FGFR1 overexpression induces EMT-like gene signatures and promotes tumor aggressiveness, while FGFR1 loss reduces tumor growth [76,77]. FGFR1 isoforms also play a role; the epithelial FGFR1 IIIb and mesenchymal FGFR1 IIIc isoforms contribute to EMT through crosstalk with TGF-β and Wnt signaling pathways, activating SNAIL and TWIST [75,78]. FGFR1-mediated MAPK activation is particularly crucial in AR-null prostate tumors, driving anti-androgen therapy resistance [49] and promoting metastasis [79,80]. Noncanonical FGFR ligands like Gremlin1 (GREM1), upregulated by ADT, activate FGFR1/MAPK signaling to induce stemness and suppress AR activity, further contributing to lineage plasticity and therapy resistance [49,50,80]. In TP53 and RB1 knockout models of prostate cancer cell plasticity, pathways involving FGFR1 and EMT expression were concurrently activated, leading to enzalutamide resistance [81]. These findings confirm that FGFR1 signaling also drives EMT and lineage plasticity in prostate cancer and serve as a potential pathway for treatment resistance. Other RTKs, such as EGFR and IGFR, also support EMT progression. EGFR enhances SNAIL stability by preventing its ubiquitination and promotes TWIST expression via HIF1α/STAT3 signaling [82,83]. RTKs synergize with TGF-β signaling, amplifying EMT and promoting autocrine production of ligands to sustain their activation [84].
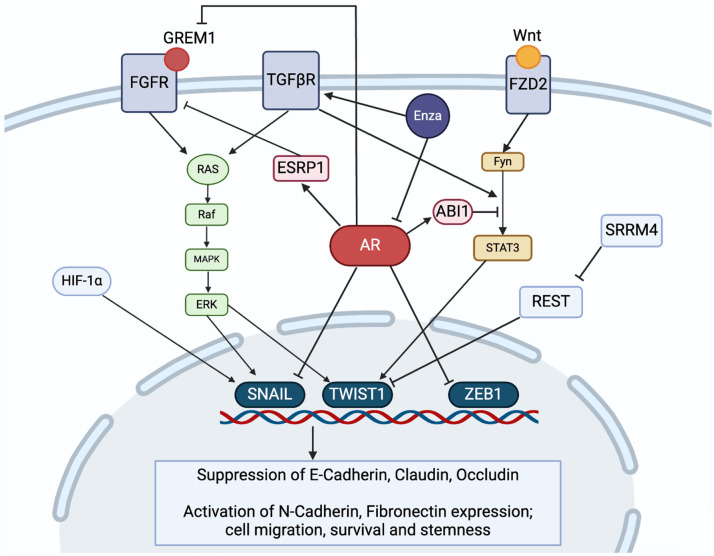
Figure 2.
Pathways of EMT regulation in prostate cancer. EMT in prostate cancer has been shown to be regulated by various pathways, including non-canonical Wnt, receptor tyrosine kinase signaling pathways such as FGFR, and TGFβ signaling. Changes in alternative splicing factors, such as ESRP1 and SRRM4 also affect EMT. The loss of AR signaling due to Enza suppression has been shown to drive EMT progression through its interactions with many of these regulators.
At the end of many signal transduction cascades lie the Signal Transducer and Activator of Transcription (STAT) family of proteins. Upon phosphorylation by tyrosine kinases, STATs are activated and enter the nucleus, where they bind DNA and promote transcription. In prostate cancer, STAT3 is of particular importance, as higher phospho-STAT3 levels are correlated with higher Gleason scores and increased bone metastases [85,86]. During prostate cancer progression, STAT3 activation has been shown to be driven as well by TGF-β1 signaling and is able to directly upregulate TWIST expression through binding to the TWIST1 promoter in a HSP27-dependent manner [87,88]. STAT3 is also part of the non-canonical, or β-catenin-independent Wnt signaling pathway. In this pathway, Wnt binds the Frizzled2 (Fzd2) receptor, which then activates STAT3 to promote EMT through its phosphorylation by Fyn tyrosine kinase [89]. In prostate cancer, non-canonical Wnt signaling was found to be elevated in high Gleason grade patient samples and promoted expression of the mesenchymal markers N-cadherin, vimentin while downregulating E-cadherin [90]. This pathway also includes the actin-cytoskeleton regulator ABI1, which interacts with Fyn and acts upstream of STAT3 (Figure 2). The loss of ABI1 was found to increase STAT3 phosphorylation, activation, and the expression of mesenchymal markers such as Slug and vimentin [91]. STAT3 has also been shown to be hyperactivated in TP53 and RB1 knockout models of prostate cancer plasticity, and promotes enzalutamide resistance through the expression of stemness markers such as SOX2 downstream of Janus kinase (JAK) activation [22,81,92]. As a result, STAT3 inhibitors were able to effectively target cancer stem cells in both in vitro and in vivo models of prostate cancer [93]. In this sense, STAT3 has been shown to be another master regulator of EMT and cancer progression through its interconnection between a wide network of pathways.
Alternative splicing in EMT
Alternative splicing also influences EMT by modulating epithelial and mesenchymal markers [94,95]. Loss of epithelial splicing regulator proteins (ESRPs) and upregulation of RBFOX2 drive mesenchymal phenotypes [64,65,95,96]. ESRPs maintain epithelial characteristics by regulating splicing events critical for cell adhesion and migration, such as FGFR2 isoforms. ESRP promotes the epithelial FGFR2 IIIb variant, while its loss enables the mesenchymal FGFR2 IIIc variant, inducing EMT and enhancing invasiveness [95,97]. ESRP1 is also able to suppress the expression of pluripotency transcription factors such as OCT4, SOX2, and Nanog [98]. Thus, the loss of ESRP in EMT changes protein-protein interaction networks, leads to the increased mesenchymal features of cell invasion and migration, and the development of pluripotency needed to undergo transdifferentiation. Another RNA splicing mediated driver of EMT is the loss of repressor element 1 silencing transcription factor (REST). In heathy tissues, REST serves to repress neuronal development and differentiation [99]. In neuroendocrine prostate cancer, the splicing factor SRRM4 can generate alternative splice isoforms of ABI1 [99] as well as nonfunctional isoform of REST, which then allows for the expression of various neuroendocrine markers [99,100]. However, REST has also been shown to repress the expression of TWIST1 (Figure 2), and thus the loss of REST by SRRM4-mediated alternative splicing can promote both neuroendocrine differentiation and EMT [101].
The EMT-MET dynamic dictates metastatic behavior of prostate tumors
The primary metastatic site for prostate cancer is the bone, and progression to metastatic prostate cancer reduced the five-year survival rate to 30% [1]. The functional contribution of cell plasticity/phenotypic conversions to further advance prostatic tumor progression to metastasis, via facilitating migration, invasion, and colonization at distant sites continues to be a topic of focus for CRPC research [13]. EMT is a key determinant of cancer progression and for metastasis, associated with increased invasive potential and therapeutic resistance of tumors. Phenotypic reprogramming via EMT, involves the adoption of mesenchymal characteristics, loss of cell polarity and tight junctions [102]. The dynamic and reversible nature of EMT, and interconversion between epithelial and mesenchymal phenotypes contributes to both the dissemination of cancer cells from the primary tumor, and re-colonization at the metastatic site. These function of EMT in these processes are impacted by signaling in the tumor microenvironment and as a result of prostate cancer treatment (both ADT and taxane chemotherapy).
Within the primary tumor, prostate cancer cells remain unable to migrate with cell-cell adhesions formed by adherens and tight junctions (E-cadherin, integrins). As the master regulator of EMT, TGF-β can modulate the tumor microenvironment to facilitate cell invasion and progression to metastasis [103,104]. TGF-β produced by tumor cells, immune cells and fibroblasts in the TME, can induce phenotypic transition to mesenchymal through transcriptional regulators of EMT including Snail, Zeb1, and Twist, leading to the degradation of adhesion molecules such as E-cadherin and detachment from the basement membrane [105,106]. Simultaneously, this master regulator of EMT activates the cancer associated fibroblasts (CAFs) in the stroma to produce pro-angiogenic factors hypoxia-inducible factor (HIF-1α), and β-catenin transcriptionally promotes expression of vascular endothelial growth factor (VEGF) [107]. Induction of factors driving vascularity and angiogenesis promote the intravasation of prostate cancer cells through the basement membrane into the bloodstream, where these mesenchymal circulating tumor cells (CTCs) can travel to other organs at adjacent or distant sites [108]. EMT regulators Snail1, Snail2, and SLUG promote the degradation of the ECM by induction of matrix metalloproteases (MMPs) MT4-MMP and MMP2, while Snail1 suppresses expression of MMP9 [109,110]. Recent studies suggest that some prostate cancer cells do not adhere to the specific roles of mesenchymal or epithelial phenotypes through progression of metastasis, and therefore there may be cells that exist in a hybrid EMT-MET state expressing both epithelial and mesenchymal markers such as E-cadherin and vimentin concurrently [111,112]. Metastatic spread can be facilitated through expression of chemoattractant proteins such as CCL2, which can be suppressed by AR-signaling and can promote EMT directly or under the regulation of TGF-β [113,114]. When the cancer cells in circulation reach the secondary organs, they must cross the endothelium to exit the bloodstream through extravasation. This process is stimulated by blood platelets, and there is some evidence for a feedback loop between TGF-β and transcription factor PRH/HHEX whereby a downregulation of PRH by TGF-β induced EMT and extravasation [115]. There is some evidence that EMT transcriptional regulator Twist1 promotes extravasation of cancer cells through integrin β1 [116]. Through reversal of EMT, prostate cancer cells re-differentiate towards the epithelial phenotype so generate secondary tumors. Tumor cells at secondary sites of solid tumors are often more poorly differentiated than the primary tumor of origin but share similar epithelial profiles [116,117]. The interplay between cancer cells and the bone microenvironment by TGF-β drives metastatic spread. TGF-β released from the bone as a result of bone remodeling promotes cancer cell proliferation and osteoclast-mediated bone resorption in the metastatic site [118].
