Abstract
Breast cancer (BrCa) is a complex and heterogeneous disease with diverse molecular subtypes, leading to varied clinical outcomes and posing significant treatment challenges. The increasing global burden of BrCa, particularly in low- and middle-income countries, underscores the urgent need for more effective therapeutic strategies. The androgen receptor (AR), expressed in a substantial proportion of breast cancer cases, has emerged as a potential biomarker and therapeutic target. In breast cancer, AR exhibits diverse functions across subtypes, often interacting with other hormone receptors, thereby influencing tumor progression and treatment responses. This intricate interplay is further complicated by the presence of constitutively expressed AR splice variants (AR-Vs) that drive resistance to AR-targeting therapies through structural rearrangements in the domains and activation of aberrant signaling pathways. Although AR-targeting drugs, initially developed for prostate cancer (PCa), have shown promise in AR-positive breast cancer, significant gaps remain in understanding AR’s precise functions and therapeutic potential. The systemic management of breast cancer is guided primarily by theranostic biomarkers; ER, PR, HER2, and Ki67 which also dictate the breast cancer classification. The ubiquitous expression of AR in BrCa and the emergence of AR-Vs can assist the management of disease complementing the standard of care. This article provides a comprehensive overview of AR and its splice variants in the context of breast cancer, highlighting their prognostic and predictive value across different subtypes looking beyond the conventional ER, PR, and HER2 status. This review also raises the possibility of using AR splice variants in predicting tumor aggressiveness. From the settings of developing nations, this may provide useful insight by integrating recent advances in AR-targeted therapies and exploring their translational potential, emphasizing the critical need for further research to optimize AR-based therapeutic strategies for breast cancer management.
Keywords: Androgen receptor, Breast cancer, Splice variants, AR-V7, Androgens, AR signaling
Background
The androgen receptor, also known as NR3C4, (nuclear receptor subfamily 3, group C, gene 4) belongs to the steroid receptor superfamily. Other members of steroid receptor groups are Estrogen Receptor (ER), Progesterone Receptor (PR), Glucocorticoid Receptor (GR), and Mineralocorticoid Receptor (MR) [1, 2]. Being a ligand-dependent transcription factor, AR mediates the biological effects of androgens, primarily testosterone and dihydrotestosterone (DHT). It plays a vital role in various physiological processes, including developing male reproductive systems, secondary sexual characteristics, and overall homeostasis. Despite being extensively studied in the context of prostate cancer, the role of AR in breast cancer has garnered increasing attention. AR having a substantial expression, complex, and multifaceted role in diverse molecular subtypes of breast cancer may influence tumor behavior, response to therapy, and clinical outcomes [3–5]. This complexity is aggravated by AR splice variants (AR-Vs) which contribute to the therapy resistance documented in prostate cancer. Given the pivotal role of AR in breast cancer, it is imperative to explore its potential as a prognostic and predictive biomarker, as well as a therapeutic target.
This review delves into the structural intricacies of the AR gene, highlighting the significance of its splice variants and their functional implications. Understanding androgen signaling, as well as the structural, functional, and clinical dimensions of AR and its variants, is essential for developing effective, tailored treatments and improving patient outcomes.
Circulating androgens in women
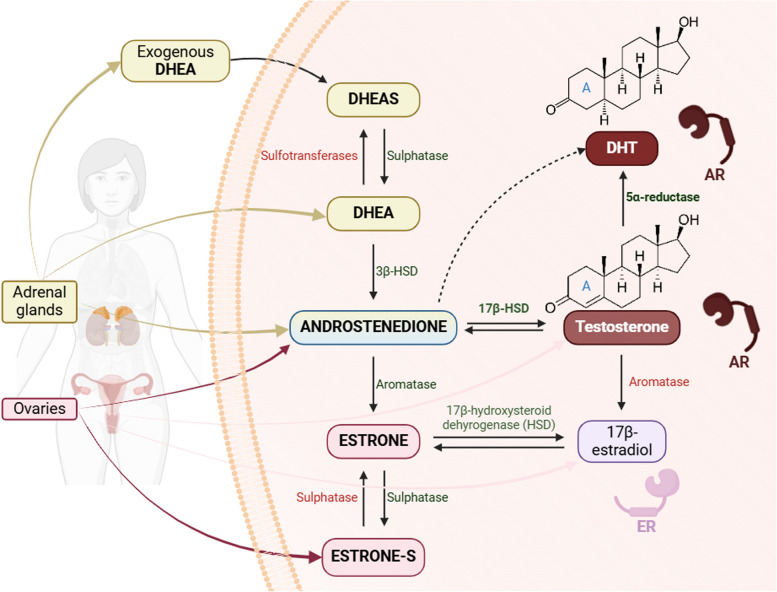
Androgens, primarily, are male sex hormones, which are also produced in smaller quantities in females and are crucial for regular reproductive health and fertility [6, 7]. They were first described in 1889, by French physiologist and Professor of Medicine Charles Edward Brown-Sequard from testicular extracts [8]. In women, circulating androgens are secreted by the adrenal glands as dehydroepiandrosterone-sulphate (DHEAS), dehydroepiandrosterone (DHEA), and androstenedione (A4), and by the ovaries as testosterone, DHEA, and A4 as outlined in Fig. 1 [9]. Peripheral tissues, such as the brain, bone, and breast, also produce testosterone and DHT [7]. Within cells, testosterone is converted to more potent androgen, DHT, by the enzyme 5α-reductase [10, 11]. The aromatase enzyme in various tissues, including mammary glands, converts testosterone to estradiol. DHT differs from testosterone by lacking a double bond in ring A [12], increasing its affinity for the AR two-fold and reducing the rate of dissociation of the AR-DHT complex five-fold compared to the AR-testosterone complex [13]. In women, circulating androgen levels decline during mid-reproductive years and continue to decrease after menopause [14]. Elevated testosterone levels are associated with an increased risk of breast cancer in pre-menopausal women.
Fig. 1.
Biosynthesis and interconversion of circulating androgens in women. Androgens, including DHEAS, DHEA, A4, and testosterone, are produced by the adrenal glands and ovaries. Testosterone and DHT (with a higher affinity for the AR) play a significant role in androgen signaling by binding to and catalyzing ligand-dependent action of AR. Created in https://BioRender.com
Structural components of AR
The AR gene, located on the X chromosome at locus Xq12, spans 186,599 base pairs and includes 8 exons, resulting in a 10,667 bp mRNA transcript encoding a 920-amino acid protein with a molecular weight of approximately 110 kDa [15, 16]. Like other steroid receptors, AR is characterized by three major functional domains.
The N-Terminal domain (NTD)
The NTD, encoded by exon 1, comprises about 60% of the AR protein, encompassing residues 1 to 555. Studies involving AR deletion mutations have shown that NTD is crucial for the function and transactivation of AR [17]. It contains the constitutively expressed Activation Function domain 1 (AF-1), which includes two separable transcription activation units: the ligand-dependent TAU-1 and ligand-independent TAU-5 [18, 19]. These subunits facilitate the transcriptional activity of AR and interaction between the NTD and the Ligand Binding Domain (LBD) [20]. This NTD-LBD interaction stabilizes the AR dimer, and ultimately, transcriptional regulation [21]. The NTD is linked to the pathogenesis of several diseases with increased mutations mapped in this region [22].
AR-NTD incorporates two polymorphic trinucleotide repeat segments, the first of which are highly variable poly-glutamine (poly-Q) repeats [23]. These poly-Q sequences regulate folding and structure within the NTD, thus affecting AR-transcriptional activity [24]. While a majority of studies have not linked poly-Q with BrCa risk, Rebbeck et al. have demonstrated that women carrying AR allele with ≥ 28 CAG repeats were at higher risk of breast cancer [25]. The NTD also harbors poly-glycine (poly-G) repeats. These, however, are researched in limited studies with no relevant correlations to breast cancer risk [26, 27].
