Abstract
1. Adult squirrel monkeys were injected intravenously with doubly labelled lysophosphatidylcholine (a mixture of 1-[1-14C]palmitoyl-sn-glycero-3-phosphorylcholine and 1-acyl-sn-glycero-3-phosphoryl[Me-3H]choline; 3H:14Cratio 3.75) complexed to albumin, and the incorporation into the brain was studied at times up to 3h. 2. After 20min, 1% of the radioactivity injected as lysophosphatidylcholine had been taken up by the brain. 3. Approx. 70% of the doubly labelled lysophosphatidylcholine taken up by both grey and white matter was converted into phosphatidylcholine, whereas about 30% was hydrolysed. 4. The absence of significant radioactivity in the phosphatidylcholine, free fatty acid and water-soluble fractions of plasma up to 30min after injection of doubly labelled lysophosphatidylcholine rules out the possibility that the rapid labelling of these compounds in brain could be due to uptake from or exchange with their counterparts in plasma. 5. The similarity between the 3H:14C ratios of brain phosphatidylcholine and injected lysophosphatidylcholine demonstrates that formation of the former occurred predominantly via direct acylation. 6. Analysis of the water-soluble products from lysophosphatidylcholine catabolism revealed that appreciable glycerophosphoryl-[Me-3H]choline did not accumulate in the brain and that radioactivity was incorporated into choline, acetylcholine, phosphorylcholine and betaine. 7. The role of plasma lysophosphatidylcholine as both a precursor of brain phosphatidylcholine and a source of free choline for the brain is discussed.
Full text
PDF


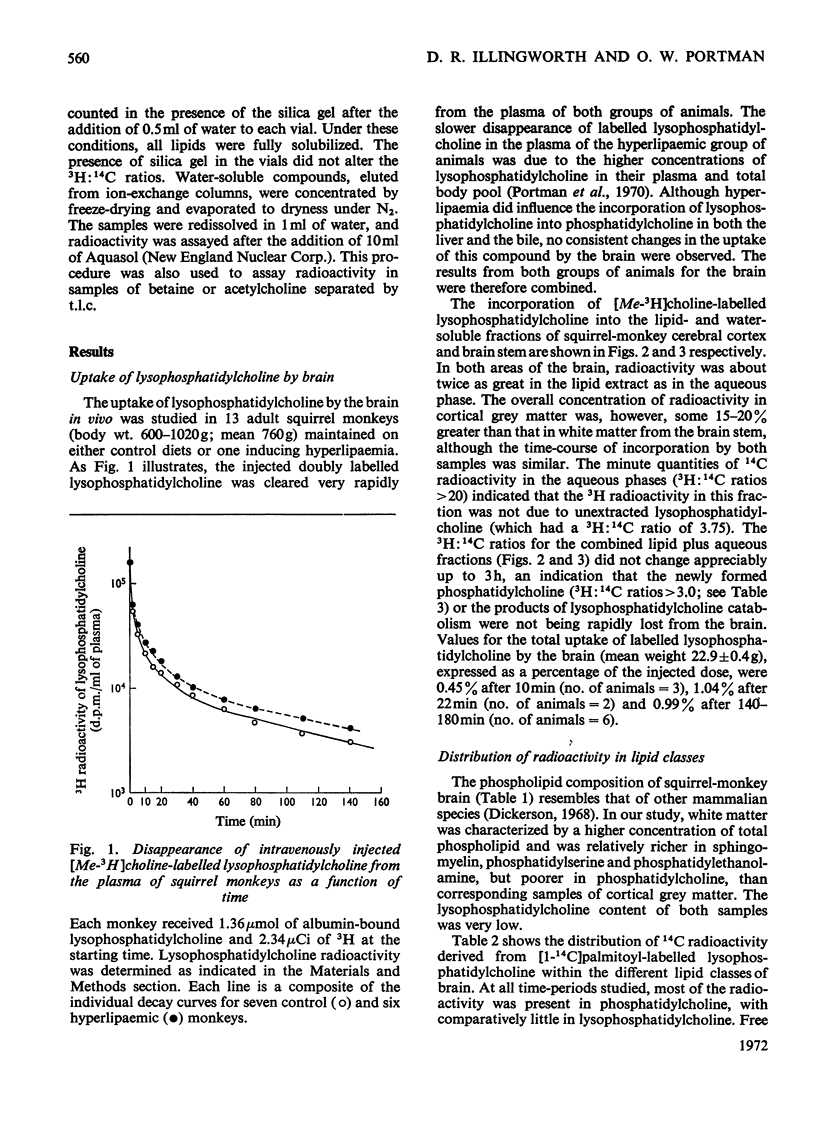


Selected References
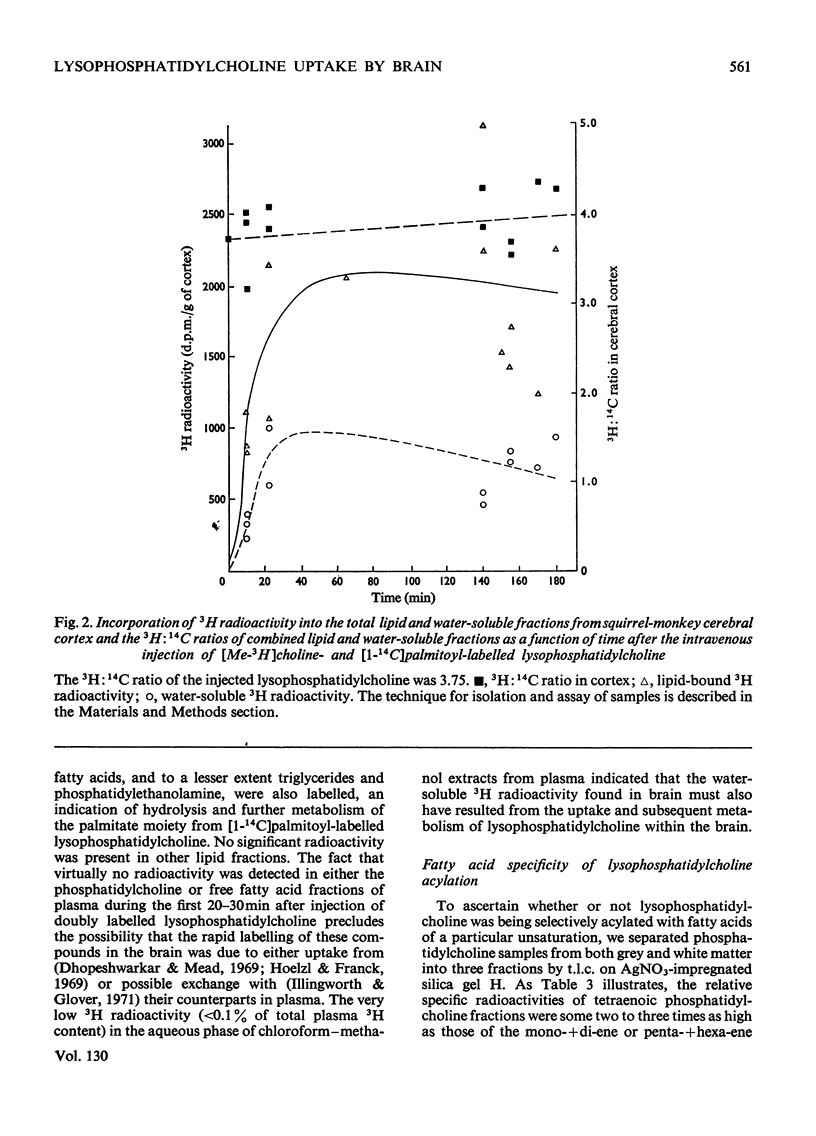