The role of androgen receptor signaling in prostate cancer EMT
The role of androgens and androgen deprivation therapies (ADT) in promoting EMT and stemness is complex and remains a topic of debate. Consistent with the understanding that prostate cancer development is androgen-driven, early studies suggested that androgens could promote the expression of EMT markers. For instance, androgen stimulation in prostate cancer cell lines has been shown to upregulate EMT transcription factors such as ZEB1 and TWIST1 [119-121]. This regulation is partly attributed to androgen response elements (AREs) upstream of the ZEB1 gene, implying direct androgen receptor (AR) involvement in its transcription [119,122]. Interestingly, TWIST1 silencing also reduces AR expression, highlighting a reciprocal regulatory relationship between the two genes [123]. Conversely, suppression of AR signaling, such as through ADT or anti-androgen treatments, has been shown to induce EMT, increase stemness, promote metastasis, and facilitate lineage switching in prostate cancer. For example, N-cadherin is consistently upregulated in castration-resistant prostate cancer (CRPC) models but not in hormone-sensitive cell lines or xenografts [124]. Furthermore, physical and chemical castration in mouse models and prostate cancer organoids induces ZEB1 expression, accompanied by increased levels of mesenchymal markers such as N-cadherin and vimentin [125]. Notably, ZEB1 engages in a negative feedback loop with AR, suggesting that ZEB1 expression is not directly promoted by AR signaling but rather by its inhibition. Supporting this, ZEB1 ARE reporter assays are active even in AR-null prostate cancer cell lines [122]. SNAIL is another transcription factor regulated by AR signaling. In the C4-2 prostate cancer model, SNAIL expression is suppressed by dihydrotestosterone (DHT) treatment, with AR binding to the SNAI1 gene to repress its transcription [126]. Conversely, enzalutamide treatment induces SNAIL expression and upregulates mesenchymal markers such as N-cadherin, vimentin, and fibronectin in a reversible manner [126].
Enzalutamide has also been shown to enhance cell invasion via activation of TGF-β1/Smad3 signaling [127]. Inhibiting TGF-β receptor 1 reverses EMT and synergizes with enzalutamide treatment in preclinical transgenic mouse models of prostate cancer [30]. Similarly, under androgen deprivation environment, such as culturing LNCaP cells in charcoal-stripped serum, can result in the upregulation of EMT markers (N-cadherin, vimentin, and SNAIL) in stem-like cell populations compared to non-stem progenitors [128].
Anti-androgen treatment can activate STAT3 and non-canonical Wnt signaling pathways, further driving EMT progression. Loss of ABI1, an androgen-responsive gene downregulated by enzalutamide, has been linked to increased STAT3 activation [91,129]. Furthermore, epithelial splicing regulators (ESRPs), which are androgen-responsive genes, are lost following ADT or anti-AR therapy [130]. Hence loss of AR signaling by ADT and anti-AR therapy could contribute to pluripotency and transdifferentiation, driving the epithelial-to-mesenchymal transition via alternative splicing in critical proteins like FGFR2 and others.
In summary, while AR signaling promotes EMT under certain conditions, AR inhibition via ADT or anti-androgen therapies often exacerbates EMT, stemness, and treatment resistance. These findings underscore the dual roles of AR signaling in prostate cancer progression and highlight the need for therapeutic strategies that address the complex interplay between EMT, stemness, and lineage plasticity.
The EMT-MET dynamics as a mechanism of therapeutic resistance
Prostate cancer, a hormonally responsive malignancy, relies on androgen signaling for growth. Standard treatments such as androgen deprivation therapy (ADT) and AR-targeted therapies (e.g., enzalutamide) aim to inhibit this pathway. However, resistance often develops, leading to castration-resistant prostate cancer (CRPC), where these therapies become ineffective (Figure 1). One mechanism underlying this progression is the induction of EMT during androgen deprivation.
A negative feedback loop between AR and ZEB1, a key transcriptional regulator of EMT, has been implicated in promoting EMT under androgen-deprived conditions [125]. In LNCaP cells cultured in androgen-depleted conditions, EMT is characterized by low E-cadherin, high vimentin, elevated ZEB1 levels, and a reduction in miR-200b, a microRNA known to inhibit ZEB1 [131-133]. Several microRNAs associated with an epithelial phenotype, including miR-143, miR-145, miR-29b, miR-34b, and the miR-200 family, have been linked to resistance to ADT and docetaxel [134]. Similarly, in LNCaP tumor xenograft models, castration-resistant tumors exhibit upregulation of EMT regulator TWIST1 and other mesenchymal markers [135]. Clinically, biopsies from prostate cancer patients show increased N-cadherin expression after three months of ADT, supporting the role of EMT in therapy resistance [136].
In response to enzalutamide, EMT can be induced via upregulation of SNAIL, a transcription factor repressed by AR, and TGF-β expression, which facilitates survival in androgen-deprived environments [126,137]. As a “master regulator” of EMT, TGF-β is heavily implicated in CRPC progression. Dysregulated TGF-β signaling, such as through a dominant negative TGF-β type II receptor (DNTGF-βRII), promotes increased tumor growth and reduced apoptosis after ADT in animal models. This aberrant signaling also enhances nuclear localization of AR and β-catenin, demonstrating crosstalk between TGF-β, AR, and EMT pathways in CRPC progression [31]. Interestingly, androgens can also induce EMT independently of TGF-β via SNAIL activation, with low AR content sensitizing cells to androgen-mediated EMT [120]. Furthermore, ADT leads to the accumulation of CCL2, a chemokine repressed by AR signaling, which is associated with EMT induction and increased resistance [113,114].
Taxane chemotherapy further contributes to the phenotypic dynamics of EMT-MET and therapeutic resistance. TGF-β signaling drives resistance to taxanes, reducing docetaxel sensitivity in prostate cancer cells [138]. Mechanistically, TGF-β induces KLF5 acetylation, which upregulates and stabilizes the pro-survival protein Bcl-2, enhancing cell survival during docetaxel treatment [139]. However, in preclinical models of androgen-sensitive prostate cancer, cabazitaxel combined with enzalutamide induces EMT reversion toward an epithelial phenotype [140]. Similarly, in patient-derived xenograft models of therapeutically resistant CRPC, cabazitaxel treatment after ADT cessation restores an epithelial phenotype in circulating tumor cells [141]. The phenotypic interconversion between EMT and MET is not only central to taxane resistance and metastasis but may also reveal windows of therapeutic vulnerability [32]. Targeting this dynamic EMT-MET process offers a promising strategy to overcome resistance, which will be explored in the next section.
Neuroendocrine differentiation in prostate cancer: EMT-MET link in emergence of treatment-induced NEPC
CRPC initially remains androgen-sensitive and is treated with androgen receptor pathway inhibitors (ARPIs) such as enzalutamide and abiraterone [10]. However, prolonged ARPI therapy often leads to androgen resistance and neuroendocrine differentiation (NED) in CRPC cells [135]. These neuroendocrine-like cells lose AR expression and acquire neuroendocrine markers, such as chromogranin A, neuron-specific enolase, and synaptophysin [15,142]. By escaping AR pathway inhibition, these cells continue to proliferate, resulting in treatment-induced neuroendocrine prostate cancer (T-NEPC), a lethal form of prostate cancer that arises in advanced stages of CRPC treatment [10]. The increasing use of potent ARPIs has contributed to a rise in T-NEPC incidence [10]. Emerging evidence suggests that epithelial-mesenchymal transition (EMT) and its reverse, mesenchymal-epithelial transition (MET), play critical roles in T-NEPC development [23,143].
T-NEPC is characterized by overexpression of oncogenes such as AURKA and MYCN, which upregulate SOX and EZH2, promoting lineage plasticity and NED. These changes inhibit AR signaling and drive aggressive tumor behavior [144]. The loss of tumor suppressor genes TP53 and RB1 further exacerbates this process by enabling unchecked cell cycle progression and resistance to ADT [10]. Additionally, molecular pathways such as PI3K-AKT-mTOR and Wnt-ABI1-STAT3 are implicated in driving NED and tumor progression.
The EMT-MET interconversion dynamic is a fundamental molecular program involved in developmental processes, such as embryonic gastrulation and prostate gland formation [11]. In T-NEPC, tumors exhibit reprogramming to a pluripotent stem cell-like state, mediated by EMT-MET cycling [11]. Neuroendocrine cells pass through hybrid transition states, co-expressing epithelial markers (e.g., E-cadherin, EPCAM) and mesenchymal markers (e.g., N-cadherin, cadherin-11) [20]. This hybrid phenotype allows rapid adaptation to environmental cues and facilitates phenotypic plasticity, a hallmark of T-NEPC.
Mechanisms driving EMT-MET interconversion in T-NEPC
Several mechanisms have been proposed as driving induction of EMT-MET interconversion dynamic in the development of T-NEPC. One of the mechanisms involves ADT-mediated induction of AR splice variants, such as for example AR-V7, which are linked to EMT activation and stem-cell-like characteristics [145]. Another key regulator of EMT-MET is the Abelson Interactor 1 (ABI1) gene. The ABI1 gene stabilizes the WAVE complex, which is critical for maintaining cell-cell adhesion. Loss of ABI1 reduces adhesion and increases cell migratory potential [91]. In prostate cancer cell models, ABI1 depletion activates non-canonical Wnt signaling, leading to STAT3 activation and transcription of EMT-related genes [129]. Importantly, ABI1 expression is suppressed by anti-AR treatments and in NEPC tumors, suggesting co-regulation between ABI1 and AR, and positioning ABI1 as a potential therapeutic target in NEPC [47].
TGF-β is a critical driver of EMT-MET interconversions, functionally activates serine-threonine membrane kinases, which phosphorylate Smad proteins. These Smad complexes translocate to the nucleus and regulate EMT-MET-related gene transcription. During ADT, AR downregulation enhances ZEB1 expression, inducing EMT and promoting mesenchymal characteristics. EMT transcription factors, such as SNAIL, SLUG, and TWIST, repress E-cadherin, disrupt epithelial integrity, and drive plasticity [23]. Additionally, TWIST suppresses apoptosis and promotes angiogenesis, further contributing to T-NEPC progression [23]. The interplay between EMT-MET dynamics, AR signaling, and lineage plasticity underscores the complex molecular mechanisms driving T-NEPC development. Understanding these processes provides a foundation for developing targeted therapies to combat this lethal form of prostate cancer.