DNA binding domain (DBD)
The DBD, comprising residues 556 to 623 and encoded by exons 2 and 3, is a cysteine-rich, highly conserved region among the steroid receptor superfamily. The crystal structure of the DBD shows two zinc fingers, each containing four cysteine residues responsible for recognizing and binding to promoter DNA sequences called the Androgen Response Element (ARE), thereby regulating gene expression. The α-helix of the zinc finger interacts with the nucleotides in the DNA major groove of ARE [28, 29].
The DBD is connected to the LBD by a flexible hinge region (residues 623–665), which includes a Nuclear Localization Signal (NLS) facilitating AR nuclear import upon ligand binding [30]. AR, being a large protein of 110 kDa, requires active transport into the nucleus upon ligand binding. Li et al. suggested that androgen binding induces a switch exposing the NLS which ultimately facilitates nuclear entry of AR by importin family of proteins [31]. The NLS contains two bundles of basic amino acids that are highly conserved in the steroid receptors superfamily [21]. One motif in the hinge region, 629-RKLKKL-634, plays a role in DNA binding, AR translocation, and coactivator recruitment [32].
Ligand binding domain (LBD)
The C-terminal LBD, encompassing residues 666 to 920 and encoded by exons 4 to 8, is crucial for recognizing and binding androgens such as testosterone and DHT. Eleven α-helices and four short β-strands making two anti-parallel β-sheets constitute the AR LBD [21]. The Activation Function domain 2 (AF-2) is located on a ligand binding pocket, which functions as the binding site for endogenous androgens. The AF-2 facilitates cross-talk between AR domains by providing interaction between the N- and C-termini of the receptor [18]. When androgens bind to the AR-LBD, the AF-2 region becomes crucial for initiating a cascade of molecular events that leads to the recruitment of coactivators, binding thereof, and transcriptional activation of androgen-responsive genes.
The LBD also interacts with various agonists and antagonists, making it a significant target for therapeutic interventions in hormone-responsive cancers, such as prostate cancer. Mutations in the LBD can alter androgen binding, receptor activation, and gene expression, potentially contributing to the development and progression of prostate cancer [33]. Due to its key role in AR signaling, the LBD is a prime target for therapeutic interventions.
AR signaling
Being a steroid hormone-activated transcription factor, AR is dependent on androgens for its activity. It, however, can catalyze its actions either by DNA binding or independently. AR is located in the cytoplasm sequestered with the Heat Shock Proteins (HSP) and other chaperons [34]. Circulating androgens (testosterone), being steroid hormones, may passively diffuse through the cell membrane. Testosterone may get converted into its active metabolite, DHT by 5α-reductase. The binding of testosterone or DHT to the ligand-binding pocket promotes conformational changes in the AR that expose the NLS.
As exhibited in Fig. 2, through DNA binding-dependent action, commonly known as classical AR signaling cascade, AR disassociates from HSP and accessory proteins. Following this, AR translocates into the nucleus, dimerizes [35], and then attaches to the androgen response element in the promoter region of target genes, causing modulations in DNA transcription. Recruiting other proteins and coactivators, AR controls gene transcription positively or negatively, leading to a cascade of molecular events such as metabolism, differentiation, proliferation, apoptosis, or angiogenesis [36, 37].
Fig. 2.
Classical AR signaling in an androgen-responsive cell. AR activation through its ligands regulates gene expression by AR binding to androgen response elements on DNA, thus initiating the transcription of AR-responsive genes. Created in https://BioRender.com
However, in addition to its DNA binding-dependent action, AR also exhibits DNA binding independent activities that play a crucial role in cellular function. In the DNA binding independent events, AR can initiate rapid, non-genomic signaling by activating second messenger pathways such as MAPK (Mitogen-Activated Protein Kinase) pathway and PI3K (Phosphoinositide 3-Kinase)/Akt pathway [38].
Clinical relevance of AR in cancer settings
The AR is necessary for the proper growth and functioning of the prostate and plays a crucial role in the development and progression of prostate cancer, one of the most common cancers in men. In their seminal work, Huggins and Hodges correlated the AR-dependent growth and survival in PCa [39]. AR expression has ubiquitously been linked to both primary and metastatic PCa, making it a key target in treatment [40]. Androgen Deprivation Therapy (ADT) is the cornerstone of advanced PCa treatment [41]. ADT approaches include using drugs that inhibit androgen production, block their interaction with AR, or through surgical removal of the testes to eliminate the primary source of androgen production [42]. Bicalutamide, an AR antagonist, competes with endogenous androgens at the LBD, inhibiting AR activation [43]. Enzalutamide, a second-generation non-steroidal AR antagonist [44], has a higher affinity for AR and inhibits nuclear translocation of AR as well [45]. It has shown a significantly low risk of relapse in a double-blind randomized phase 3 trial [46]. With different modes of action focussing on AR signaling, AR antagonists are essential components of the treatment of prostate cancer and are useful in the management of this disease.
However, the efficacy of ADT can be limited by the development of resistance over time, leading to an advanced form of PCa, known as Castration-Resistant Prostate Cancer (CRPC) [47]. CRPC remains androgen-driven through multiple mechanisms such as AR truncations, mutations, overexpression, amplification, and extragonadal androgen synthesis, including within cancer tissue itself [48]. Several reports have linked constitutively expressed androgen receptor splice variants (AR-Vs) as a potential cause of treatment resistance in CRPC [49–53].
Alternative splicing in cancer
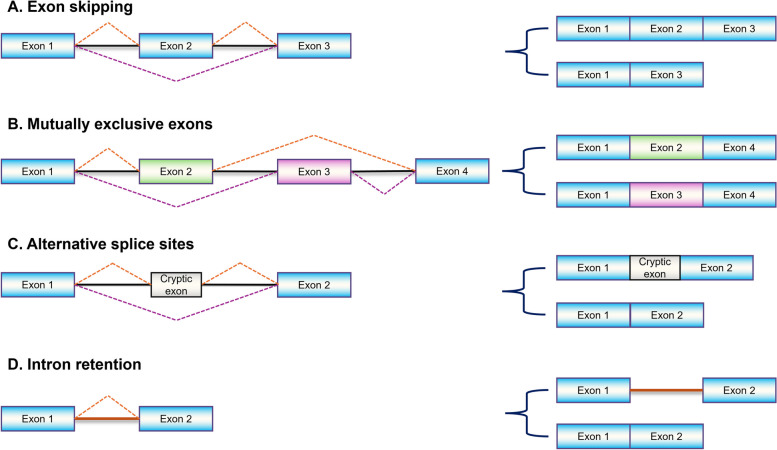
Alternative splicing (AS) is a fundamental molecular process joining exons in various combinations, prevalent in over 90% of human genes [54]. This process creates multiple mRNA isoforms from a single gene, contributing to genetic diversity [55–58]. Splicing mechanisms are orchestrated mainly by exon skipping, mutually exclusive exons, alternative splice sites, and intron retention as illustrated in Fig. 3 [59–62].
Fig. 3.
Commonly observed alternative splicing patterns. Differential combinations of exonic and intronic sequences generate full-length transcript or splice variants. Exons and introns are represented by colored boxes and horizontal lines, respectively while splicing arrangement is represented by dotted lines
Dysregulation of splicing factors commonly exhibited by cancer cells changes the splicing patterns, contributing to tumorigenesis [63–65]. AS can produce oncogenic isoforms that promote cell proliferation, inhibit apoptosis, and enhance metastasis [66]. Dysregulation of normal proliferation and apoptotic pathways takes place by aberrant splicing patterns in proteins such as the TP53 gene, which encodes the p53 tumor suppressor protein [67]. Certain splice variants of the BCL-X gene, a member of the BCL-2 family that regulates apoptosis, are involved in the evasion of apoptosis, metastasis, and resistance against chemo- or targeted therapies [68]. The AS of genes such as CD44 and VEGF-A have been reported to aggravate Epithelial-Mesenchymal Transition and angiogenesis, respectively [69]. Splice variants in several crucial genes can modify signaling pathways that regulate cell cycle, growth, differentiation, and survival facilitating tumor progression [70].