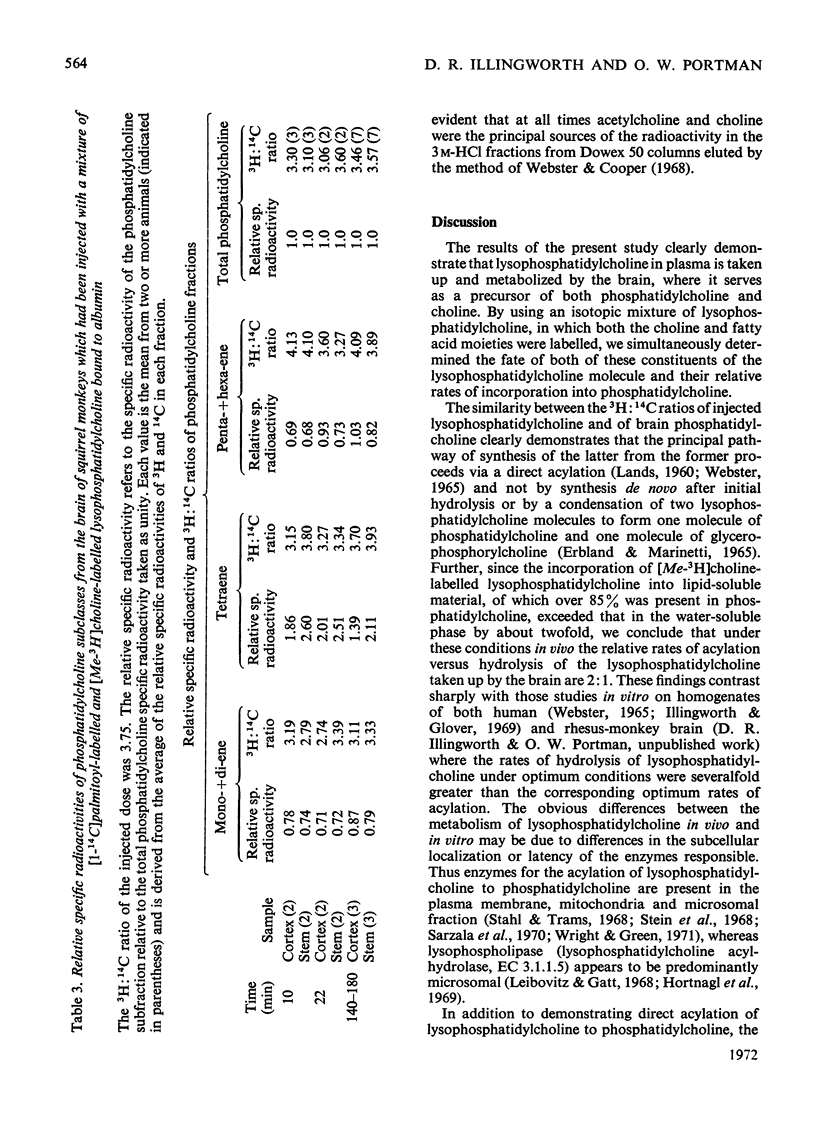
These references are in PubMed. This may not be the complete list of references from this article.
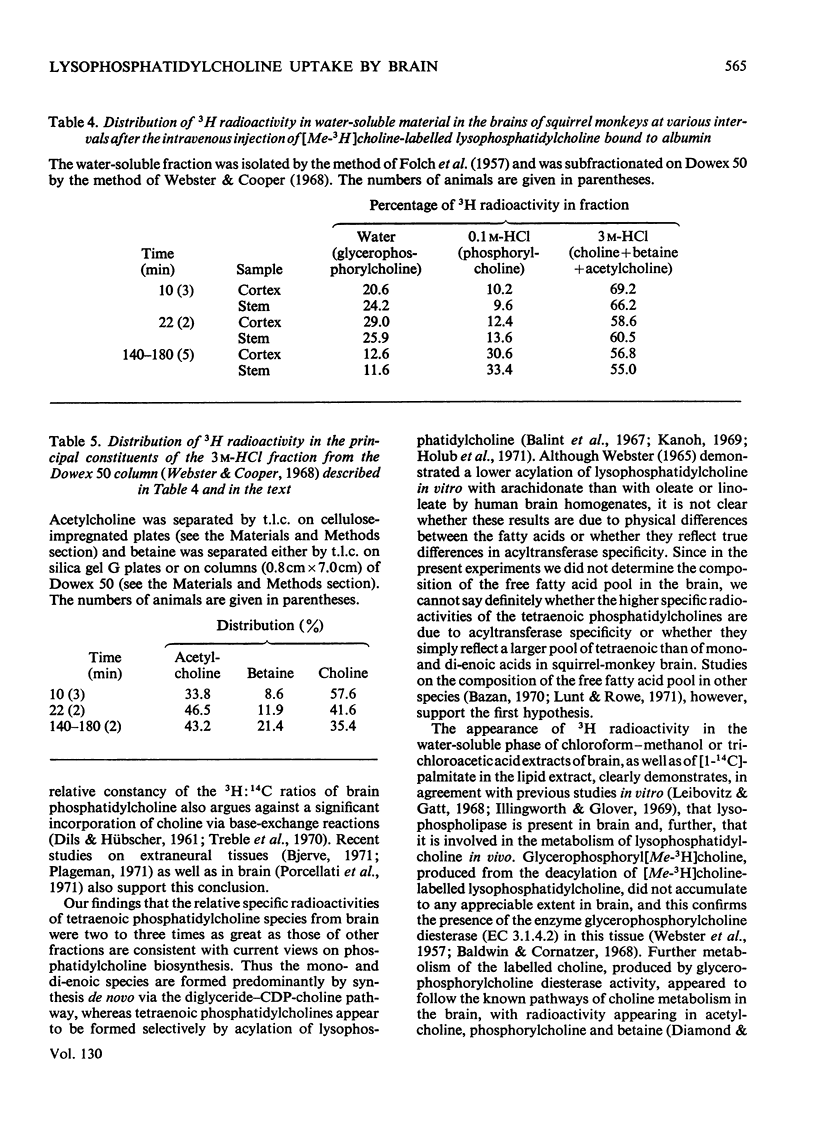
- Akesson B., Elovson J., Arvidson G. Initial incorporation into rat liver glycerolipids of intraportally injected (9,10-3H2)palmitic acid. Biochim Biophys Acta. 1970 Oct 6;218(1):44–56. doi: 10.1016/0005-2760(70)90091-3. [DOI] [PubMed] [Google Scholar]
- Ansell G. B., Spanner S. Studies on the origin of choline in the brain of the rat. Biochem J. 1971 May;122(5):741–750. doi: 10.1042/bj1220741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell G. B., Spanner S. The metabolism of [Me-14C]choline in the brain of the rat in vivo. Biochem J. 1968 Nov;110(2):201–206. doi: 10.1042/bj1100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell G. B., Spanner S. The metabolism of labelled ethanolamine in the brain of the rat in vivo. J Neurochem. 1967 Sep;14(9):873–885. doi: 10.1111/j.1471-4159.1967.tb09576.x. [DOI] [PubMed] [Google Scholar]
- Arvidson G. A. Structural and metabolic heterogeneity of rat liver glycerophosphatides. Eur J Biochem. 1968 May;4(4):478–486. doi: 10.1111/j.1432-1033.1968.tb00237.x. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
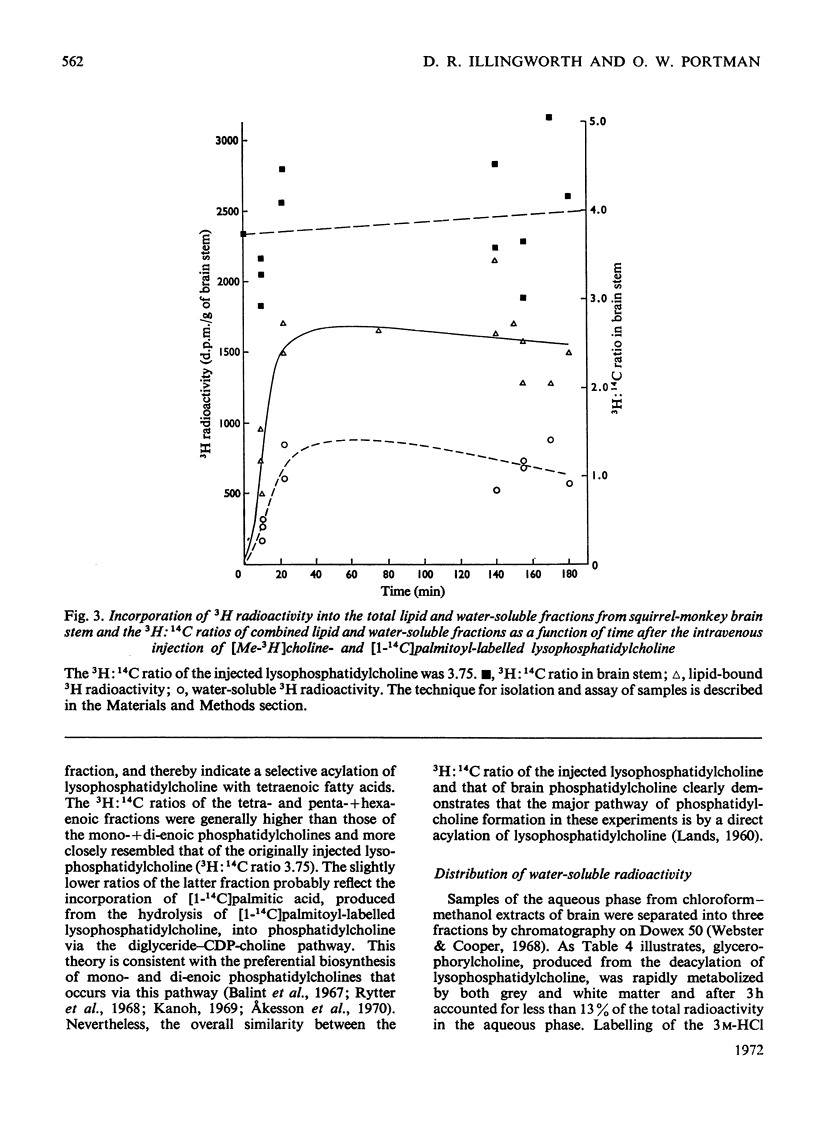
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baldwin J. J., Cornatzer W. E. Rat kidney glycerylphosphorylcholine diesterase. Biochim Biophys Acta. 1968 Oct 22;164(2):195–204. doi: 10.1016/0005-2760(68)90146-x. [DOI] [PubMed] [Google Scholar]
- Balint J. A., Beeler D. A., Treble D. H., Spitzer H. L. Studies in the biosynthesis of hepatic and biliary lecithins. J Lipid Res. 1967 Sep;8(5):486–493. [PubMed] [Google Scholar]