Translational impact of lineage plasticity: in therapy and biomarker development
Targeting EMT-MET to overcome resistance: preclinical studies
Exploiting the dynamic interplay of EMT-MET in prostate cancer progression and therapeutic resistance offers a promising opportunity to enhance treatment responses and improve patient survival [9]. Understanding windows of susceptibility during these transitions may help optimize standard-of-care treatments without requiring significant regulatory changes.
Preclinical studies from our group have shown that cabazitaxel promotes MET, driving prostate cancer cells toward epithelial phenotypes [13]. This phenotypic shift reduces tumor invasiveness and may sensitize epithelial populations to further therapeutic interventions [32,146,147]. For example, androgen deprivation therapy (ADT), which targets the N-terminal transcriptional activity of AR, induces EMT-MET interconversions, leading to improved responses to docetaxel in preclinical models [148]. Sequencing ADT before taxane chemotherapy, such as cabazitaxel, has shown efficacy in androgen-sensitive prostate cancer [149]. However, this strategy is less effective in castration-resistant models. In recent studies, cabazitaxel administered after ADT cessation promoted an epithelial phenotype in patient-derived xenograft (PDX) models of CRPC, providing additional rationale for its use in therapeutic sequencing [141].
Upfront treatment with taxane chemotherapy, as demonstrated in the STAMPEDE trials, supports this approach clinically. Administering docetaxel within nine weeks of initiating ADT resulted in a 10-month survival benefit, a 22% reduction in mortality risk, and delayed metastatic progression [150,151]. Targeting transcriptional regulators of EMT, such as TWIST, has also shown promise in enhancing taxane sensitivity, creating therapeutically vulnerable phenotypes [152]. While directly targeting EMT transcription factors poses challenges due to potential off-target effects, research has shifted toward inhibiting downstream effectors. For instance, monoclonal antibodies against the mesenchymal marker N-cadherin delayed tumor growth and progression in preclinical models by reducing activity in downstream pathways, including IL-6, IL-8, and AKT signaling [124]. Early clinical trials using Exherin (ADH-1), an antagonistic N-cadherin peptide, have shown delayed progression to castration resistance with modest improvements in CRPC outcomes [136,153].
Further research is needed to elucidate the precise role of EMT-MET dynamics in the development of treatment-induced neuroendocrine prostate cancer (T-NEPC). Targeting programs related to neuroendocrine differentiation (NED) and EMT-MET cycling may offer novel therapeutic avenues. Promising approaches include inhibitors of MYCN/AURKA, EZH2 [15,20] and the Wnt-ABI1-STAT3 pathway, as well as blockers of IL-6-STAT3 and mTOR signaling [91]. These strategies hold potential to mitigate therapeutic resistance and improve outcomes in aggressive, treatment-resistant prostate cancer.
Clinical trials exploiting EMT-targeted therapy
Efforts to target epithelial-mesenchymal transition (EMT) pathways in metastatic castration-resistant prostate cancer (mCRPC) have focused on impairing EMT-MET signaling to improve progression-free survival (PFS). To date, 22 clinical trials have investigated EMT-targeted therapies in mCRPC (Table 1). While these studies demonstrate the potential for EMT-targeted approaches, they also underscore the challenges associated with these therapies.
Table 1.
Summary of clinical trials exploring epithelial-mesenchymal transition targeted therapy
| NCT Number | Year | Drug | Target | Phase | Design | Status | Outcome of Interest | Findings |
|---|---|---|---|---|---|---|---|---|
| NCT00992186 | 2012 | Carlumab | CCL2 | Phase 2 | Single-arm | Completed | Progression-free survival of patients with CRPC who were previously treated with Docetaxel | Carlumab was well tolerated, but did not show antitumor activity as a monotherapy against CRPC |
| NCT00433446 | 2013 | Siltuximab | Interleukin 6 | Phase 2 | Single-arm | Completed | Progression-free survival after receiving Siltuximab in patients with CRPC who received prior taxane therapy | Siltuximab resulted in 3.8% PSA response rate, and 23% of patients had stable disease as per the RECIST criteria. Baseline elevated IL6 was associated with poorer prognosis |
| NCT00385827 | 2014 | Siltuximab | Interleukin 6 | Phase 2 | Two-arm nonrandomized | Terminated | Progression-free survival after receiving Siltuximab + mitoxantrone/prednisone versus mitoxantrone/prednisone alone | Though Siltuximab was well tolerated, there was no improvement in progression-free survival and so the study was terminated |
| NCT00401765 | 2014 | Siltuximab | Interleukin 6 | Phase 1 | Single-arm | Completed | Safety and efficacy of using Siltuximab in combination with Docetaxel in patients with CRPC | No results posted |
| NCT00487786 | 2016 | Apatorsen | Heat Shock Protein 27 inhibitor | Phase 1 | Single-arm | Completed | Safety and Efficacy of Apatorsen monotherapy in patients with CRPC, breast, ovary, lung, or bladder cancer | Apatorsen was well tolerated and can be safely administered |
| NCT01120470 | 2019 | Apatorsen | Heat Shock Protein 27 inhibitor | Phase 2 | Two-arm | Completed | Progression-free survival of apatorsen with prednisone compared to prednisone alone in chemo-naïve patients | The addition of apatorsen did not increase progression-free survival when compared to prednisone alone |
| NCT01681433 | 2022 | Apatorsen | Heat Shock Protein 27 inhibitor | Phase 2 | Two-arm | Terminated | Progression-free survival of patients receiving Abiraterone and prednisone with apatorsen compared to abiraterone and prednisone alone | Study terminated due to lack of accrual |
| NCT01270880 | 2018 | Ganetespib | Heat Shock Protein 90 inhibitor | Phase 1 | Single-arm | Completed | Six-month progression-free survival after Ganetespib in men with CRPC who received prior docetaxel therapy | As a single therapy, Ganetespib did not prolong progression free survival. All patients had disease progression by 6 months |
| NCT01685268 | 2019 | Onalespib | Heat Shock Protein 90 inhibitor | Phase 1 and 2 | Two-arm stratified by regimens | Completed | Tolerability and antitumor activity of onalespib in combination with abiraterone/prednisone | Onalespib with abiraterone/prednisone showed mild evidence of biological effect, but no clinical effect |
| NCT02065323 | 2015 | Dovitinib | Receptor Tyrosine Kinase inhibition | Phase 2 | Two-arm | Withdrawn | Progression-free survival in patients with mCRPC after Dovitinib therapy with ADT in comparison to ADT alone | Terminated due to budgetary considerations and length of development |
| NCT01741116 | 2018 | Dovitinib | Receptor Tyrosine Kinase inhibition | Phase 2 | Single-arm | Completed | Progression-free survival in patients with mCRPC after 16 weeks of Dovitinib therapy | Dovitinib showed modest anti-tumor activity with manageable toxicity. Patients who were chemo-naïve benefitted more |
| NCT01994590 | 2019 | Dovitinib | Receptor Tyrosine Kinase inhibition | Phase 2 | Single-arm | Terminated | Progression-free survival in patients with mCRPC after Dovitinib therapy in combination with Abiraterone | Study terminated as sponsor stopped supplying the drug |
| NCT00744497 | 2016 | Dasatinib | Tyrosine kinase inhibitor | Phase 3 | RCT | Completed | Overall survival after Docetaxel + Dasatinib in comparison to Placebo | The addition of dasatinib did not increase overall survival |
| NCT03761225 | 2021 | Masitinib | Tyrosine kinase inhibitor | Phase 3 | RCT | Completed | Progression-free survival after Docetaxel + Masitinib in comparison to Docetaxel + Placebo | No results posted |
| NCT01990196 | 2023 | Degarelix, Enzalutamide, Trametinib, Dasatinib | Src and/or MEK inhibition of tyrosine kinase | Phase 2 | Two-arm nonrandomized | Active, not recruiting | Change in molecular signature after AR inhibition with and without SRC and/or MEK inhibition | No results posted |
| NCT00887640 | 2014 | Temsirolimus | mTOR, VEGF | Phase 2 | Single-arm | Terminated | Impact of Temsirolimus on CTC count at 8 weeks | Study terminated |
| NCT03480646 | 2021 | CPI-1205 | EZH2 inhibitor | Phase 1 and 2 | Two-arm | Unknown | To assess safety and efficacy of CPI-1205, and to assess its antitumor activity in combination with enzalutamide or abiraterone/prednisone | Unknown |
| NCT03460977 | 2024 | Mevrometostat | EZH2 inhibitor | Phase 1 and 2 | Single-arm | Recruiting, preliminary findings posted | Safety and efficacy of Mevrometostat in combination with enzalutamide + ADT | Mevremostat + enzalutamide shows promising activity in combination with ADT with manageable adverse effect profile |
| NCT04179864 | 2024 | Tazemetostat | EZH2 inhibitor | Phase 1 and 2 | Single-arm | Active, not recruiting | Safety and efficacy of Tazemetostat in combination with Enzalutamide or Abiraterone/Prednisone | No results posted |
| NCT05413421 | 2023 | ORIC-944 | Polycomb receptor complex 2 inhibitor | Phase 1 | Single-arm | Recruiting | Safety and preliminary antitumor activity of ORIC-944 in patients with mCRPC | No results posted |
| NCT02204943 | 2018 | Radium-223 | Osteoblastic plasticity of CRPC | Phase 2 | Single-arm | Completed | Impact of Radium-223 on CTC B-ALP levels | CTC B-ALP expression was decreased in 31% of men |
| NCT02452008 | 2024 | Galunisertib | TGF-β Receptor Inhibitor | Phase 2 | RCT | Active, not recruiting | Progression-free survival after enzalutamide + Galunisertib compared to enzalutamide alone | No results posted |
CCL2 and interleukin-6 (IL-6) inhibitors
The earliest trials, initiated in 2012, explored CCL2 inhibition using Carlumab in patients with mCRPC who had failed docetaxel therapy (NCT00992186). Unfortunately, Carlumab monotherapy did not improve PFS [154]. Subsequent trials shifted focus to IL-6 inhibitors, such as Siltuximab. While Siltuximab showed modest anti-tumor activity in patients pretreated with taxanes (NCT00433446) [155], a phase 2 non-randomized trial combining Siltuximab with mitoxantrone/prednisone demonstrated no improvement in PFS compared to mitoxantrone/prednisone alone (NCT00385827). Consequently, this trial was discontinued due to lack of efficacy. The results of a Siltuximab-docetaxel combination trial are still pending (NCT00401765).