AR splice variants
Among AR splice variants, AR-Vs, particularly AR variant 7 (AR-V7), are extensively studied for their clinical relevance as prognostic and potential predictive biomarkers. AR-Vs lacking the LBD contribute to drug resistance in ADT, as anti-androgen drugs target the LBD. The earliest report of AR-Vs comes from Gregory et al., who in 2001 provided the first evidence of AR-Vs’ endogenic expression in PCa cell lines. They observed shorter molecular weight bands in the 80–90 kDa range on AR immunoblots, which were more prominent in CRPC cell lines [71]. AR45, an NTD truncated isoform, was identified in 2005, predominantly in the heart and other tissues, including the prostate, breast, and lung [72]. In the late 2000s, AR splice variants gained prominence, especially after the discovery of several variants lacking the LBD (except for AR-45). These variants can potentially facilitate constant AR activity. Identifying these variants laid the groundwork for subsequent studies crucial in comprehending CRPC’s molecular mechanisms [73, 74].
Hu et al. in their landmark work, identified cryptic exons (CE1, CE2, CE3) in intron 3, upstream to the DBD of AR. Alternative splicing involving these cryptic exons leads to seven novel AR-Vs (AR-V1 to AR-V7), which lack the DBD. AR-V1 and AR-V7 are abundantly expressed in clinical prostate cancer cases, with approximately 20-fold higher levels observed in CRPC [75]. Watson et al. in 2010, using conventional and next-generation sequencing approaches not only validated the known AR-V1 and AR-V7 but also detected several structurally diverse AR isoforms. They also reported the upregulation of AR-V1 and AR-V7 after castration at both mRNA and protein levels [76]. The discovery of AR-V1 and AR-V7 was confirmed by Guo et al. and further established by immunoblot in different cell lines [77]. AR-Vs validated and annotated on the NCBI database (as of November 2024) are summarized in Fig. 4 [15].
Fig. 4.
Organization of the AR gene and its splice variants annotated on NCBI. Located on the X chromosome, the AR primary transcript translates into AR-FL and various AR splice variants. Except for AR-TV2, most of the AR-Vs have ligand binding domain truncated. This figure presents the structural complexity of the AR, highlighting its relevance in cancer biology and potential therapeutic targeting. Created in https://BioRender.com
Depending on their conditional expression, most AR-Vs can be categorized into three groups: (1) constitutively expressed, (2) conditionally expressed, and (3) inactive [73]. The most extensively studied variant is AR-V7 in terms of clinical relevance. Since its discovery, AR-V7 has garnered more attention due to its constitutively high expression in CRPC cases [73, 78], and nuclear transport [79]. Additionally, AR-V7 expression significantly goes up following AR-FL signaling suppression due to androgen depletion, AR-FL knockdown, or enzalutamide treatment in VCaP and LNCaP95 cells but not in LNCaP and CWR22Rv1 cells. This indicates that the control of AR variant levels may rely on the specific cellular context alongside the cell-specific roles of AR splice variants [80]. Some AR-Vs exhibit conditional transcriptional activity, dependent on specific cellular contexts. For example, AR-V1 and AR-V9 are active in AR-FL-expressing cells (LNCaP) but not in AR-FL-negative cells (PC3) [81]. Inactive AR-Vs such as AR-V13 and AR-V14 are reported to have a partially truncated AR-LBD [81].
Mode of action of AR-Vs
AR-Vs exhibit varied nuclear transport capabilities due to the loss of the NLS. Immunofluorescent staining evidences the constitutive nuclear localization of AR-V7 even in the absence of androgens, while AR-V1, AR-V9, and AR-V13 remain cytoplasmic [75, 76, 81]. To explore the nature of AR-V7 nuclear transport and its transcriptional function, Chan et al. demonstrated a resemblance between unique regions in the AR-V7 C-terminal and AR-NLS. Mutations in some residues of this unique region compromised the AR-V7 nuclear transport [82].
Homodimerization is essential for the optimal transcriptional activity of AR-FL, involving intramolecular N-terminal and C-terminal interactions leading to nuclear transport and downstream actions [83]. Similar to AR-FL, AR-Vs require dimerization for transactivation of target genes, which can occur through homodimerization or heterodimerization with other AR-Vs [84]. AR-FL and AR-V7 heterodimerization can facilitate AR-FL nuclear localization and transcriptional activity regardless of androgens [52]. However, there is no consensus on the binding sites of AR-FL homodimer and AR-FL/AR-V7 heterodimer, with some studies showing overlapping regions and others indicating unique bindings [85–88]. AR-V7 binds to unique transcription sites through interactions with co-regulators like HOXV13 and ZFX [87, 88]. The absence of the LBD in AR-V7 is likely to substantially alter its 3D structure in AR-FL/AR-V7 heterodimers, exposing or masking regions that interact with co-regulators. Thus, while AR-FL and AR-V7 share some targets, AR-V7 may regulate distinct gene sets [75, 77, 80, 87, 89].
Clinical implications of AR-Vs
Prognostic and predictive value of AR-Vs
AR-Vs, especially AR-V7, have emerged as valuable prognostic markers for predicting resistance to AR-targeted therapies like abiraterone and enzalutamide. AR-V7 mRNA expression in circulating tumor cells (CTCs) predicts outcomes in CRPC patients, with AR-V7-positive CTCs showing resistance to next-generation AR-directed agents and less favorable clinical outcomes [51, 90]. Elevated levels of AR-V7 in hormone-naïve PCa correlate with an increased risk of relapse after radical prostatectomy [75, 77].
Prior studies in CTCs have examined how AR-V7-positive patients responded to taxanes versus enzalutamide or abiraterone. Taxanes were more effective in AR-V7-positive patients, especially in terms of Prostate-specific antigen (PSA) - Progression-Free Survival (PFS) and PFS, affirming CTC AR-V7’s predictive potential [91]. However, the prognostic value of AR-Vs in tissues is less clear compared to liquid biopsy, Zhu et al. have observed increased expression of AR-V7 and AR-V7/AR-FL ratio as the PCa progresses to CRPC [92, 93]. These results reinforce AR-V7 as a prognostic marker linked to poor outcomes in metastatic CRPC patients treated with abiraterone or enzalutamide [94].
Therapeutic potential of AR-Vs
Given AR-Vs’ significance in CRPC progression, there is a growing need for AR-Vs targeting therapies. While taxanes are currently the primary treatment choice for AR-V7-positive CRPC patients, research is underway to develop new drugs that target AR-Vs [95]. Such approaches aim to disrupt AR-Vs signaling, either by targeting AR-NTD [96], or inhibition of protein expression [97], reducing AR-Vs expression [98] or prevention at DNA-binding [99].
Ji et al. discuss NTD-targeting agents under development with a broad spectrum of effects on AR-FL and other AR-Vs containing AR-NTD [100]. For instance, EPI, non-steroidal AR antagonists that directly target the NTD of AR (which is also present in other AR-Vs) and prevent coactivators from binding to it, are under investigation [101, 102]. EPI-7386, one such novel drug is currently in clinical trials to evaluate its efficacy, both as a monotherapy and in combination with enzalutamide, for AR-V7-positive CRPC patients [95]. Additionally, niclosamide, an FDA-approved anti-helminthic drug, was found to selectively inhibit AR-V7 protein expression through enhanced protein degradation in preclinical studies [103]. Although not all of these compounds will likely find their way into clinics, these new medications offered novel and promising ways to efficiently target AR-Vs [104].
The study of AR splice variants in CRPC is still in the early stages despite significant interest in the years since their discovery. AR-Vs, notably AR-V7, are consistently detectable in various biospecimens and have negative prognostic implications. Although further clinical investigations with multiple treatment arms are needed to confirm the predictive value of AR-Vs, some results indicate that CTC-based AR-V7 may be a useful predictive biomarker when choosing between AR-targeted therapies and taxanes in treatment decisions [91, 105, 106].