- Bazán N. G., Jr Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochim Biophys Acta. 1970 Oct 6;218(1):1–10. doi: 10.1016/0005-2760(70)90086-x. [DOI] [PubMed] [Google Scholar]
- Belfrage P., Elovson J., Olivecrona T. Radioactivity in blood and liver partial glycerides, and liver phospholipids after intravenous administration to carbohydrate-fed rats of chyle containing double-labeled triglycerides. Biochim Biophys Acta. 1965 Jul 7;106(1):45–55. doi: 10.1016/0005-2760(65)90094-9. [DOI] [PubMed] [Google Scholar]
- Bjerve K. S. The Ca(2+) stimulated incorporation of choline into microsomal lecithin subspecies in vitro. FEBS Lett. 1971 Sep 15;17(1):14–16. doi: 10.1016/0014-5793(71)80551-3. [DOI] [PubMed] [Google Scholar]
- Chida N., Arakawa T. Metabolism of phosphatidylcholine in brain and liver of developing rats. Tohoku J Exp Med. 1971 Aug;104(4):359–371. doi: 10.1620/tjem.104.359. [DOI] [PubMed] [Google Scholar]
- DILS R. R., HUBSCHER G. Metabolism of phospholipids. III. The effect of calcium ions on the incorporation of labelled choline into rat-liver microsomes. Biochim Biophys Acta. 1961 Jan 29;46:505–513. doi: 10.1016/0006-3002(61)90581-9. [DOI] [PubMed] [Google Scholar]
- Dhopeshwarkar G. A., Mead J. F. Fatty acid uptake by the brain. II. Incorporation of [I-14C] palmitic acid into the adult rat brain. Biochim Biophys Acta. 1969 Dec 17;187(4):461–467. [PubMed] [Google Scholar]
- Diamond I. Choline metabolism in brain. The role of choline transport and the effects of phenobarbital. Arch Neurol. 1971 Apr;24(4):333–339. doi: 10.1001/archneur.1971.00480340065007. [DOI] [PubMed] [Google Scholar]
- Diamond I., Kennedy E. P. Carrier-mediated transport of choline into synaptic nerve endings. J Biol Chem. 1969 Jun 25;244(12):3258–3263. [PubMed] [Google Scholar]
