Abstract
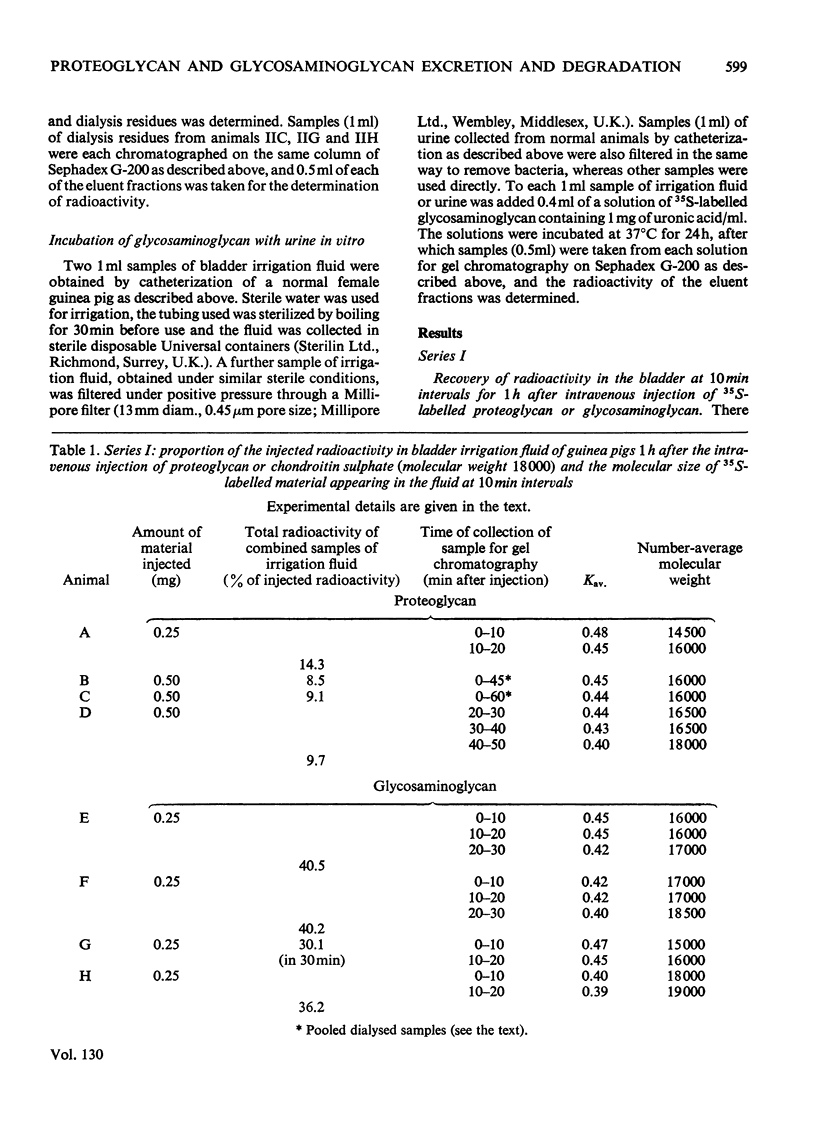
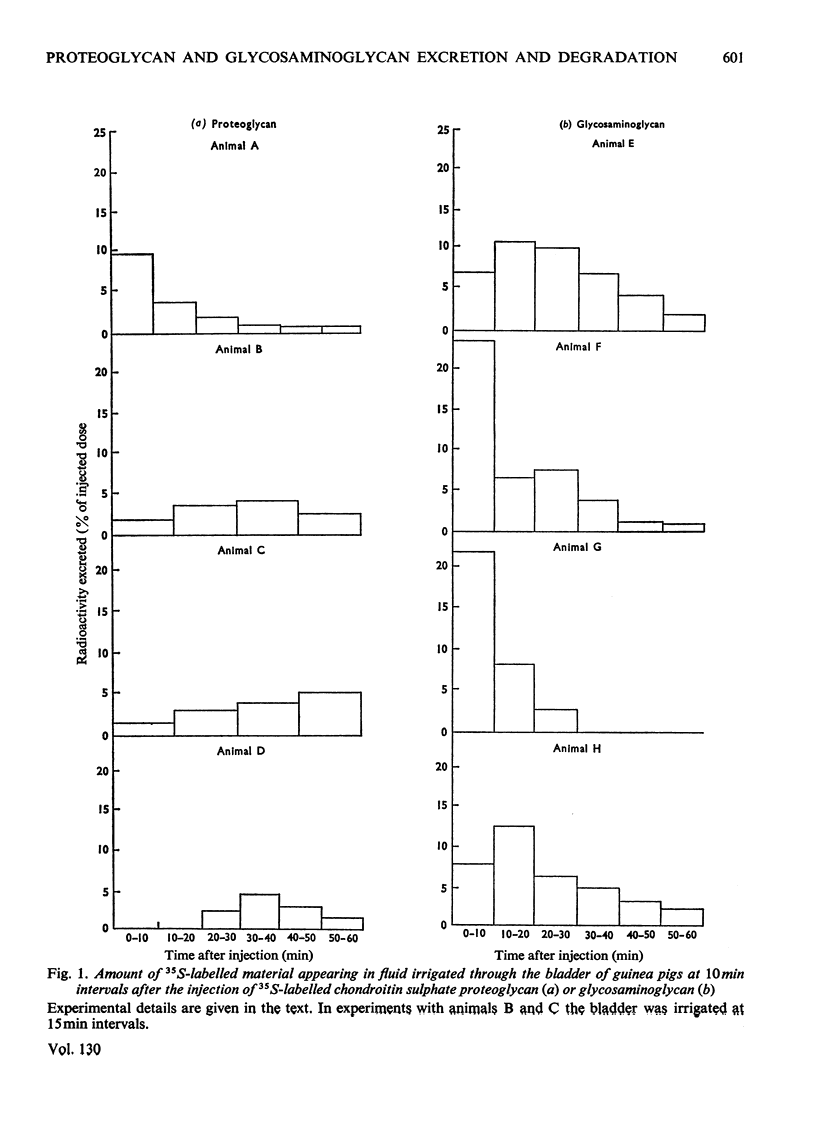
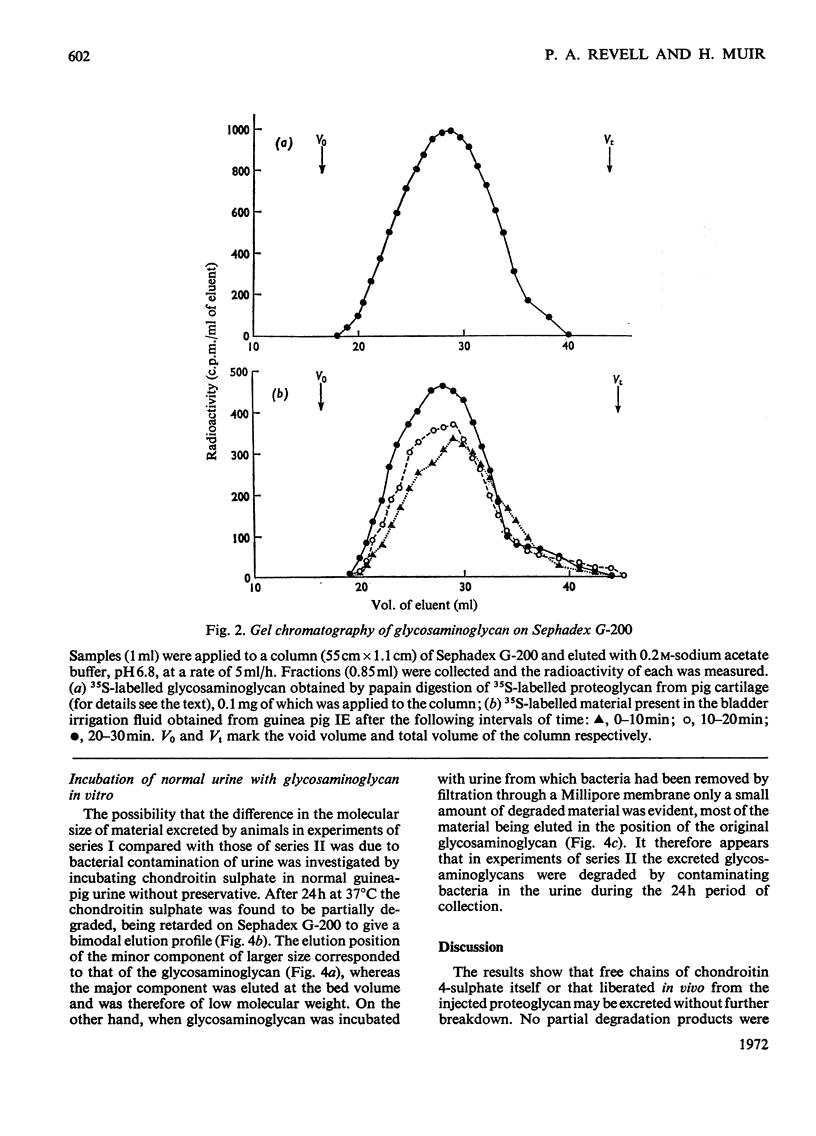
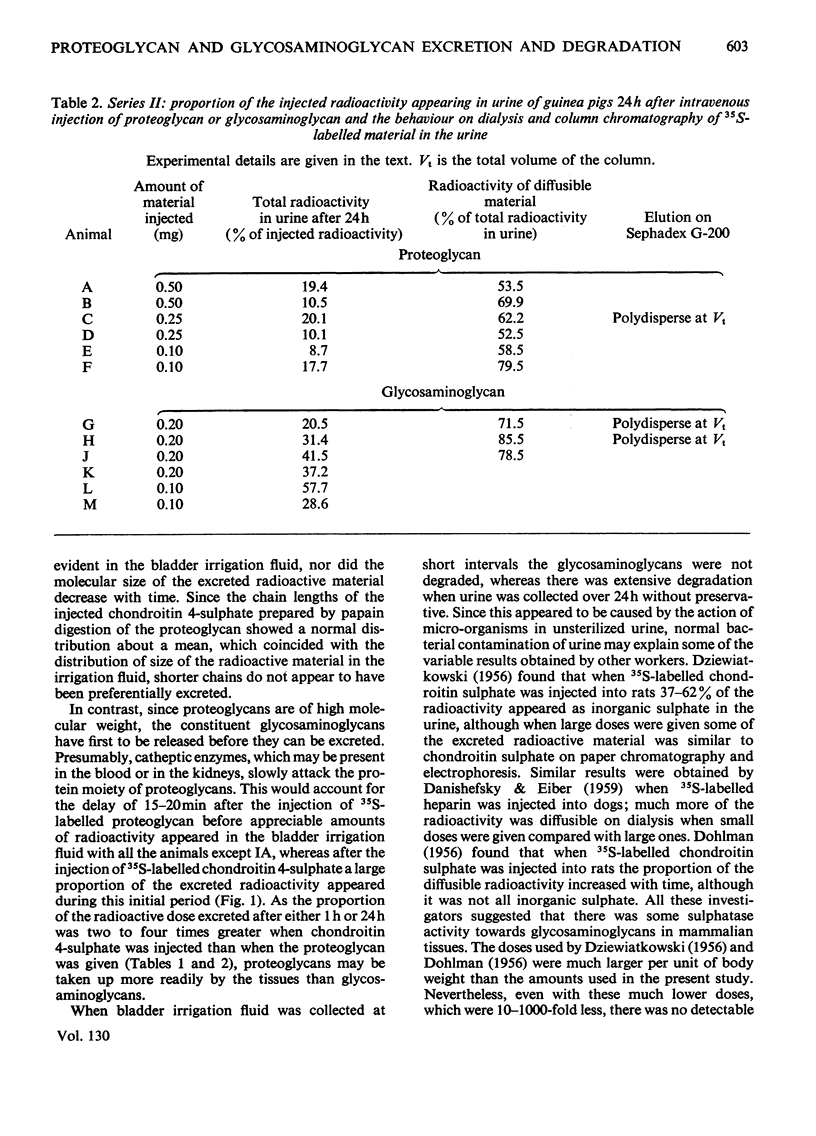
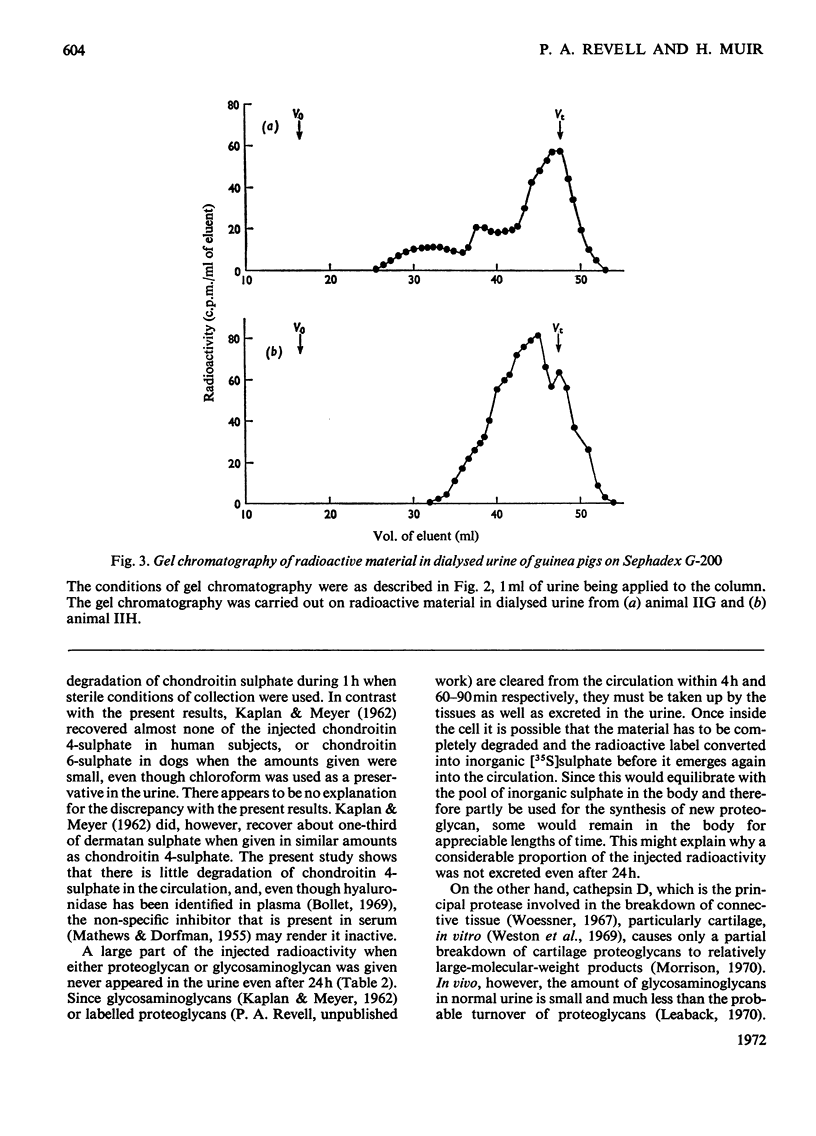
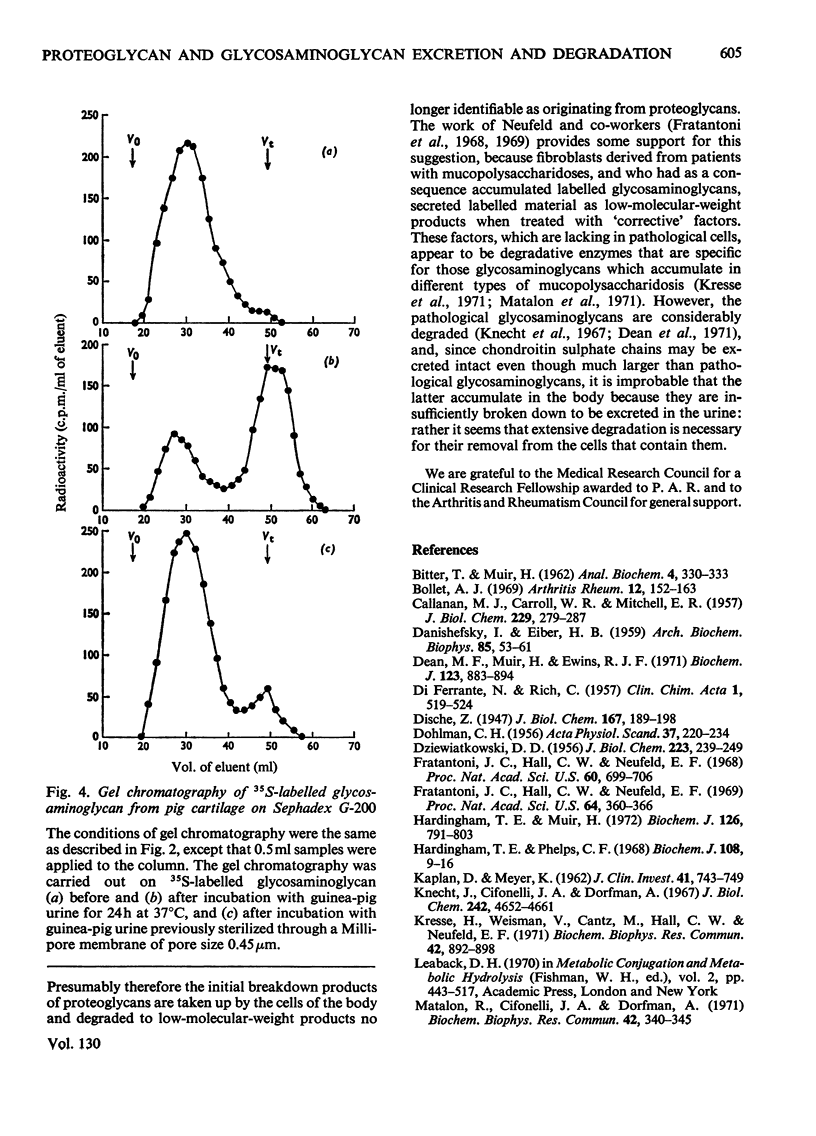
The excretion and degradation was studied of 35S-labelled 4-chondroitin sulphate injected into guinea pigs in the form of proteoglycan isolated from cartilage and in the form of free chondroitin 4-sulphate prepared from the same proteoglycan by proteolysis. When the proteoglycan was injected there was a delay of about 15–20min before significant amounts or radioactivity were excreted, whereas after injection of chondroitin 4-sulphate a considerable amount of radioactivity was excreted within 10min and a much higher proportion of the radioactive dose was excreted in 1h or 24h compared with the proteoglycan. In both cases, however, a major part of the radioactivity was not excreted even in 24h. Sterile conditions were used to collect the radioactive material directly from the bladder. When chondroitin 