AR in breast cancer
The heterogeneous nature of breast cancer poses significant treatment challenges, necessitating new therapeutic regimens in addition to currently employed drugs. Keeping aside methodological variability such as AR localization, IHC cut-off, and staining protocol used, AR is reported to be expressed in 70 − 90% of clinical breast cancer [3–5]. This ubiquitous expression pattern positions AR as an emerging marker and therapeutic target. Immunohistochemistry of AR in the prostate and different subtypes of breast cancer tissues is illustrated in Fig. 5.
Fig. 5.
Immunohistochemical staining of AR in the prostate and different subtypes of breast cancer at different magnifications. The varying intensity of AR expression across different subtypes highlights the receptor’s differential expression patterns and potential diagnostic significance. IHC performed with anti-AR mAb (MA5-16412, Invitrogen 1:400)
AR expression and relevance in different BrCa subtypes
Although there are similarities between prostate and breast cancers due to the hormone-driven nature of both diseases, there are considerable differences in AR signaling pathways and their downstream effects [107]. In PCa, AR functions as a primary driver of tumor growth, with AR-Vs such as AR-V7 contributing to therapeutic resistance. In contrast, AR’s role in BrCa varies by subtype, acting as a tumor suppressor in ER-positive subtypes, and a potential oncogene in AR-positive Triple-Negative Breast Cancer (TNBC) [108, 109]. These nuanced roles underscore the need to explore the subtype-specific implications of AR in BrCa while leveraging insights from PCa to guide therapeutic development.
Luminal type
A comprehensive systematic review of 19 studies involving 7,693 women found that AR co-expressed with ER-positive breast cancer in 74.8% of cases versus 31.8% in ER-negative tumors [110]. In further analyses based on molecular subtypes, Luminal types, characterized by ER-positivity, displayed varying levels of AR expression, ranging from 50 to 90% [111]. In breast cancer, there is a complex interplay between AR and ER. Normal breast tissue expresses two types of ER involved in reproductive organ development and other cellular processes. Among the two ERs, ERα and ERβ, with estradiol being their natural ligand, ERβ is dominant in normal breast. However, in breast cancer, ERα expression increases, contributing to tumorigenesis [112].
Preclinical studies indicate varying proliferative effects linked to the ratios of ERα and AR and the availability of their ligands [113–116]. Importantly, testosterone can also be peripherally converted to estradiol via the aromatase enzyme, adding complexity to the AR-ER relationship [7, 117, 118]. Panet-Raymond et al. have demonstrated an interaction between AR and ERα that might alter both receptors’ transcriptional activity, with AR having a greater impact on ERα transactivation [114]. Studies on AR activity in breast cancer carcinogenesis, primarily conducted in vitro using breast cancer cell lines like MCF-7, T47-D, and BT20, have demonstrated that AR antagonism has an antiproliferative effect [119–121]. This highlights the intricate and varying responses to hormones and their antagonists in breast cancer cells which could depend on the several proteins that interact with AR [122].
HER2 enriched
AR expression in HER2-enriched subtypes ranges from 20 to 60% [3, 111, 123]. Preclinical studies suggest that the molecular apocrine subtype (ER-negative, AR-positive) may proliferate in the presence of androgens, driven by intricate interactions between the AR and the HER2 signal transduction pathway, independent of the ER pathway [124]. HER2 kinase signaling is essential for full AR activity at low androgen levels, enhancing AR’s DNA binding and stability [125]. HER2-enriched and AR-positive cases correlate with poor clinical outcomes, including lower DFS and OS [126]. Another study finds an association between high AR mRNA with shorter DFS and OS in HER2-positive ER-negative patients [36]. DHT treatment in AR-positive HER2-enriched cell lines like MDA-MB-453 promotes growth through oncogenic Wnt and HER3 activation [127].
Additional research in cell lines and tissue samples emphasized the cross-regulation of specific genes between AR and HER2. This led to increased expression of steroid response genes and enhanced cell proliferation when either AR or HER2 was stimulated. Notably, the anti-androgen flutamide and HER2 inhibition demonstrated pro-apoptotic effects with a synergistic impact when used in combination [128]. The findings from these studies revealed that AR contributes to the carcinogenesis of HER2-enriched breast cancer.
TNBC
TNBC subtype displays comparatively lower AR expression (20–50%) [111, 123]. Traditionally, TNBC has exhibited poor outcomes, with a median overall survival of around 13 months in metastatic cases and a shorter time from disease recurrence to death compared to other breast cancers [129, 130]. TNBC remains biologically diverse despite its common features, complicating therapeutic target identification [131]. The Luminal Androgen Receptor (LAR) subtype, characterized by enriched AR signaling, accounts for 22% of TNBC cases [132, 133], and demonstrated increased sensitivity to the AR antagonist bicalutamide in in vitro studies [134]. AR-positive TNBC patients have lower survival rates than AR-negative ones [135]. Notably, even non-LAR TNBC cell lines showed involvement in AR signaling. AR was observed to up-regulate the EGFR ligand amphiregulin, promoting cell proliferation through the EGFR pathway, and the anti-androgen enzalutamide blocked this effect [136]. Thus, AR consistently promotes cellular proliferation in non-luminal types, making it a potential therapeutic target.
Prognostic and predictive value of AR in BrCa
Patients with ERα-positive and AR-positive tumors exhibit better outcomes due to AR competing with ERα for Estrogen Response Elements (ERE) [137], impairing ERα-induced transcription and promoting apoptosis [126, 135]. Clinical studies validate these findings, indicating that ERα-positive AR-positive patients have improved outcomes [126, 135, 138]. In contrast, in ERα-negative tumors, AR binds to androgen-responsive elements, promoting cell proliferation and tumor growth [138]. Ravaioli et al. report in ductal carcinoma in situ (DCIS), AR expression is higher in relapsed tumors, while ERα is higher in non-relapsed cases [139]. Thus, AR, along with hormone receptors (HR) may serve as a better prognostic marker to predict relapse in DCIS patients [140, 141].
The role of AR in luminal breast cancers is debated, with some studies identifying it as an independent prognostic biomarker [110, 142]. The AR/ERα ratio serves as a prognostic tool for in situ relapse or progression to invasive subtypes [140]. It can predict endocrine therapy response, with high nuclear AR/ERα ratios linked to hormone therapy failure, and enzalutamide effectively reduces tumor growth in hormone therapy-resistant cases [143]. However, in advanced breast cancers, AR is not a strong predictor of first-line anti-estrogen therapy effectiveness, with progesterone receptor and Ki-67 being more reliable predictors [111]. In ERα-positive, HER2-positive breast cancers, AR-positivity correlates with smaller, less aggressive tumors [144]. AR promotes growth in ERα-negative and HER2-positive cancers by affecting HER3 gene expression [127].
Clinical data suggested AR might not be a suitable biomarker for endocrine therapy selection. On the other hand, studies supported AR’s predictive value for AR inhibitors, especially in TNBC [145–147]. In TNBC, AR-positivity is associated with better disease-free and overall survival, particularly in the LAR TNBC subgroup [148]. Antiandrogen therapy may benefit AR-positive TNBC patients, whereas AR-negative, EGFR-positive TNBCs are high-risk and benefit from chemotherapy [149]. ERβ is expressed in approximately 30% of TNBC and can downregulate AR expression [150].
Treatment options
AR activation inhibits cellular proliferation in most ER-positive breast cancers but stimulates growth in ER-negative cases. This suggests a potential use of AR agonists or antagonists in treatment. In ER-positive and some ER-negative BrCa cases where AR activation prevents tumor growth, androgens have been employed for therapy [151], but they come with side effects [152]. Selective AR modulators (SARMs) like non-steroidal anabolic agent, enobosarm (GTx-024), with minimal side effects, have gained attention for ER-positive advanced BrCa treatment [153]. SARMs were shown to reduce tumor load by 90% in in vivo studies conducted in MDA-MB-453 cells [154].
AR antagonists, such as bicalutamide and enzalutamide, are commonly used for advanced breast cancer, especially in tamoxifen-resistant and TNBC cases, with positive clinical trial results [155–157]. For TNBC and HER2-enriched BrCa, AR can promote growth when combined with other factors involving cell cycle progression and intracellular pathways for survival, proliferation, and invasiveness. Combining AR antagonists with pathway inhibitors may be a promising approach to achieve optimal outcomes [158–160]. There are several clinical trials (listed in Table 1) modulating AR activity using AR inhibitors as monotherapy or combined with other medications in breast cancer settings.