- Erbland J. F., Marinetti G. V. The enzymatic acylation and hydrolysis of lysolecithin. Biochim Biophys Acta. 1965 Jul 7;106(1):128–138. doi: 10.1016/0005-2760(65)90101-3. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- Holub B. J., Breckenridge W. C., Kuksis A. Studies of differential turnover of palmitoyl and stearoyl species of glycerophosphatides using labeled unsaturated acids. Lipids. 1971 May;6(5):307–313. [PubMed] [Google Scholar]
- Hörtnagl H., Winkler H., Hörtnagl H. The subcellular distribution of lysophospholipase in bovine adrenal medulla. Eur J Biochem. 1969 Sep;10(2):243–248. doi: 10.1111/j.1432-1033.1969.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Illingworth D. R., Glover J. Lecithin: cholesterol acyl transferase activity in human cerebrospinal fluid. Biochim Biophys Acta. 1970 Dec 16;220(3):610–613. doi: 10.1016/0005-2744(70)90292-5. [DOI] [PubMed] [Google Scholar]
- Illingworth D. R., Glover J. The composition of lipids in cerebrospinal fluid of children and adults. J Neurochem. 1971 May;18(5):769–776. doi: 10.1111/j.1471-4159.1971.tb12006.x. [DOI] [PubMed] [Google Scholar]
- Illingworth D. R., Portman O. W. Exchange of phospholipids between low and high density lipoproteins of squirrel monkeys. J Lipid Res. 1972 Mar;13(2):220–227. [PubMed] [Google Scholar]
- Kanoh H. Biosynthesis of molecular species of phosphatidyl choline and phosphatidyl ethanolamine from radioactive precursors in rat liver slices. Biochim Biophys Acta. 1969 Jun 10;176(4):756–763. [PubMed] [Google Scholar]
- LANDS W. E. Metabolism of glycerolipids. 2. The enzymatic acylation of lysolecithin. J Biol Chem. 1960 Aug;235:2233–2237. [PubMed] [Google Scholar]