Heat Shock Protein (Hsp) inhibitors
Hsp inhibitors targeting Hsp27 and Hsp90 were explored for their potential to disrupt EMT. Apatorsen, an Hsp27 inhibitor, demonstrated good tolerability with minimal adverse effects in a phase 1 trial (NCT00487786) [156], leading to phase 2 trials. However, in a non-randomized trial, Apatorsen combined with prednisone did not improve PFS compared to prednisone alone (NCT01120470). Another trial combining Apatorsen with abiraterone and prednisone was terminated due to low patient accrual (NCT01681433). Hsp90 inhibitors Ganetespib and Onalespib were also evaluated, but neither showed clinical efficacy as monotherapy or in combination therapy (NCT01270880, NCT01685268).
Receptor tyrosine kinase (RTK) and tyrosine kinase (TK) inhibitors
Given the central role of RTKs in EMT signaling, RTK and TK inhibitors have also been investigated. Dovitinib, an RTK inhibitor, showed modest anti-tumor activity in a trial where chemo-naïve patients benefitted the most (NCT01741116) [157]. However, another Dovitinib trial combining it with ADT was discontinued due to budgetary constraints (NCT02065323). Similarly, direct TK inhibitors such as Dasatinib (NCT00744497, NCT01990196) and Masitinib (NCT03761225) did not yield favorable results [158].
Emerging therapies and future directions
Hsp inhibition has also been investigated in the context of EZH2, a downstream effector of Hsp that promotes EMT. Drugs such as CPI-1205, Mevrometostat, and Tezmetostat are currently being studied in mCRPC. Preliminary results with Mevrometostat combined with enzalutamide are promising (NCT03480646, NCT03460977, NCT04179864). Similarly, trials investigating TGF-β receptor inhibitors (Galunisertib, NCT02452008) and polycomb receptor complex 2 inhibitors (ORIC-944, NCT05413421) are ongoing (Table 1).
The limited success of EMT-targeted therapies can be attributed to several factors. Most clinical trials targeted single EMT pathways, which may have allowed compensation by alternative pathways, limiting efficacy. Additionally, economic constraints led to the termination of some trials, highlighting the financial challenges of developing novel drugs. Despite these setbacks, these therapies were generally well-tolerated, suggesting that EMT-targeted strategies could be safely integrated into mCRPC treatment regimens. Future clinical trials should focus on combining drugs that target convergent EMT pathways to enhance efficacy while maintaining cost-effectiveness. Moreover, given the role of TGF-β receptor activity and long non-coding RNAs in regulating cancer cell apoptosis, future studies could prioritize apoptosis as a primary endpoint to better evaluate therapeutic effectiveness [104,159-162]. Future clinical trials exploring the combination of drugs blocking convergent EMT pathways, which are cost-effective, and can demonstrate the efficacy of mono- or combination therapies may provide mCRPC patients with an alternate and safe form of therapy, thereby blocking progression to advanced disease and improving survival.
Acknowledgements
This work was supported by DoD HT94252410044 (LK); NCI R21 CA260381 (LK); and NIH/NCI R01 CA232574 (NK).
Disclosure of conflict of interest
None.
Abbreviations
- ADT
Androgen deprivation therapy
- AR
Androgen receptor
- ARPIs
Androgen receptor pathway inhibitors
- CAFs
Cancer associated fibroblasts
- CRPC
Castration resistant prostate cancer
- CTCs
Circulating tumor cells
- ECM
Extracellular matrix
- EMT
Epithelial-mesenchymal transition
- ESRP
Epithelial splicing receptor protein
- LHRH
Leuteinising hormone-releasing hormone
- mCRPC
Metastatic castration resistant prostate cancer
- MET
Mesenchymal-epithelial transition
- NED
Neuroendocrine differentiation
- NEPC
Neuroendocrine prostate cancer
- PCa
Prostate cancer
- PDX
Patient derived xenograft
- PIN
Prostatic intraepithelial neoplasia
- PSA
Prostate specific antigen
- RTKs
Receptor tyrosine kinases
- STAT
Signal Transducer and Activator of Transcription
- TFs
Transcription factors
- TK
Tyrosine kinase
- TME
Tumor microenvironment
- T-NEPC
Treatment induced neuroendocrine prostate cancer
References
- 1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 2.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–62. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 3.Rennebeck G, Martelli M, Kyprianou N. Anoikis and survival connections in the tumor microenvironment: is there a role in prostate cancer metastasis? Cancer Res. 2005;65:11230–5. doi: 10.1158/0008-5472.CAN-05-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyprianou N. ASK-ing EMT not to spread cancer. Proc Natl Acad Sci U S A. 2010;107:2731–2. doi: 10.1073/pnas.0914721107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–51. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong D, Banerjee S, Ahmad A, Li Y, Wang Z, Sethi S, Sarkar FH. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS One. 2010;5:e12445. doi: 10.1371/journal.pone.0012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15:4792–8. doi: 10.1158/1078-0432.CCR-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akamatsu S, Inoue T, Ogawa O, Gleave ME. Clinical and molecular features of treatment-related neuroendocrine prostate cancer. Int J Urol. 2018;25:345–51. doi: 10.1111/iju.13526. [DOI] [PubMed] [Google Scholar]
- 11.Grant CM, Kyprianou N. Epithelial mesenchymal transition (EMT) in prostate growth and tumor progression. Transl Androl Urol. 2013;2:202–11. doi: 10.3978/j.issn.2223-4683.2013.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies AH, Beltran H, Zoubeidi A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat Rev Urol. 2018;15:271–86. doi: 10.1038/nrurol.2018.22. [DOI] [PubMed] [Google Scholar]
- 13.Beltran H, Hruszkewycz A, Scher HI, Hildesheim J, Isaacs J, Yu EY, Kelly K, Lin D, Dicker A, Arnold J, Hecht T, Wicha M, Sears R, Rowley D, White R, Gulley JL, Lee J, Diaz Meco M, Small EJ, Shen M, Knudsen K, Goodrich DW, Lotan T, Zoubeidi A, Sawyers CL, Rudin CM, Loda M, Thompson T, Rubin MA, Tawab-Amiri A, Dahut W, Nelson PS. The role of lineage plasticity in prostate cancer therapy resistance. Clin Cancer Res. 2019;25:6916–24. doi: 10.1158/1078-0432.CCR-19-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein JI, Amin MB, Beltran H, Lotan TL, Mosquera JM, Reuter VE, Robinson BD, Troncoso P, Rubin MA. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol. 2014;38:756–67. doi: 10.1097/PAS.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, Wang Y, Sheikh KL, Terry S, Tagawa ST, Dhir R, Nelson JB, de la Taille A, Allory Y, Gerstein MB, Perner S, Pienta KJ, Chinnaiyan AM, Wang Y, Collins CC, Gleave ME, Demichelis F, Nanus DM, Rubin MA. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1:487–95. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–40. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 17.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Fléchon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 18.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies A, Nouruzi S, Ganguli D, Namekawa T, Thaper D, Linder S, Karaoğlanoğlu F, Omur ME, Kim S, Kobelev M, Kumar S, Sivak O, Bostock C, Bishop J, Hoogstraat M, Talal A, Stelloo S, van der Poel H, Bergman AM, Ahmed M, Fazli L, Huang H, Tilley W, Goodrich D, Feng FY, Gleave M, He HH, Hach F, Zwart W, Beltran H, Selth L, Zoubeidi A. An androgen receptor switch underlies lineage infidelity in treatment-resistant prostate cancer. Nat Cell Biol. 2021;23:1023–34. doi: 10.1038/s41556-021-00743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, Goodrich MM, Labbé DP, Gomez EC, Wang J, Long HW, Xu B, Brown M, Loda M, Sawyers CL, Ellis L, Goodrich DW. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355:78–83. doi: 10.1126/science.aah4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, Wongvipat J, Ku SY, Gao D, Cao Z, Shah N, Adams EJ, Abida W, Watson PA, Prandi D, Huang CH, de Stanchina E, Lowe SW, Ellis L, Beltran H, Rubin MA, Goodrich DW, Demichelis F, Sawyers CL. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355:84–8. doi: 10.1126/science.aah4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dicken H, Hensley PJ, Kyprianou N. Prostate tumor neuroendocrine differentiation via EMT: the road less traveled. Asian J Urol. 2019;6:82–90. doi: 10.1016/j.ajur.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broster SA, Kyprianou N. Epithelial-mesenchymal transition in prostatic disease. Future Oncol. 2015;11:3197–206. doi: 10.2217/fon.15.253. [DOI] [PubMed] [Google Scholar]