Table 1.
Ongoing clinical trials targeting AR-positive breast cancer
| Study identifier | Registration Date | Title | Breast cancer subtype | Trial agent | Agent class | Phase | Enrolment (n) | Primary outcome |
|---|---|---|---|---|---|---|---|---|
| NCT02605486 | 2015-11-11 | Palbociclib in Combination with Bicalutamide for the Treatment of AR(+) Metastatic Breast Cancer | AR + BrCa | Palbociclib + Bicalutamide | CDK4/6 inhibitor | I/II | 46 | PFS |
| NCT02750358 | 2016-05-19 | Feasibility Study of Adjuvant Enzalutamide for the Treatment of Early Stage AR (+) Triple Negative Breast Cancer | AR + TNBC | Enzalutamide | AR inhibitor | II | 50 | TDR |
| NCT02955394 | 2017-09-21 | Preoperative Fulvestrant with or Without Enzalutamide in ER+/Her2- Breast Cancer | AR+/ER+/Her2- BrCa | Fulvestrant + Enzalutamide | SERD | II | 61 | PEPI score |
| NCT03090165 | 2018-05-07 | Ribociclib and Bicalutamide in AR + TNBC | AR + TNBC | Ribociclib + Bicalutamide | CDK4/6 inhibitor | I/II | 37 | MTD, CBR |
| NCT03650894 | 2019-04-03 | Nivolumab, Ipilimumab, and Bicalutamide in Human Epidermal Growth Factor (HER) 2 Negative Breast Cancer Patients | HER2- | Nivolumab + Bicalutamide and Ipilimumab | anti-PD-1 + AR inhibitor and anit-CTLA4 | II | 30 | CBR |
| NCT04360941 | 2020-08-11 | PAveMenT: Palbociclib and Avelumab in Metastatic AR + Triple Negative Breast Cancer (PAveMenT) | AR + TNBC | Palbociclib + Avelumab | CDK4/6 inhibitor and immunotherapy drug | I | 45 | MTD, ORR |
| NCT04947189 | 2022-11-01 | Seviteronel in Combination with Chemotherapy in Androgen-receptor Positive Metastatic Triple-negative Breast Cancer (4CAST) | AR + TNBC | Seviteronel + Dexamethasone and Docetaxel | AR inhibitor | I and II | 65 | DLT |
| NCT05095207 | 2021-09-20 | Abemaciclib in Combination with Bicalutamide for Androgen Receptor-positive, HER2-negative Metastatic Breast Cancer | AR + HER2 - | Abemaciclib + Bicalutamide | CDK4/6 inhibitor | IB/II | 60 | DLT, RP2D |
| NCT05573126 | 2023-01-11 | Study to Evaluate EP0062 in Patients with Relapsed Locally Advanced or Metastatic Androgen Receptor Positive (AR+)/HER2-/ER + Breast Cancer | AR+/HER2-/ER + BrCa | EP0062 | SARM | I/II | 128 | DLT, MTD, AE, and SAE |
| NCT05673694 | 2023-03-08 | To Evaluate a Phase Ia/Ib Clinical Study of EG017 in Patients with Advanced Breast Cancer | AR+, ER+, HER2- | EG017 | SARM | Ia/Ib | 70 | DLTs, AE, and SAE |
| NCT05954442 | 2023-09-13 | Everolimus With Investigator’s Choice of Chemotherapy in Advanced Triple-Negative Breast Cancer (TNBC) With LAR Subtype (BCTOP-T-M03) | AR + TNBC | Everolimus + Investigator’s Choice of Chemotherapy | mTOR inhibitor | III | 203 | PFS |
| NCT06099769 | 2023-10-18 | A Study of Enzalutamide, Enzalutamide in Combination with Mifepristone, or Chemotherapy in People with Metastatic Breast Cancer | AR + TNBC or ER-low BrCa | Mifepristone ± Enzalutamide ± Chemotherapy | GR and AR inhibitors | II | 201 | PFS |
| NCT06365788 | 2024-04-08 | Bicalutamide and Abemaciclib in Inoperable or Metastatic Androgen Receptor-positive Triple-negative Breast Cancer (ABBICAR) | AR + TNBC | Abemaciclib + Bicalutamide | CDK4/6 inhibitor | II | 53 | DCR |
Abbreviations: PFS Progression-free survival, TDR Treatment discontinuation rate, PEPI Preoperative Endocrine Prognostic Index, CBR Clinical Benefit Rate, MTD Maximum Tolerated Dose, ORR Objective Response Rate, DLT Dose Limiting Toxicity, RP2D Recommended Phase II Dose, AE Adverse Events, SAE Serious Adverse Events, DCR Disease Control Rate
AR-Vs in breast cancer
With the significant prevalence of AR in breast cancer several AR-targeted therapies are employed at the clinical stage. Despite exhibiting encouraging outcomes, it leads to medication resistance in certain patients, eventually worsening the disease. Although the mechanisms driving treatment resistance are still being investigated, there is a chance that they are similar to those seen in prostate cancer. One such mechanism is attributed to the generation and overexpression of AR splice variants [76]. While AR-Vs testing has been well-established in the clinical management of PCa, its application in BrCa remains in its infancy. Pioneering works by Hu and Hickey et al. demonstrated several AR-Vs in different classes of breast cancer and cell lines [161, 162]. AR-V7, the most prominently expressed variant, is linked to resistance to ADT, correlates with the HER2-enriched subtype, and is supported in PCa cells as well [125, 127, 128]. Another study conducted by Ferguson et al. reviewed the prevalence and clinicopathologic features associated with AR-V7 in a sizable BrCa cohort. Their report reassures AR-V7 testing for AR-positive cases in both primary and metastatic settings. This study further encourages the consideration of AR-V7 as a predictive biomarker for the benefit of AR antagonists [163]. Recently we have reported the association of AR-V7 with aggressive clinicopathological parameters and poor overall and disease-free survival in breast cancer patients [164]. Armstrong et al. discussed the strategies targeting AR-Vs, the efficacy of which is under evaluation [165]. Such therapeutics can be extended to AR-Vs positive BrCa settings.
The clinical utility of AR-V7 testing in different subtypes of BrCa is limited by the lack of attention to the clinical utility of AR-V7 and robust validation studies. The detection of AR-V7 in CTCs through Immunohistochemistry (IHC) or qRT-PCR as a tool for non-invasive biomarker development has been unexplored in breast cancer [166]. Current research relies predominantly on preclinical works, cell lines, and small patient cohorts with limited diversity, which restricts the generalizability of findings. Moreover, there is limited evidence on whether AR-V7 expression correlates consistently with therapeutic resistance in BrCa, as observed in PCa. Despite these challenges, the advancement of the EPI series of drugs that target AR-NTD has paved the way for targeted therapies with AR-V7-positive BrCa [167]. Moving forward, larger, well-designed clinical studies are needed to establish AR-V7 as a reliable biomarker for therapeutic decision-making in BrCa. Understanding the mechanisms and signaling pathways associated with these variants is crucial for developing targeted therapies that can effectively combat hormone-resistant breast cancer.
Conclusion
The AR is a significant player in breast cancer pathophysiology, with its diverse roles and interactive landscape across the different molecular subtypes. The advent of AR-Vs in mediating therapeutic resistance necessitates further exploration in the context of breast cancer. The development of SARMs and AR-NTD antagonist drugs has opened new avenues for targeted therapy with the potential for improved efficacy and fewer side effects. Future research should focus more on developing predictive biomarkers to stratify patient selection for anti-AR therapies, exploring AR signaling pathways in various breast cancer subtypes, and the interplay between AR and other hormone receptors. Additionally, understanding the functional significance of AR-Vs in breast cancer progression and treatment resistance will be essential to optimize therapeutic strategies. These efforts hold the promise of advancing precision medicine approaches, improving prognostic and predictive accuracy, and ultimately enhancing outcomes for breast cancer patients.