- LONG C., PENNY I. F. The structure of the naturally occurring phosphoglycerides. III. Action of moccasin-venom phospholipase A on ovolecithin and related substances. Biochem J. 1957 Feb;65(2):382–389. doi: 10.1042/bj0650382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovitz Z., Gatt S. Isolation of lysophospholipase, free of phospholipase activity, from rat brain. Biochim Biophys Acta. 1968 Oct 22;164(2):439–441. doi: 10.1016/0005-2760(68)90173-2. [DOI] [PubMed] [Google Scholar]
- Lunt G. G., Rowe C. E. The effect of cholinergic substances on the production of unesterified fatty acids in brain. Brain Res. 1971 Dec 10;35(1):215–220. doi: 10.1016/0006-8993(71)90606-8. [DOI] [PubMed] [Google Scholar]
- Nelson G. J. The phospholipid composition of plasma in various mammalian species. Lipids. 1967 Jul;2(4):323–328. doi: 10.1007/BF02532119. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. Choline metabolism and membrane formation in rat hepatoma cells grown in suspension culture. 3. Choline transport and uptake by simple diffusion and lack of direct exchange with phosphatidylcholine. J Lipid Res. 1971 Nov;12(6):715–724. [PubMed] [Google Scholar]
- Porcellati G., Arienti G., Pirotta M., Giorgini D. Base-exchange reactions for the synthesis of phospholipids in nervous tissue: the incorporation of serine and ethanolamine into the phospholipids of isolated brain microsomes. J Neurochem. 1971 Aug;18(8):1395–1417. doi: 10.1111/j.1471-4159.1971.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Portman O. W., Alexander M. Changes in arterial subfractions with aging and atherosclerosis. Biochim Biophys Acta. 1972 Mar 23;260(3):460–474. doi: 10.1016/0005-2760(72)90061-6. [DOI] [PubMed] [Google Scholar]