- 25.Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–85. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 26.Farfán N, Ocarez N, Castellón EA, Mejía N, de Herreros AG, Contreras HR. The transcriptional factor ZEB1 represses Syndecan 1 expression in prostate cancer. Sci Rep. 2018;8:11467. doi: 10.1038/s41598-018-29829-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Oquendo M, Gibbons DL. Regulation of ZEB1 function and molecular associations in tumor progression and metastasis. Cancers (Basel) 2022;14:1864. doi: 10.3390/cancers14081864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aigner K, Dampier B, Descovich L, Mikula M, Sultan A, Schreiber M, Mikulits W, Brabletz T, Strand D, Obrist P, Sommergruber W, Schweifer N, Wernitznig A, Beug H, Foisner R, Eger A. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26:6979–88. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gheldof A, Hulpiau P, van Roy F, De Craene B, Berx G. Evolutionary functional analysis and molecular regulation of the ZEB transcription factors. Cell Mol Life Sci. 2012;69:2527–41. doi: 10.1007/s00018-012-0935-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paller C, Pu H, Begemann DE, Wade CA, Hensley PJ, Kyprianou N. TGF-β receptor I inhibitor enhances response to enzalutamide in a pre-clinical model of advanced prostate cancer. Prostate. 2019;79:31–43. doi: 10.1002/pros.23708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pu H, Begemann DE, Kyprianou N. Aberrant TGF-β signaling drives castration-resistant prostate cancer in a male mouse model of prostate tumorigenesis. Endocrinology. 2017;158:1612–22. doi: 10.1210/en.2017-00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stylianou N, Lehman ML, Wang C, Fard AT, Rockstroh A, Fazli L, Jovanovic L, Ward M, Sadowski MC, Kashyap AS, Buttyan R, Gleave ME, Westbrook TF, Williams ED, Gunter JH, Nelson CC, Hollier BG. A molecular portrait of epithelial-mesenchymal plasticity in prostate cancer associated with clinical outcome. Oncogene. 2019;38:913–34. doi: 10.1038/s41388-018-0488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith BN, Odero-Marah VA. The role of Snail in prostate cancer. Cell Adh Migr. 2012;6:433–41. doi: 10.4161/cam.21687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olmeda D, Moreno-Bueno G, Flores JM, Fabra A, Portillo F, Cano A. SNAI1 is required for tumor growth and lymph node metastasis of human breast carcinoma MDA-MB-231 cells. Cancer Res. 2007;67:11721–31. doi: 10.1158/0008-5472.CAN-07-2318. [DOI] [PubMed] [Google Scholar]
- 35.Jin H, Yu Y, Zhang T, Zhou X, Zhou J, Jia L, Wu Y, Zhou BP, Feng Y. Snail is critical for tumor growth and metastasis of ovarian carcinoma. Int J Cancer. 2010;126:2102–11. doi: 10.1002/ijc.24901. [DOI] [PubMed] [Google Scholar]
- 36.Min AL, Choi JY, Woo HY, Kim JD, Kwon JH, Bae SH, Yoon SK, Shin SH, Chung YJ, Jung CK. High expression of Snail mRNA in blood from hepatocellular carcinoma patients with extra-hepatic metastasis. Clin Exp Metastasis. 2009;26:759–67. doi: 10.1007/s10585-009-9275-6. [DOI] [PubMed] [Google Scholar]
- 37.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 38.Vesuna F, van Diest P, Chen JH, Raman V. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem Biophys Res Commun. 2008;367:235–41. doi: 10.1016/j.bbrc.2007.11.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng G, Chen Y, Guo C, Yin L, Han Y, Li Y, Fu Y, Cai C, Shen H, Zeng S. BMP4 promotes the metastasis of gastric cancer by inducing epithelial-mesenchymal transition via ID1. J Cell Sci. 2020;133:jcs237222. doi: 10.1242/jcs.237222. [DOI] [PubMed] [Google Scholar]
- 40.Pang MF, Georgoudaki AM, Lambut L, Johansson J, Tabor V, Hagikura K, Jin Y, Jansson M, Alexander JS, Nelson CM, Jakobsson L, Betsholtz C, Sund M, Karlsson MC, Fuxe J. TGF-β1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene. 2016;35:748–60. doi: 10.1038/onc.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gogola S, Rejzer M, Bahmad HF, Abou-Kheir W, Omarzai Y, Poppiti R. Epithelial-to-mesenchymal transition-related markers in prostate cancer: from bench to bedside. Cancers (Basel) 2023;15:2309. doi: 10.3390/cancers15082309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Y, Kyprianou N. Overexpression of transforming growth factor (TGF) beta1 type II receptor restores TGF-beta1 sensitivity and signaling in human prostate cancer cells. Cell Growth Differ. 1998;9:185–93. [PubMed] [Google Scholar]
- 43.Huang F, Chen YG. Regulation of TGF-β receptor activity. Cell Biosci. 2012;2:9. doi: 10.1186/2045-3701-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du Z, Lovly CM. Mechanisms of receptor tyrosine kinase activation in cancer. Mol Cancer. 2018;17:58. doi: 10.1186/s12943-018-0782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graham TR, Zhau HE, Odero-Marah VA, Osunkoya AO, Kimbro KS, Tighiouart M, Liu T, Simons JW, O’Regan RM. Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008;68:2479–88. doi: 10.1158/0008-5472.CAN-07-2559. [DOI] [PubMed] [Google Scholar]
- 46.Yang X, Chrisman H, Weijer CJ. PDGF signalling controls the migration of mesoderm cells during chick gastrulation by regulating N-cadherin expression. Development. 2008;135:3521–30. doi: 10.1242/dev.023416. [DOI] [PubMed] [Google Scholar]
- 47.Drake JM, Graham NA, Stoyanova T, Sedghi A, Goldstein AS, Cai H, Smith DA, Zhang H, Komisopoulou E, Huang J, Graeber TG, Witte ON. Oncogene-specific activation of tyrosine kinase networks during prostate cancer progression. Proc Natl Acad Sci U S A. 2012;109:1643–8. doi: 10.1073/pnas.1120985109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tatarov O, Mitchell TJ, Seywright M, Leung HY, Brunton VG, Edwards J. SRC family kinase activity is up-regulated in hormone-refractory prostate cancer. Clin Cancer Res. 2009;15:3540–9. doi: 10.1158/1078-0432.CCR-08-1857. [DOI] [PubMed] [Google Scholar]
- 49.Bluemn EG, Coleman IM, Lucas JM, Coleman RT, Hernandez-Lopez S, Tharakan R, Bianchi-Frias D, Dumpit RF, Kaipainen A, Corella AN, Yang YC, Nyquist MD, Mostaghel E, Hsieh AC, Zhang X, Corey E, Brown LG, Nguyen HM, Pienta K, Ittmann M, Schweizer M, True LD, Wise D, Rennie PS, Vessella RL, Morrissey C, Nelson PS. Androgen receptor pathway-independent prostate cancer is sustained through FGF signaling. Cancer Cell. 2017;32:474–89. e6. doi: 10.1016/j.ccell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70:6945–56. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 51.Patel S, Alam A, Pant R, Chattopadhyay S. Wnt signaling and its significance within the tumor microenvironment: novel therapeutic insights. Front Immunol. 2019;10:2872. doi: 10.3389/fimmu.2019.02872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002;26:463–76. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- 53.Zucchini-Pascal N, Peyre L, Rahmani R. Crosstalk between beta-catenin and snail in the induction of epithelial to mesenchymal transition in hepatocarcinoma: role of the ERK1/2 pathway. Int J Mol Sci. 2013;14:20768–92. doi: 10.3390/ijms141020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu ZQ, Li XY, Hu CY, Ford M, Kleer CG, Weiss SJ. Canonical Wnt signaling regulates Slug activity and links epithelial-mesenchymal transition with epigenetic Breast Cancer 1, Early Onset (BRCA1) repression. Proc Natl Acad Sci U S A. 2012;109:16654–9. doi: 10.1073/pnas.1205822109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fendrich V, Waldmann J, Esni F, Ramaswamy A, Mullendore M, Buchholz M, Maitra A, Feldmann G. Snail and Sonic Hedgehog activation in neuroendocrine tumors of the ileum. Endocr Relat Cancer. 2007;14:865–74. doi: 10.1677/ERC-07-0108. [DOI] [PubMed] [Google Scholar]
- 56.Li X, Deng W, Nail CD, Bailey SK, Kraus MH, Ruppert JM, Lobo-Ruppert SM. Snail induction is an early response to Gli1 that determines the efficiency of epithelial transformation. Oncogene. 2006;25:609–21. doi: 10.1038/sj.onc.1209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jing J, Wu Z, Wang J, Luo G, Lin H, Fan Y, Zhou C. Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies. Signal Transduct Target Ther. 2023;8:315. doi: 10.1038/s41392-023-01559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nolan KD, Franco OE, Hance MW, Hayward SW, Isaacs JS. Tumor-secreted Hsp90 subverts polycomb function to drive prostate tumor growth and invasion. J Biol Chem. 2015;290:8271–82. doi: 10.1074/jbc.M115.637496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–14. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 61.Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018;18:128–34. doi: 10.1038/nrc.2017.118. [DOI] [PubMed] [Google Scholar]
- 62.Saitoh M. Transcriptional regulation of EMT transcription factors in cancer. Semin Cancer Biol. 2023;97:21–9. doi: 10.1016/j.semcancer.2023.10.001. [DOI] [PubMed] [Google Scholar]
- 63.Yu X, He T, Tong Z, Liao L, Huang S, Fakhouri WD, Edwards DP, Xu J. Molecular mechanisms of TWIST1-regulated transcription in EMT and cancer metastasis. EMBO Rep. 2023;24:e56902. doi: 10.15252/embr.202356902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meng J, Chen S, Han JX, Qian B, Wang XR, Zhong WL, Qin Y, Zhang H, Gao WF, Lei YY, Yang W, Yang L, Zhang C, Liu HJ, Liu YR, Zhou HG, Sun T, Yang C. Twist1 regulates vimentin through Cul2 circular RNA to promote EMT in hepatocellular carcinoma. Cancer Res. 2018;78:4150–62. doi: 10.1158/0008-5472.CAN-17-3009. [DOI] [PubMed] [Google Scholar]
- 65.Zhu GH, Huang C, Feng ZZ, Lv XH, Qiu ZJ. Hypoxia-induced snail expression through transcriptional regulation by HIF-1α in pancreatic cancer cells. Dig Dis Sci. 2013;58:3503–15. doi: 10.1007/s10620-013-2841-4. [DOI] [PubMed] [Google Scholar]