Acknowledgements
The authors are grateful to All India Institute of Medical Sciences, New Delhi for providing institutional and logistic support to this study. The authors would like to acknowledge BioRender for their image preparation tool.
Abbreviations
- ADT
Androgen Deprivation Therapy
- AF
Activation Function
- AR
Androgen Receptor
- ARE
Androgen Response Element
- AS
Alternative Splicing
- bp
Base Pairs
- CE
Cryptic Exon
- CRPC
Castration-Resistant Prostate Cancer
- CTC
Circulating Tumor Cells
- DBD
DNA Binding Domain
- DCIS
Ductal Carcinoma in situ
- DFS
Disease-Free Survival
- DHEA
Dehydroepiandrosterone
- DHEAS
Dehydroepiandrosterone Sulphate
- DHT
Dihydrotestosterone
- EGFR
Epidermal Growth Factor Receptor
- ER
Estrogen Receptor
- HER2
Human Epidermal Growth Factor Receptor 2
- IHC
Immunohistochemistry
- LAR
Luminal Androgen Receptor
- LBD
Ligand Binding Domain
- NLS
Nuclear Localization Signal
- NTD
N-Terminal Domain
- OS
Overall Survival
- PCa
Prostate Cancer
- PFS
Progression-Free Survival
- PR
Progesterone Receptor
- PSA
Prostate-Specific Antigen
- qRT-PCR
Quantitative Reverse Transcription Polymerase Chain Reaction
- SARM
Selective Androgen Receptor Modulator
- SERD
Selective Estrogen Receptor Degrader
- TNBC
Triple-Negative Breast Cancer
Authors' contributions
TPS conceptualized the idea, created illustrations, and wrote the primary manuscript and drafts. RD and SK conceptualized, monitored, and oversaw the whole study. All authors read and approved the final manuscript.
Funding
The preparation of this review article required no funding.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
No ethics approval and consent to participate were required for this review article, as it does not involve primary data collection involving human participants or animals.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ruby Dhar, Email: rubydhar@gmail.com.
Subhradip Karmakar, Email: subhradip.k@aiims.edu.
References
- 1.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63(1):451–86. [DOI] [PubMed] [Google Scholar]
- 2.Committee Nuclear Receptors Nomenclature. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97(2):161–3. 10.1016/s0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- 3.Collins LC, Cole KS, Marotti JD, Hu R, Schnitt SJ, Tamimi RM. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the nurses’ health study. Mod Pathol. 2011;24(7):924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. 2010;23(2):205–12. [DOI] [PubMed] [Google Scholar]
- 5.Moinfar F, Okcu M, Tsybrovskyy O, Regitnig P, Lax SF, Weybora W, et al. Androgen receptors frequently are expressed in breast carcinomas: potential relevance to new therapeutic strategies. Cancer. 2003;98(4):703–11. [DOI] [PubMed] [Google Scholar]
- 6.Walters KA, Simanainen U, Handelsman DJ. Molecular insights into androgen actions in male and female reproductive function from androgen receptor knockout models. Hum Reprod Update. 2010;16(5):543–58. [DOI] [PubMed] [Google Scholar]
- 7.Labrie F, Luu-The V, Labrie C, Bélanger A, Simard J, Lin SX, et al. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003;24(2):152–82. [DOI] [PubMed] [Google Scholar]
- 8.Medvei VC. The history of clinical endocrinology: a comprehensive account of endocrinology from earliest times to the present day. CRC Press; 1993. p. 576. Medical. Retrieved from https://books.google.co.in/books/about/The_History_of_Clinical_Endocrinology_A.html?id=zRxQImynEsoC&redir_esc=y.
- 9.McNamara KM, Moore NL, Hickey TE, Sasano H, Tilley WD. Complexities of androgen receptor signalling in breast cancer. Endocr Relat Cancer. 2014;21(4):T161-181. [DOI] [PubMed] [Google Scholar]
- 10.Baulieu EE, Lasnizki I, Robel P. Metabolism of testosterone and action of metabolites on prostate glands grown in organ culture. Nature. 1968;219(5159):1155–6. [DOI] [PubMed] [Google Scholar]
- 11.Bruchovsky N, Wilson JD. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968;243(8):2012–21. [PubMed] [Google Scholar]
- 12.Brinkmann AO. Molecular basis of androgen insensitivity. Mol Cell Endocrinol. 2001;179(1–2):105–9. [DOI] [PubMed] [Google Scholar]
- 13.Grino PB, Griffin JE, Wilson JD. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology. 1990;126(2):1165–72. [DOI] [PubMed] [Google Scholar]
- 14.Kotsopoulos J, Narod SA. Androgens and breast cancer. Steroids. 2012;77(1):1–9. [DOI] [PubMed] [Google Scholar]
- 15.AR androgen receptor [Homo sapiens (human)] - gene - NCBI. Available from: https://www.ncbi.nlm.nih.gov/gene/367. Cited 2022 Jun 27.
- 16.Yang X, Guo Z, Sun F, Li W, Alfano A, Shimelis H, et al. Novel membrane-associated androgen receptor splice variant potentiates proliferative and survival responses in prostate cancer cells. J Biol Chem. 2011;286(41):36152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simental JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem. 1991;266(1):510–8. [PubMed] [Google Scholar]
- 18.Monaghan AE, McEwan IJ. A sting in the tail: the N-terminal domain of the androgen receptor as a drug target. Asian J Androl. 2016;18(5):687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McEwan IJ. Molecular mechanisms of androgen receptor-mediated gene regulation: structure-function analysis of the AF-1 domain. Endocr Relat Cancer. 2004;11(2):281–93. [DOI] [PubMed] [Google Scholar]
- 20.Jenster G, van der Korput HA, van Vroonhoven C, van der Kwast TH, Trapman J, Brinkmann AO. Domains of the human androgen receptor involved in steroid binding, transcriptional activation, and subcellular localization. Mol Endocrinol. 1991;5(10):1396–404. [DOI] [PubMed] [Google Scholar]
- 21.Tan ME, Li J, Xu HE, Melcher K, Yong E. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacol Sin. 2015;36(1):3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottlieb B, Beitel LK, Nadarajah A, Paliouras M, Trifiro M. The androgen receptor gene mutations database: 2012 update. Hum Mutat. 2012;33(5):887–94. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki M, Kaneuchi M, Sakuragi N, Fujimoto S, Carroll PR, Dahiya R. The polyglycine and polyglutamine repeats in the androgen receptor gene in Japanese and Caucasian populations. Biochem Biophys Res Commun. 2003;312(4):1244–7. [DOI] [PubMed] [Google Scholar]
- 24.Davies P, Watt K, Kelly SM, Clark C, Price NC, McEwan IJ. Consequences of poly-glutamine repeat length for the conformation and folding of the androgen receptor amino-terminal domain. J Mol Endocrinol. 2008;41(5):301–14. [DOI] [PubMed] [Google Scholar]
- 25.Rebbeck TR, Kantoff PW, Krithivas K, Neuhausen S, Blackwood MA, Godwin AK, et al. Modification of BRCA1-associated breast cancer risk by the polymorphic androgen-receptor CAG repeat. Am J Hum Genet. 1999;64(5):1371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadouri L, Easton DF, Edwards S, Hubert A, Kote-Jarai Z, Glaser B, et al. CAG and GGC repeat polymorphisms in the androgen receptor gene and breast cancer susceptibility in BRCA1/2 carriers and non-carriers. Br J Cancer. 2001;85(1):36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suter NM, Malone KE, Daling JR, Doody DR, Ostrander EA. Androgen receptor (CAG)n and (GGC)n polymorphisms and breast cancer risk in a population-based case-control study of young women1. Cancer Epidemiol Biomarkers Prev. 2003;12(2):127–35. [PubMed] [Google Scholar]
- 28.Shaffer PL, Jivan A, Dollins DE, Claessens F, Gewirth DT. Structural basis of androgen receptor binding to selective androgen response elements. Proc Natl Acad Sci U S A. 2004;101(14):4758–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenster G, Trapman J, Brinkmann AO. Nuclear import of the human androgen receptor. Biochem J. 1993;293(Pt 3):761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ni L, Llewellyn R, Kesler CT, Kelley JB, Spencer A, Snow CJ, et al. Androgen induces a switch from cytoplasmic Retention to Nuclear Import of the androgen receptor. Mol Cell Biol. 2013;33(24):4766–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haelens A, Tanner T, Denayer S, Callewaert L, Claessens F. The hinge region regulates DNA binding, nuclear translocation, and transactivation of the androgen receptor. Cancer Res. 2007;67(9):4514–23. [DOI] [PubMed] [Google Scholar]
- 33.McDonald S, Brive L, Agus DB, Scher HI, Ely KR. Ligand responsiveness in human prostate cancer: structural analysis of mutant androgen receptors from LNCaP and CWR22 tumors. Cancer Res. 2000;60(9):2317–22. [PubMed] [Google Scholar]
- 34.Veldscholte J, Berrevoets CA, Zegers ND, van der Kwast TH, Grootegoed JA, Mulder E. Hormone-induced dissociation of the androgen receptor-heat-shock protein complex: use of a new monoclonal antibody to distinguish transformed from nontransformed receptors. Biochemistry. 1992;31(32):7422–30. [DOI] [PubMed] [Google Scholar]
- 35.Androgen receptor isoform 1 [homo sapiens] - protein - NCBI. Available from: https://www.ncbi.nlm.nih.gov/protein/NP_000035.2. Cited 2023 Nov 24.