4-sulphate was injected, the molecular sizes of injected and excreted materials were similar, as assessed by gel chromatography on Sephadex G-200, whereas when proteoglycan was injected the molecular size of the excreted labelled material was similar to that of the chondroitin 4-sulphate chains in the original proteoglycan. In neither case did the size of the excreted labelled material change with time over 1h, and low-molecular-weight labelled material was virtually absent. In contrast, when urine was collected for 24h without preservative the labelled material in it was extensively degraded after either the proteoglycan or chondroitin 4-sulphate had been given. Chondroitin 4-sulphate became similarly degraded when incubated with non-sterile urine, but not when the urine was passed through a bacterial filter, suggesting that degradation was caused by contaminating micro-organisms in the experiments in which urine was collected for 24 h. It is concluded that chondroitin 4-sulphate chains of about 18000 molecular weight can be excreted readily as such, whereas intact proteoglycans must be degraded to free glycosaminoglycans first, although both are taken up by the tissues more rapidly than they are excreted.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bollet A. J. An essay on the biology of osteoarthritis. Arthritis Rheum. 1969 Apr;12(2):152–163. doi: 10.1002/art.1780120212. [DOI] [PubMed] [Google Scholar]
- CALLANAN M. J., CARROLL W. R., MITCHELL E. R. Physical and chemical properties of protamine from the sperm of salmon (Oncorhynchus tschawytscha). J Biol Chem. 1957 Nov;229(1):279–287. [PubMed] [Google Scholar]
- DANISHEFSKY I., EIBER H. B. Studies on the metabolism of heparin. Arch Biochem Biophys. 1959 Nov;85:53–61. doi: 10.1016/0003-9861(59)90446-1. [DOI] [PubMed] [Google Scholar]
- DI FERRANTE N., RICH C. The mucopolysaccharide of normal human urine. Clin Chim Acta. 1956 Nov-Dec;1(6):519–524. doi: 10.1016/0009-8981(56)90039-0. [DOI] [PubMed] [Google Scholar]
- DOHLMAN C. H. The fate of the sulfate group of chondroitin sulfate after administration to rats. Acta Physiol Scand. 1956 Sep 26;37(2-3):220–234. doi: 10.1111/j.1748-1716.1956.tb01358.x. [DOI] [PubMed] [Google Scholar]