- 66.Bery F, Figiel S, Kouba S, Fontaine D, Guéguinou M, Potier-Cartereau M, Vandier C, Guibon R, Bruyère F, Fromont G, Mahéo K. Hypoxia promotes prostate cancer aggressiveness by upregulating EMT-Activator Zeb1 and SK3 channel expression. Int J Mol Sci. 2020;21:4786. doi: 10.3390/ijms21134786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Derynck R, Akhurst RJ. Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat Cell Biol. 2007;9:1000–4. doi: 10.1038/ncb434. [DOI] [PubMed] [Google Scholar]
- 68.Nakazawa M, Kyprianou N. Epithelial-mesenchymal-transition regulators in prostate cancer: androgens and beyond. J Steroid Biochem Mol Biol. 2017;166:84–90. doi: 10.1016/j.jsbmb.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 69.Kim IY, Ahn HJ, Lang S, Oefelein MG, Oyasu R, Kozlowski JM, Lee C. Loss of expression of transforming growth factor-β receptors is associated with poor prognosis in prostate cancer patients. Clin Cancer Res. 1998;4:1625–30. [PubMed] [Google Scholar]
- 70.Hao Y, Bjerke GA, Pietrzak K, Melhuish TA, Han Y, Turner SD, Frierson HF Jr, Wotton D. TGFβ signaling limits lineage plasticity in prostate cancer. PLoS Genet. 2018;14:e1007409. doi: 10.1371/journal.pgen.1007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang K, Pascal LE, Li F, Chen W, Dhir R, Balasubramani GK, DeFranco DB, Yoshimura N, He D, Wang Z. Tight junction protein claudin-1 is downregulated by TGF-β1 via MEK signaling in benign prostatic epithelial cells. Prostate. 2020;80:1203–15. doi: 10.1002/pros.24046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lamouille S, Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;178:437–51. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–39. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamidi A, Song J, Thakur N, Itoh S, Marcusson A, Bergh A, Heldin CH, Landström M. TGF-β promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85α. Sci Signal. 2017;10:eaal4186. doi: 10.1126/scisignal.aal4186. [DOI] [PubMed] [Google Scholar]
- 75.Sahadevan K, Darby S, Leung HY, Mathers ME, Robson CN, Gnanapragasam VJ. Selective over-expression of fibroblast growth factor receptors 1 and 4 in clinical prostate cancer. J Pathol. 2007;213:82–90. doi: 10.1002/path.2205. [DOI] [PubMed] [Google Scholar]
- 76.Acevedo VD, Gangula RD, Freeman KW, Li R, Zhang Y, Wang F, Ayala GE, Peterson LE, Ittmann M, Spencer DM. Inducible FGFR-1 activation leads to irreversible prostate adenocarcinoma and an epithelial-to-mesenchymal transition. Cancer Cell. 2007;12:559–71. doi: 10.1016/j.ccr.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Yang F, Zhang Y, Ressler SJ, Ittmann MM, Ayala GE, Dang TD, Wang F, Rowley DR. FGFR1 is essential for prostate cancer progression and metastasis. Cancer Res. 2013;73:3716–24. doi: 10.1158/0008-5472.CAN-12-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carstens JL, Shahi P, Van Tsang S, Smith B, Creighton CJ, Zhang Y, Seamans A, Seethammagari M, Vedula I, Levitt JM, Ittmann MM, Rowley DR, Spencer DM. FGFR1-WNT-TGF-β signaling in prostate cancer mouse models recapitulates human reactive stroma. Cancer Res. 2014;74:609–20. doi: 10.1158/0008-5472.CAN-13-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Labanca E, Yang J, Shepherd PDA, Wan X, Starbuck MW, Guerra LD, Anselmino N, Bizzotto JA, Dong J, Chinnaiyan AM, Ravoori MK, Kundra V, Broom BM, Corn PG, Troncoso P, Gueron G, Logothethis CJ, Navone NM. Fibroblast growth factor receptor 1 drives the metastatic progression of prostate cancer. Eur Urol Oncol. 2022;5:164–75. doi: 10.1016/j.euo.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 80.Cheng C, Wang J, Xu P, Zhang K, Xin Z, Zhao H, Ji Z, Zhang M, Wang D, He Y, Jing N, Fan L, Liu K, Li F, Liu C, Gong Y, Cui S, Sun Z, Sun D, Yao X, Li H, Zhang J, Zhang P, Dong B, Xue W, Qian X, Gao WQ, Zhu HH. Gremlin1 is a therapeutically targetable FGFR1 ligand that regulates lineage plasticity and castration resistance in prostate cancer. Nat Cancer. 2022;3:565–80. doi: 10.1038/s43018-022-00380-3. [DOI] [PubMed] [Google Scholar]
- 81.Chan JM, Zaidi S, Love JR, Zhao JL, Setty M, Wadosky KM, Gopalan A, Choo ZN, Persad S, Choi J, LaClair J, Lawrence KE, Chaudhary O, Xu T, Masilionis I, Linkov I, Wang S, Lee C, Barlas A, Morris MJ, Mazutis L, Chaligne R, Chen Y, Goodrich DW, Karthaus WR, Pe’er D, Sawyers CL. Lineage plasticity in prostate cancer depends on JAK/STAT inflammatory signaling. Science. 2022;377:1180–91. doi: 10.1126/science.abn0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu ZC, Chen XH, Song HX, Wang HS, Zhang G, Wang H, Chen DY, Fang R, Liu H, Cai SH, Du J. Snail regulated by PKC/GSK-3β pathway is crucial for EGF-induced epithelial-mesenchymal transition (EMT) of cancer cells. Cell Tissue Res. 2014;358:491–502. doi: 10.1007/s00441-014-1953-2. [DOI] [PubMed] [Google Scholar]
- 83.Cho KH, Choi MJ, Jeong KJ, Kim JJ, Hwang MH, Shin SC, Park CG, Lee HY. A ROS/STAT3/HIF-1α signaling cascade mediates EGF-induced TWIST1 expression and prostate cancer cell invasion. Prostate. 2014;74:528–36. doi: 10.1002/pros.22776. [DOI] [PubMed] [Google Scholar]
- 84.Grünert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol. 2003;4:657–65. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- 85.Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A, Muro-Cacho C, Livingston S, Karras J, Pow-Sang J, Jove R. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–66. [PubMed] [Google Scholar]
- 86.Don-Doncow N, Marginean F, Coleman I, Nelson PS, Ehrnström R, Krzyzanowska A, Morrissey C, Hellsten R, Bjartell A. Expression of STAT3 in prostate cancer metastases. Eur Urol. 2017;71:313–6. doi: 10.1016/j.eururo.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cho KH, Jeong KJ, Shin SC, Kang J, Park CG, Lee HY. STAT3 mediates TGF-β1-induced TWIST1 expression and prostate cancer invasion. Cancer Lett. 2013;336:167–73. doi: 10.1016/j.canlet.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 88.Shiota M, Bishop JL, Nip KM, Zardan A, Takeuchi A, Cordonnier T, Beraldi E, Bazov J, Fazli L, Chi K, Gleave M, Zoubeidi A. Hsp27 regulates epithelial mesenchymal transition, metastasis, and circulating tumor cells in prostate cancer. Cancer Res. 2013;73:3109–19. doi: 10.1158/0008-5472.CAN-12-3979. [DOI] [PubMed] [Google Scholar]
- 89.Gujral TS, Chan M, Peshkin L, Sorger PK, Kirschner MW, MacBeath G. A noncanonical Frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Cell. 2014;159:844–56. doi: 10.1016/j.cell.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sandsmark E, Hansen AF, Selnæs KM, Bertilsson H, Bofin AM, Wright AJ, Viset T, Richardsen E, Drabløs F, Bathen TF, Tessem MB, Rye MB. A novel non-canonical Wnt signature for prostate cancer aggressiveness. Oncotarget. 2017;8:9572–86. doi: 10.18632/oncotarget.14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nath D, Li X, Mondragon C, Post D, Chen M, White JR, Hryniewicz-Jankowska A, Caza T, Kuznetsov VA, Hehnly H, Jamaspishvili T, Berman DM, Zhang F, Kung SHY, Fazli L, Gleave ME, Bratslavsky G, Pandolfi PP, Kotula L. Abi1 loss drives prostate tumorigenesis through activation of EMT and non-canonical WNT signaling. Cell Commun Signal. 2019;17:120. doi: 10.1186/s12964-019-0410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deng S, Wang C, Wang Y, Xu Y, Li X, Johnson NA, Mukherji A, Lo UG, Xu L, Gonzalez J, Metang LA, Ye J, Tirado CR, Rodarte K, Zhou Y, Xie Z, Arana C, Annamalai V, Liu X, Vander Griend DJ, Strand D, Hsieh JT, Li B, Raj G, Wang T, Mu P. Ectopic JAK-STAT activation enables the transition to a stem-like and multilineage state conferring AR-targeted therapy resistance. Nat Cancer. 2022;3:1071–87. doi: 10.1038/s43018-022-00431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Canesin G, Maggio V, Palominos M, Stiehm A, Contreras HR, Castellón EA, Morote J, Paciucci R, Maitland NJ, Bjartell A, Hellsten R. STAT3 inhibition with galiellalactone effectively targets the prostate cancer stem-like cell population. Sci Rep. 2020;10:13958. doi: 10.1038/s41598-020-70948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pradella D, Naro C, Sette C, Ghigna C. EMT and stemness: flexible processes tuned by alternative splicing in development and cancer progression. Mol Cancer. 2017;16:8. doi: 10.1186/s12943-016-0579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roy Burman D, Das S, Das C, Bhattacharya R. Alternative splicing modulates cancer aggressiveness: role in EMT/metastasis and chemoresistance. Mol Biol Rep. 2021;48:897–914. doi: 10.1007/s11033-020-06094-y. [DOI] [PubMed] [Google Scholar]