- 36.Venema CM, Bense RD, Steenbruggen TG, Nienhuis HH, Qiu SQ, van Kruchten M, et al. Consideration of breast cancer subtype in targeting the androgen receptor. Pharmacol Ther. 2019;200:135–47. [DOI] [PubMed] [Google Scholar]
- 37.ichi Takayama K, Inoue S. Transcriptional network of androgen receptor in prostate cancer progression. Int J Urol. 2013;20(8):756–68. [DOI] [PubMed] [Google Scholar]
- 38.Estrada M, Espinosa A, Müller M, Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144(8):3586–97. [DOI] [PubMed] [Google Scholar]
- 39.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate*. J Urol. 2002;168(1):9–12. [DOI] [PubMed] [Google Scholar]
- 40.Aurilio G, Cimadamore A, Mazzucchelli R, Lopez-Beltran A, Verri E, Scarpelli M, et al. Androgen receptor signaling pathway in prostate cancer: from genetics to clinical applications. Cells. 2020;9(12): 2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grossmann M, Cheung AS, Zajac JD. Androgens and prostate cancer; pathogenesis and deprivation therapy. Best Pract Res Clin Endocrinol Metab. 2013;27(4):603–16. [DOI] [PubMed] [Google Scholar]
- 42.Perlmutter MA, Lepor H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev Urol. 2007;9(Suppl 1):S3-8. [PMC free article] [PubMed] [Google Scholar]
- 43.Furr BJ, Tucker H. The preclinical development of bicalutamide: pharmacodynamics and mechanism of action. Urology. 1996;47(1A Suppl):13–25 discussion 29–32. [DOI] [PubMed] [Google Scholar]
- 44.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rathkopf D, Scher HI. Androgen receptor antagonists in castration-resistant prostate cancer. Cancer J. 2013;19(1):43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beltran H, Beer TM, Carducci MA, de Bono J, Gleave M, Hussain M, et al. New therapies for castration-resistant prostate cancer: efficacy and safety. Eur Urol. 2011;60(2):279–90. [DOI] [PubMed] [Google Scholar]
- 48.Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15(15):4792–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17(18):5913–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nadiminty N, Tummala R, Liu C, Yang J, Lou W, Evans CP, et al. NF-κB2/p52 induces resistance to enzalutamide in prostate cancer: role of androgen receptor and its variants. Mol Cancer Ther. 2013;12(8):1629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao B, Qi Y, Zhang G, Xu D, Zhan Y, Alvarez X, et al. Androgen receptor splice variants activating the full-length receptor in mediating resistance to androgen-directed therapy. Oncotarget. 2014;5(6):1646–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto Y, Loriot Y, Beraldi E, Zhang F, Wyatt AW, Al Nakouzi N, et al. Generation 2.5 antisense oligonucleotides targeting the androgen receptor and its splice variants suppress enzalutamide-resistant prostate cancer cell growth. Clin Cancer Res. 2015;21(7):1675–87. [DOI] [PubMed] [Google Scholar]
- 54.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456(7221):470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robert C, Watson M. The incredible complexity of RNA splicing. Genome Biol. 2016;17:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roy B, Haupt LM, Griffiths LR. Review: alternative splicing (AS) of genes as an approach for generating protein complexity. Curr Genomics. 2013;14(3):182–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Graveley BR. Alternative splicing: increasing diversity in the proteomic world. Trends Genet. 2001;17(2):100–7. [DOI] [PubMed] [Google Scholar]
- 58.Bush SJ, Chen L, Tovar-Corona JM, Urrutia AO. Alternative splicing and the evolution of phenotypic novelty. Philos Trans R Soc B Biol Sci. 2017;372(1713):20150474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40(12):1413–5. [DOI] [PubMed] [Google Scholar]
- 60.Sammeth M, Foissac S, Guigó R. A general definition and nomenclature for alternative splicing events. PLoS Comput Biol. 2008;4(8):e1000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim P, Yang M, Yiya K, Zhao W, Zhou X. ExonSkipDB: functional annotation of exon skipping event in human. Nucleic Acids Res. 2020;48(D1):D896-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sultan M, Schulz MH, Richard H, Magen A, Klingenhoff A, Scherf M, et al. A global view of gene activity and alternative splicing by deep sequencing of the human transcriptome. Science. 2008;321(5891):956–60. [DOI] [PubMed] [Google Scholar]
- 63.Song X, Zeng Z, Wei H, Wang Z. Alternative splicing in cancers: from aberrant regulation to new therapeutics. Semin Cell Dev Biol. 2018;75:13–22. [DOI] [PubMed] [Google Scholar]
- 64.Tang JY, Lee JC, Hou MF, Wang CL, Chen CC, Huang HW, et al. Alternative splicing for diseases, cancers, drugs, and databases. Sci World J. 2013;2013:e703568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cooper TA, Wan L, Dreyfuss G. RNA and disease. Cell. 2009;136(4):777–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Y, Huang M, Liu X, Huang Y, Liu C, Zhu J, et al. Alternative splicing of mRNA in colorectal cancer: new strategies for tumor diagnosis and treatment. Cell Death Dis. 2021;12(8):752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belluti S, Rigillo G, Imbriano C. Transcription factors in cancer: when alternative splicing determines opposite cell fates. Cells. 2020;9(3): 760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuan CY, Roth KA, Flavell RA, Rakic P, Kuan CY, Roth KA, et al. Mechanisms of programmed cell death in the developing brain. Trends Neurosci. 2000;23(7):291–7. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Qian J, Gu C, Yang Y. Alternative splicing and cancer: a systematic review. Sig Transduct Target Ther. 2021;6(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi S, Cho N, Kim EM, Kim KK. The role of alternative pre-mRNA splicing in cancer progression. Cancer Cell Int. 2023;23(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gregory CW, He B, Wilson EM. The putative androgen receptor-A form results from in vitro proteolysis. J Mol Endocrinol. 2001;27(3):309–19. [DOI] [PubMed] [Google Scholar]
- 72.Ahrens-Fath I, Politz O, Geserick C, Haendler B. Androgen receptor function is modulated by the tissue-specific AR45 variant. FEBS J. 2005;272(1):74–84. [DOI] [PubMed] [Google Scholar]
- 73.Lu C, Luo J. Decoding the androgen receptor splice variants. Transll Androl Urol. 2013;2(3):17886–17186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wadosky KM, Koochekpour S. Androgen receptor splice variants and prostate cancer: from bench to bedside. Oncotarget. 2017;8(11):18550–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A. 2010;107(39):16759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, et al. A novel androgen receptor splice variant is upregulated during prostate cancer progression and promotes androgen-depletion-resistant growth. Cancer Res. 2009;69(6):2305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haile S, Sadar MD. Androgen receptor and its splice variants in prostate cancer. Cell Mol Life Sci. 2011;68(24):3971–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Messner EA, Steele TM, Tsamouri MM, Hejazi N, Gao AC, Mudryj M, et al. The androgen receptor in prostate cancer: effect of structure, ligands and spliced variants on therapy. Biomedicines. 2020;8(10): 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu R, Lu C, Mostaghel EA, Yegnasubramanian S, Gurel M, Tannahill C, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72(14):3457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu R, Isaacs WB, Luo J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate. 2011;71(15):1656–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan SC, Li Y, Dehm SM. Androgen receptor splice variants activate androgen receptor target genes and support aberrant prostate cancer cell growth independent of canonical androgen receptor nuclear localization signal. J Biol Chem. 2012;287(23):19736–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Royen ME, van Cappellen WA, de Vos C, Houtsmuller AB, Trapman J. Stepwise androgen receptor dimerization. J Cell Sci. 2012;125(8):1970–9. [DOI] [PubMed] [Google Scholar]