- Portman O. W., Alexander M. Lysophosphatidylcholine concentrations and metabolism in aortic intima plus inner media: effect of nutritionally induced atherosclerosis. J Lipid Res. 1969 Mar;10(2):158–165. [PubMed] [Google Scholar]
- Portman O. W., Alexander M., Maruffo C. A. Nutritional control of arterial lipid composition in squirrel monkeys: major ester classes and types of phospholipids. J Nutr. 1967 Jan;91(1):35–46. doi: 10.1093/jn/91.1.35. [DOI] [PubMed] [Google Scholar]
- Portman O. W., Alexander M. Metabolism of sphingolipids by normal and atherosclerotic aorta of squirrel monkeys. J Lipid Res. 1970 Jan;11(1):23–30. [PubMed] [Google Scholar]
- Portman O. W., Soltys P., Alexander M., Osuga T. Metabolism of lysolecithin in vivo: effects of hyperlipemia and atherosclerosis in squirrel monkeys. J Lipid Res. 1970 Nov;11(6):596–604. [PubMed] [Google Scholar]
- Rytter D., Miller J. E., Cornatzer W. E. Specificity for incorporation of choline and ethanolamine into rat-liver microsomal lecithins. Biochim Biophys Acta. 1968 Mar 4;152(2):418–421. doi: 10.1016/0005-2760(68)90054-4. [DOI] [PubMed] [Google Scholar]
- Sarzala M. G., Van Golde L. M., De Kruyff B., Van Deenen L. L. The intramitochondrial distribution of some enzymes involved in the biosynthesis of rat-liver phospholipids. Biochim Biophys Acta. 1970 Feb 10;202(1):106–119. doi: 10.1016/0005-2760(70)90222-5. [DOI] [PubMed] [Google Scholar]
- Schuberth J., Sparf B., Sundwall A. A technique for the study of acetylcholine turnover in mouse brain in vivo. J Neurochem. 1969 May;16(5):695–700. doi: 10.1111/j.1471-4159.1969.tb06447.x. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl W. L., Trams E. G. Synthesis of lipids by liver plasma membranes. Incorporation of acyl-coenzyme A derivatives into membrane lipids in vitro. Biochim Biophys Acta. 1968 Dec 10;163(4):459–471. doi: 10.1016/0005-2736(68)90075-8. [DOI] [PubMed] [Google Scholar]