- 96.Venables JP, Brosseau JP, Gadea G, Klinck R, Prinos P, Beaulieu JF, Lapointe E, Durand M, Thibault P, Tremblay K, Rousset F, Tazi J, Abou Elela S, Chabot B. RBFOX2 is an important regulator of mesenchymal tissue-specific splicing in both normal and cancer tissues. Mol Cell Biol. 2013;33:396–405. doi: 10.1128/MCB.01174-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kwabi-Addo B, Ropiquet F, Giri D, Ittmann M. Alternative splicing of fibroblast growth factor receptors in human prostate cancer. Prostate. 2001;46:163–72. doi: 10.1002/1097-0045(20010201)46:2<163::aid-pros1020>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 98.Fagoonee S, Bearzi C, Di Cunto F, Clohessy JG, Rizzi R, Reschke M, Tolosano E, Provero P, Pandolfi PP, Silengo L, Altruda F. The RNA binding protein ESRP1 fine-tunes the expression of pluripotency-related factors in mouse embryonic stem cells. PLoS One. 2013;8:e72300. doi: 10.1371/journal.pone.0072300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Y, Donmez N, Sahinalp C, Xie N, Wang Y, Xue H, Mo F, Beltran H, Gleave M, Wang Y, Collins C, Dong X. SRRM4 drives neuroendocrine transdifferentiation of prostate adenocarcinoma under androgen receptor pathway inhibition. Eur Urol. 2017;71:68–78. doi: 10.1016/j.eururo.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 100.Li Y, Zhang Q, Lovnicki J, Chen R, Fazli L, Wang Y, Gleave M, Huang J, Dong X. SRRM4 gene expression correlates with neuroendocrine prostate cancer. Prostate. 2019;79:96–104. doi: 10.1002/pros.23715. [DOI] [PubMed] [Google Scholar]
- 101.Chang YT, Lin TP, Campbell M, Pan CC, Lee SH, Lee HC, Yang MH, Kung HJ, Chang PC. REST is a crucial regulator for acquiring EMT-like and stemness phenotypes in hormone-refractory prostate cancer. Sci Rep. 2017;7:42795. doi: 10.1038/srep42795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 103.Tu WH, Thomas TZ, Masumori N, Bhowmick NA, Gorska AE, Shyr Y, Kasper S, Case T, Roberts RL, Shappell SB, Moses HL, Matusik RJ. The loss of TGF-beta signaling promotes prostate cancer metastasis. Neoplasia. 2003;5:267–77. doi: 10.1016/S1476-5586(03)80058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pu H, Collazo J, Jones E, Gayheart D, Sakamoto S, Vogt A, Mitchell B, Kyprianou N. Dysfunctional transforming growth factor-beta receptor II accelerates prostate tumorigenesis in the TRAMP mouse model. Cancer Res. 2009;69:7366–74. doi: 10.1158/0008-5472.CAN-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ricciardi M, Zanotto M, Malpeli G, Bassi G, Perbellini O, Chilosi M, Bifari F, Krampera M. Epithelial-to-mesenchymal transition (EMT) induced by inflammatory priming elicits mesenchymal stromal cell-like immune-modulatory properties in cancer cells. Br J Cancer. 2015;112:1067–75. doi: 10.1038/bjc.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–14. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang Y, Hong W, Wei X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J Hematol Oncol. 2022;15:129. doi: 10.1186/s13045-022-01347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang CH, Yang WH, Chang SY, Tai SK, Tzeng CH, Kao JY, Wu KJ, Yang MH. Regulation of membrane-type 4 matrix metalloproteinase by SLUG contributes to hypoxia-mediated metastasis. Neoplasia. 2009;11:1371–82. doi: 10.1593/neo.91326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ota I, Li XY, Hu Y, Weiss SJ. Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proc Natl Acad Sci U S A. 2009;106:20318–23. doi: 10.1073/pnas.0910962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liao TT, Yang MH. Hybrid epithelial/mesenchymal state in cancer metastasis: clinical significance and regulatory mechanisms. Cells. 2020;9:623. doi: 10.3390/cells9030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kolijn K, Verhoef EI, van Leenders GJ. Morphological and immunohistochemical identification of epithelial-to-mesenchymal transition in clinical prostate cancer. Oncotarget. 2015;6:24488–98. doi: 10.18632/oncotarget.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsai YC, Chen WY, Abou-Kheir W, Zeng T, Yin JJ, Bahmad H, Lee YC, Liu YN. Androgen deprivation therapy-induced epithelial-mesenchymal transition of prostate cancer through downregulating SPDEF and activating CCL2. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1717–27. doi: 10.1016/j.bbadis.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 114.Izumi K, Fang LY, Mizokami A, Namiki M, Li L, Lin WJ, Chang C. Targeting the androgen receptor with siRNA promotes prostate cancer metastasis through enhanced macrophage recruitment via CCL2/CCR2-induced STAT3 activation. EMBO Mol Med. 2013;5:1383–401. doi: 10.1002/emmm.201202367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marcolino E, Siddiqui YH, van den Bosch M, Poole AW, Jayaraman PS, Gaston K. Blood platelets stimulate cancer extravasation through TGFβ-mediated downregulation of PRH/HHEX. Oncogenesis. 2020;9:10. doi: 10.1038/s41389-020-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Papanikolaou S, Vourda A, Syggelos S, Gyftopoulos K. Cell plasticity and prostate cancer: the role of epithelial-mesenchymal transition in tumor progression, invasion, metastasis and cancer therapy resistance. Cancers (Basel) 2021;13:2795. doi: 10.3390/cancers13112795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buijs JT, Stayrook KR, Guise TA. The role of TGF-β in bone metastasis: novel therapeutic perspectives. Bonekey Rep. 2012;1:96. doi: 10.1038/bonekey.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Anose BM, Sanders MM. Androgen receptor regulates transcription of the ZEB1 transcription factor. Int J Endocrinol. 2011;2011:903918. doi: 10.1155/2011/903918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu ML, Kyprianou N. Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells. FASEB J. 2010;24:769–77. doi: 10.1096/fj.09-136994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eide T, Ramberg H, Glackin C, Tindall D, Taskén KA. TWIST1, a novel androgen-regulated gene, is a target for NKX3-1 in prostate cancer cells. Cancer Cell Int. 2013;13:4. doi: 10.1186/1475-2867-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mooney SM, Parsana P, Hernandez JR, Liu X, Verdone JE, Torga G, Harberg CA, Pienta KJ. The presence of androgen receptor elements regulates ZEB1 expression in the absence of androgen receptor. J Cell Biochem. 2015;116:115–23. doi: 10.1002/jcb.24948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shiota M, Yokomizo A, Tada Y, Inokuchi J, Kashiwagi E, Masubuchi D, Eto M, Uchiumi T, Naito S. Castration resistance of prostate cancer cells caused by castration-induced oxidative stress through Twist1 and androgen receptor overexpression. Oncogene. 2010;29:237–50. doi: 10.1038/onc.2009.322. [DOI] [PubMed] [Google Scholar]
- 124.Tanaka H, Kono E, Tran CP, Miyazaki H, Yamashiro J, Shimomura T, Fazli L, Wada R, Huang J, Vessella RL, An J, Horvath S, Gleave M, Rettig MB, Wainberg ZA, Reiter RE. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat Med. 2010;16:1414–20. doi: 10.1038/nm.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun Y, Wang BE, Leong KG, Yue P, Li L, Jhunjhunwala S, Chen D, Seo K, Modrusan Z, Gao WQ, Settleman J, Johnson L. Androgen deprivation causes epithelial-mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res. 2012;72:527–36. doi: 10.1158/0008-5472.CAN-11-3004. [DOI] [PubMed] [Google Scholar]
- 126.Miao L, Yang L, Li R, Rodrigues DN, Crespo M, Hsieh JT, Tilley WD, de Bono J, Selth LA, Raj GV. Disrupting androgen receptor signaling induces snail-mediated epithelial-mesenchymal plasticity in prostate cancer. Cancer Res. 2017;77:3101–12. doi: 10.1158/0008-5472.CAN-16-2169. [DOI] [PubMed] [Google Scholar]
- 127.Lin TH, Lee SO, Niu Y, Xu D, Liang L, Li L, Yeh SD, Fujimoto N, Yeh S, Chang C. Differential androgen deprivation therapies with anti-androgens casodex/bicalutamide or MDV3100/Enzalutamide versus anti-androgen receptor ASC-J9(R) Lead to promotion versus suppression of prostate cancer metastasis. J Biol Chem. 2013;288:19359–69. doi: 10.1074/jbc.M113.477216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhifang M, Liang W, Wei Z, Bin H, Rui T, Nan W, Shuhai Z. The androgen receptor plays a suppressive role in epithelial-mesenchymal transition of human prostate cancer stem progenitor cells. BMC Biochem. 2015;16:13. doi: 10.1186/s12858-015-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Porter BA, Li X, Arya N, Zhang F, Kung SHY, Fazli L, Oo HZ, Li Y, Marincin K, Kukkonen K, Urhonen H, Ortiz MA, Kemraj AP, Corey E, Dong X, Kuznetsov VA, Nykter M, Gleave ME, Bratslavsky G, Urbanucci A, Frueh D, Bah A, Kotula L. ABI1 regulates transcriptional activity of Androgen Receptor by novel DNA and AR binding mechanism. bioRxiv. 2023 2023.05.26.542350. [Google Scholar]
- 130.Munkley J, Li L, Krishnan SRG, Hysenaj G, Scott E, Dalgliesh C, Oo HZ, Maia TM, Cheung K, Ehrmann I, Livermore KE, Zielinska H, Thompson O, Knight B, McCullagh P, McGrath J, Crundwell M, Harries LW, Daugaard M, Cockell S, Barbosa-Morais NL, Oltean S, Elliott DJ. Androgen-regulated transcription of ESRP2 drives alternative splicing patterns in prostate cancer. Elife. 2019;8:e47678. doi: 10.7554/eLife.47678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tso CL, McBride WH, Sun J, Patel B, Tsui KH, Paik SH, Gitlitz B, Caliliw R, van Ophoven A, Wu L, deKernion J, Belldegrun A. Androgen deprivation induces selective outgrowth of aggressive hormone-refractory prostate cancer clones expressing distinct cellular and molecular properties not present in parental androgen-dependent cancer cells. Cancer J. 2000;6:220–33. [PubMed] [Google Scholar]