- DZIEWIATKOWSKI D. D. Some aspects of the metabolism of chondroitin sulfate-S35 in the rat. J Biol Chem. 1956 Nov;223(1):239–249. [PubMed] [Google Scholar]
- Dean M. F., Muir H., Ewins R. J. Hurler's, Hunter's and Morquio's syndromes. A biochemical study in the light of current views of the underlying defects. Biochem J. 1971 Aug;123(5):883–894. doi: 10.1042/bj1230883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratantoni J. C., Hall C. W., Neufeld E. F. The defect in Hurler and Hunter syndromes. II. Deficiency of specific factors involved in mucopolysaccharide degradation. Proc Natl Acad Sci U S A. 1969 Sep;64(1):360–366. doi: 10.1073/pnas.64.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratantoni J. C., Hall C. W., Neufeld E. F. The defect in Hurler's and Hunter's syndromes: faulty degradation of mucopolysaccharide. Proc Natl Acad Sci U S A. 1968 Jun;60(2):699–706. doi: 10.1073/pnas.60.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Biosynthesis of proteoglycans in cartilage slices. Fractionation by gel chromatography and equilibrium density-gradient centrifugation. Biochem J. 1972 Feb;126(4):791–803. doi: 10.1042/bj1260791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Phelps C. F. The tissue content and turnover rates of intermediates in the biosynthesis of glycosaminoglycans in young rat skin. Biochem J. 1968 Jun;108(1):9–16. doi: 10.1042/bj1080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN D., MEYER K. The fate of injected mucopolysac-charides. J Clin Invest. 1962 Apr;41:743–749. doi: 10.1172/JCI104532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht J., Cifonelli J. A., Dorfman A. Structural studies on heparitin sulfate of normal and Hurler tissues. J Biol Chem. 1967 Oct 25;242(20):4652–4661. [PubMed] [Google Scholar]
- Kresse H., Wiesmann U., Cantz M., Hall C. W., Neufeld E. F. Biochemical heterogeneity of the Sanfilippo syndrome: preliminary characterization of two deficient factors. Biochem Biophys Res Commun. 1971 Mar 5;42(5):892–898. doi: 10.1016/0006-291x(71)90514-6. [DOI] [PubMed] [Google Scholar]