- Stein Y., Stein O. Metabolism of labeled lysolecithin, lysophosphatidyl ethanolamine and lecithin in the rat. Biochim Biophys Acta. 1966 Feb 1;116(1):95–107. doi: 10.1016/0005-2760(66)90095-6. [DOI] [PubMed] [Google Scholar]
- Stein Y., Widnell C., Stein O. Acylation of lysophosphatides by plasma membrane fractions of rat liver. J Cell Biol. 1968 Oct;39(1):185–192. doi: 10.1083/jcb.39.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer S., Eder H. A. Transport of lysolecithin by albumin in human and rat plasma. J Lipid Res. 1965 Oct;6(4):506–511. [PubMed] [Google Scholar]
- Treble D. H., Frumkin S., Balint J. A., Beeler D. A. The entry of choline into lecithin, in vivo, by base exchange. Biochim Biophys Acta. 1970 Feb 10;202(1):163–171. doi: 10.1016/0005-2760(70)90227-4. [DOI] [PubMed] [Google Scholar]
- WEBSTER G. R., MARPLES E. A., THOMPSON R. H. Glycerylphosphorylcholine diesterase activity of nervous tissue. Biochem J. 1957 Feb;65(2):374–377. doi: 10.1042/bj0650374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster G. R. The acylation of lysophosphatides with long-chain fatty acids by rat brain and other tissues. Biochim Biophys Acta. 1965 Jun 1;98(3):512–519. doi: 10.1016/0005-2760(65)90147-5. [DOI] [PubMed] [Google Scholar]
- Wright J. D., Green C. The role of the plasma membrane in fatty acid uptake by rat liver parenchymal cells. Biochem J. 1971 Aug;123(5):837–844. doi: 10.1042/bj1230837. [DOI] [PMC free article] [PubMed] [Google Scholar]