- 132.Singh S, Sadacharan S, Su S, Belldegrun A, Persad S, Singh G. Overexpression of vimentin: role in the invasive phenotype in an androgen-independent model of prostate cancer. Cancer Res. 2003;63:2306–11. [PubMed] [Google Scholar]
- 133.Xu G, Wu J, Zhou L, Chen B, Sun Z, Zhao F, Tao Z. Characterization of the small RNA transcriptomes of androgen dependent and independent prostate cancer cell line by deep sequencing. PLoS One. 2010;5:e15519. doi: 10.1371/journal.pone.0015519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li F, Mahato RI. MicroRNAs and drug resistance in prostate cancers. Mol Pharm. 2014;11:2539–52. doi: 10.1021/mp500099g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shiota M, Itsumi M, Takeuchi A, Imada K, Yokomizo A, Kuruma H, Inokuchi J, Tatsugami K, Uchiumi T, Oda Y, Naito S. Crosstalk between epithelial-mesenchymal transition and castration resistance mediated by Twist1/AR signaling in prostate cancer. Endocr Relat Cancer. 2015;22:889–900. doi: 10.1530/ERC-15-0225. [DOI] [PubMed] [Google Scholar]
- 136.Jennbacken K, Tesan T, Wang W, Gustavsson H, Damber JE, Welén K. N-cadherin increases after androgen deprivation and is associated with metastasis in prostate cancer. Endocr Relat Cancer. 2010;17:469–79. doi: 10.1677/ERC-10-0015. [DOI] [PubMed] [Google Scholar]
- 137.Liu Q, Tong D, Liu G, Xu J, Do K, Geary K, Zhang D, Zhang J, Zhang Y, Li Y, Bi G, Lan W, Jiang J. Metformin reverses prostate cancer resistance to enzalutamide by targeting TGF-β1/STAT3 axis-regulated EMT. Cell Death Dis. 2017;8:e3007. doi: 10.1038/cddis.2017.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Marín-Aguilera M, Codony-Servat J, Kalko SG, Fernández PL, Bermudo R, Buxo E, Ribal MJ, Gascón P, Mellado B. Identification of docetaxel resistance genes in castration-resistant prostate cancer. Mol Cancer Ther. 2012;11:329–39. doi: 10.1158/1535-7163.MCT-11-0289. [DOI] [PubMed] [Google Scholar]
- 139.Li Y, Zhang B, Xiang L, Xia S, Kucuk O, Deng X, Boise LH, Dong JT. TGF-β causes Docetaxel resistance in Prostate Cancer via the induction of Bcl-2 by acetylated KLF5 and Protein Stabilization. Theranostics. 2020;10:7656–70. doi: 10.7150/thno.44567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martin SK, Pu H, Penticuff JC, Cao Z, Horbinski C, Kyprianou N. Multinucleation and mesenchymal-to-epithelial transition alleviate resistance to combined cabazitaxel and antiandrogen therapy in advanced prostate cancer. Cancer Res. 2016;76:912–26. doi: 10.1158/0008-5472.CAN-15-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Archer M, Begemann D, Gonzalez-Kozlova E, Nepali PR, Labanca E, Shepherd P, Dogra N, Navone N, Kyprianou N. Kinesin facilitates phenotypic targeting of therapeutic resistance in advanced prostate cancer. Mol Cancer Res. 2024;22:730–45. doi: 10.1158/1541-7786.MCR-23-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Parimi V, Goyal R, Poropatich K, Yang XJ. Neuroendocrine differentiation of prostate cancer: a review. Am J Clin Exp Urol. 2014;2:273–85. [PMC free article] [PubMed] [Google Scholar]
- 143.Quintanal-Villalonga Á, Chan JM, Yu HA, Pe’er D, Sawyers CL, Sen T, Rudin CM. Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat Rev Clin Oncol. 2020;17:360–71. doi: 10.1038/s41571-020-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li Q, Zhang CS, Zhang Y. Molecular aspects of prostate cancer with neuroendocrine differentiation. Chin J Cancer Res. 2016;28:122–9. doi: 10.3978/j.issn.1000-9604.2016.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ge R, Wang Z, Montironi R, Jiang Z, Cheng M, Santoni M, Huang K, Massari F, Lu X, Cimadamore A, Lopez-Beltran A, Cheng L. Epigenetic modulations and lineage plasticity in advanced prostate cancer. Ann Oncol. 2020;31:470–9. doi: 10.1016/j.annonc.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 146.Dudas J, Ladanyi A, Ingruber J, Steinbichler TB, Riechelmann H. Epithelial to mesenchymal transition: a mechanism that fuels cancer radio/chemoresistance. Cells. 2020;9:428. doi: 10.3390/cells9020428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–69. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 148.Martin SK, Banuelos CA, Sadar MD, Kyprianou N. N-terminal targeting of androgen receptor variant enhances response of castration resistant prostate cancer to taxane chemotherapy. Mol Oncol. 2014;9:628–39. doi: 10.1016/j.molonc.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Begemann D, Wang Y, Yang W, Kyprianou N. Androgens modify therapeutic response to cabazitaxel in models of advanced prostate cancer. Prostate. 2020;80:926–37. doi: 10.1002/pros.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Carthon BC, Antonarakis ES. The STAMPEDE trial: paradigm-changing data through innovative trial design. Transl Cancer Res. 2016;5(Suppl):S485–S490. doi: 10.21037/tcr.2016.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Clarke NW, Ali A, Ingleby FC, Hoyle A, Amos CL, Attard G, Brawley CD, Calvert J, Chowdhury S, Cook A, Cross W, Dearnaley DP, Douis H, Gilbert D, Gillessen S, Jones RJ, Langley RE, MacNair A, Malik Z, Mason MD, Matheson D, Millman R, Parker CC, Ritchie AWS, Rush H, Russell JM, Brown J, Beesley S, Birtle A, Capaldi L, Gale J, Gibbs S, Lydon A, Nikapota A, Omlin A, O’Sullivan JM, Parikh O, Protheroe A, Rudman S, Srihari NN, Simms M, Tanguay JS, Tolan S, Wagstaff J, Wallace J, Wylie J, Zarkar A, Sydes MR, Parmar MKB, James ND. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol. 2019;30:1992–2003. doi: 10.1093/annonc/mdz396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C, Zhang X, Chua CW, Chan KW, Chan FL, Glackin C, Wong YC, Wang X. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res. 2005;65:5153–62. doi: 10.1158/0008-5472.CAN-04-3785. [DOI] [PubMed] [Google Scholar]
- 153.Yarom N, Stewart D, Malik R, Wells J, Avruch L, Jonker DJ. Phase I clinical trial of Exherin (ADH-1) in patients with advanced solid tumors. Curr Clin Pharmacol. 2013;8:81–8. [PubMed] [Google Scholar]
- 154.Pienta KJ, Machiels JP, Schrijvers D, Alekseev B, Shkolnik M, Crabb SJ, Li S, Seetharam S, Puchalski TA, Takimoto C, Elsayed Y, Dawkins F, de Bono JS. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest New Drugs. 2013;31:760–8. doi: 10.1007/s10637-012-9869-8. [DOI] [PubMed] [Google Scholar]
- 155.Dorff TB, Goldman B, Pinski JK, Mack PC, Lara PN Jr, Van Veldhuizen PJ Jr, Quinn DI, Vogelzang NJ, Thompson IM Jr, Hussain MH. Clinical and correlative results of SWOG S0354: a phase II trial of CNTO328 (siltuximab), a monoclonal antibody against interleukin-6, in chemotherapy-pretreated patients with castration-resistant prostate cancer. Clin Cancer Res. 2010;16:3028–34. doi: 10.1158/1078-0432.CCR-09-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chi KN, Yu EY, Jacobs C, Bazov J, Kollmannsberger C, Higano CS, Mukherjee SD, Gleave ME, Stewart PS, Hotte SJ. A phase I dose-escalation study of apatorsen (OGX-427), an antisense inhibitor targeting heat shock protein 27 (Hsp27), in patients with castration-resistant prostate cancer and other advanced cancers. Ann Oncol. 2016;27:1116–22. doi: 10.1093/annonc/mdw068. [DOI] [PubMed] [Google Scholar]
- 157.Choi YJ, Kim HS, Park SH, Kim BS, Kim KH, Lee HJ, Song HS, Shin DY, Lee HY, Kim HG, Lee KH, Lee JL, Park KH. Phase II study of dovitinib in patients with castration-resistant prostate cancer (KCSG-GU11-05) Cancer Res Treat. 2018;50:1252–9. doi: 10.4143/crt.2017.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Araujo JC, Trudel GC, Saad F, Armstrong AJ, Yu EY, Bellmunt J, Wilding G, McCaffrey J, Serrano SV, Matveev VB, Efstathiou E, Oudard S, Morris MJ, Sizer B, Goebell PJ, Heidenreich A, de Bono JS, Begbie S, Hong JH, Richardet E, Gallardo E, Paliwal P, Durham S, Cheng S, Logothetis CJ. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol. 2013;14:1307–16. doi: 10.1016/S1470-2045(13)70479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yuen HF, Chua CW, Chan YP, Wong YC, Wang X, Chan KW. Significance of TWIST and E-cadherin expression in the metastatic progression of prostatic cancer. Histopathology. 2007;50:648–58. doi: 10.1111/j.1365-2559.2007.02665.x. [DOI] [PubMed] [Google Scholar]
- 160.Wang D, Ding L, Wang L, Zhao Y, Sun Z, Karnes RJ, Zhang J, Huang H. LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget. 2015;6:41045–55. doi: 10.18632/oncotarget.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Huang G, Osmulski PA, Bouamar H, Mahalingam D, Lin CL, Liss MA, Kumar AP, Chen CL, Thompson IM, Sun LZ, Gaczynska ME, Huang TH. TGF-β signal rewiring sustains epithelial-mesenchymal transition of circulating tumor cells in prostate cancer xenograft hosts. Oncotarget. 2016;7:77124–37. doi: 10.18632/oncotarget.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guo Y, Kyprianou N. Restoration of transforming growth factor beta signaling pathway in human prostate cancer cells suppresses tumorigenicity via induction of caspase-1-mediated apoptosis. Cancer Res. 1999;59:1366–71. [PubMed] [Google Scholar]