- MATHEWS M. B., DORFMAN A. Inhibition of hyaluronidase. Physiol Rev. 1955 Apr;35(2):381–402. doi: 10.1152/physrev.1955.35.2.381. [DOI] [PubMed] [Google Scholar]
- Matalon R., Cifonelli J. A., Dorfman A. L-iduronidase in cultured human fibroblasts and liver. Biochem Biophys Res Commun. 1971 Jan 22;42(2):340–345. doi: 10.1016/0006-291x(71)90108-2. [DOI] [PubMed] [Google Scholar]
- SMITH W. S., KERBY G. P. Urinary excretion of acid mucopolysaccharides by rabbits injected with chelating agents and with chondroitin sulfate. Proc Soc Exp Biol Med. 1960 Mar;103:562–565. doi: 10.3181/00379727-103-25596. [DOI] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of molecular weight dispersion in chondroitin sulphate on a microgram level. Biochim Biophys Acta. 1969 Feb 18;177(1):152–154. doi: 10.1016/0304-4165(69)90076-2. [DOI] [PubMed] [Google Scholar]
- Weston P. D., Barrett A. J., Dingle J. T. Specific inhibition of cartilage breakdown. Nature. 1969 Apr 19;222(5190):285–286. doi: 10.1038/222285b0. [DOI] [PubMed] [Google Scholar]