- 84.Xu D, Zhan Y, Qi Y, Cao B, Bai S, Xu W, et al. Androgen receptor splice variants dimerize to transactivate target genes. Cancer Res. 2015;75(17):3663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo J, Attard G, Balk SP, Bevan C, Burnstein K, Cato L, et al. Role of androgen receptor variants in prostate cancer: report from the 2017 mission androgen receptor variants meeting. Eur Urol. 2018;73(5):715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cato L, de Tribolet-Hardy J, Lee I, Rottenberg JT, Coleman I, Melchers D, et al. ARv7 represses tumor-suppressor genes in castration-resistant prostate cancer. Cancer Cell. 2019;35(3):401-413.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Basil P, Robertson MJ, Bingman WE, Dash AK, Krause WC, Shafi AA, et al. Cistrome and transcriptome analysis identifies unique androgen receptor (AR) and AR-V7 splice variant chromatin binding and transcriptional activities. Sci Rep. 2022;12(1):5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cai L, Tsai YH, Wang P, Wang J, Li D, Fan H, et al. ZFX mediates non-canonical oncogenic functions of the androgen receptor splice variant 7 in castrate-resistant prostate cancer. Mol Cell. 2018;72(2):341-e3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krause WC, Shafi AA, Nakka M, Weigel NL. Androgen receptor and its splice variant, AR-V7, differentially regulate FOXA1 sensitive genes in LNCaP prostate cancer cells. Int J Biochem Cell Biol. 2014;54:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Zhu Y, et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35(19):2149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Nakazawa M, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1(5):582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cancer Genome Atlas Research Network. The Molecular Taxonomy of Primary Prostate Cancer. Cell. 2015;163(4):1011–25. 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dan R, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Armstrong AJ, Halabi S, Luo J, Nanus DM, Giannakakou P, Szmulewitz RZ, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol. 2019;37(13):1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kanayama M, Lu C, Luo J, Antonarakis ES. AR splicing variants and resistance to AR targeting agents. Cancers (Basel). 2021;13(11):2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Myung JK, Banuelos CA, Fernandez JG, Mawji NR, Wang J, Tien AH, et al. An androgen receptor N-terminal domain antagonist for treating prostate cancer. J Clin Invest. 2013;123(7):2948–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li X, Liu Z, Xu X, Blair CA, Sun Z, Xie J, et al. Kava components down-regulate expression of AR and AR splice variants and reduce growth in patient-derived prostate cancer xenografts in mice. PLoS One. 2012;7(2): e31213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mashima T, Okabe S, Seimiya H. Pharmacological targeting of constitutively active truncated androgen receptor by nigericin and suppression of hormone-refractory prostate cancer cell growth. Mol Pharmacol. 2010;78(5):846–54. [DOI] [PubMed] [Google Scholar]
- 99.Dalal K, Roshan-Moniri M, Sharma A, Li H, Ban F, Hessein M, et al. Selectively targeting the DNA-binding domain of the androgen receptor as a prospective therapy for prostate cancer. J Biol Chem. 2014;289(38):26417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ji Y, Zhang R, Han X, Zhou J. Targeting the N-terminal domain of the androgen receptor: the effective approach in therapy of CRPC. Eur J Med Chem. 2023;247:115077. [DOI] [PubMed] [Google Scholar]
- 101.Andersen RJ, Mawji NR, Wang J, Wang G, Haile S, Myung JK, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17(6). Available from: https://pubmed.ncbi.nlm.nih.gov/20541699/. Cited 2023 Oct 20. [DOI] [PubMed]
- 102.Antonarakis ES, Chandhasin C, Osbourne E, Luo J, Sadar MD, Perabo F. Targeting the N-terminal domain of the androgen receptor: a new approach for the treatment of advanced prostate cancer. Oncologist. 2016;21(12):1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu C, Lou W, Zhu Y, Nadiminty N, Schwartz CT, Evans CP, et al. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration resistant prostate cancer. Clin Cancer Res. 2014;20(12):3198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cao S, Zhan Y, Dong Y. Emerging data on androgen receptor splice variants in prostate cancer. Endocr related Cancer. 2016;23(12):T199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scher HI, Lu D, Schreiber NA, Louw J, Graf RP, Vargas HA, et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2016;2(11):1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scher HI, Graf RP, Schreiber NA, Jayaram A, Winquist E, McLaughlin B, et al. Assessment of the validity of nuclear-localized androgen receptor splice variant 7 in circulating tumor cells as a predictive biomarker for castration-resistant prostate cancer. JAMA Oncol. 2018;4(9):1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Michmerhuizen AR, Spratt DE, Pierce LJ, Speers CW. ARe we there yet? Understanding androgen receptor signaling in breast cancer. NPJ Breast Cancer. 2020;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iacopetta D, Rechoum Y, Fuqua SA. The role of androgen receptor in breast cancer. Drug Discov Today Dis Mech. 2012;9(1–2):e19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Michael P, Roversi G, Brown K, Sharifi N. Adrenal steroids and resistance to hormonal blockade of prostate and breast cancer. Endocrinology. 2022;164(3):bqac218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vera-Badillo FE, Templeton AJ, de Gouveia P, Diaz-Padilla I, Bedard PL, Al-Mubarak M, et al. Androgen receptor expression and outcomes in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(1):djt319. [DOI] [PubMed] [Google Scholar]
- 111.Bronte G, Rocca A, Ravaioli S, Puccetti M, Tumedei MM, Scarpi E, et al. Androgen receptor in advanced breast cancer: is it useful to predict the efficacy of anti-estrogen therapy? BMC Cancer. 2018;18(1):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rondón-Lagos M, Villegas VE, Rangel N, Sánchez MC, Zaphiropoulos PG. Tamoxifen resistance: emerging molecular targets. Int J Mol Sci. 2016;17(8):1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Szelei J, Jimenez J, Soto AM, Luizzi MF, Sonnenschein C. Androgen-induced inhibition of proliferation in human breast cancer MCF7 cells transfected with androgen receptor. Endocrinology. 1997;138(4):1406–12. [DOI] [PubMed] [Google Scholar]
- 114.Panet-Raymond V, Gottlieb B, Beitel LK, Pinsky L, Trifiro MA. Interactions between androgen and estrogen receptors and the effects on their transactivational properties. Mol Cell Endocrinol. 2000;167(1–2):139–50. [DOI] [PubMed] [Google Scholar]
- 115.Need EF, Selth LA, Harris TJ, Birrell SN, Tilley WD, Buchanan G. Research resource: interplay between the genomic and transcriptional networks of androgen receptor and estrogen receptor α in luminal breast cancer cells. Mol Endocrinol. 2012;26(11):1941–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rahim B, O’Regan R. AR signaling in breast cancer. Cancers (Basel). 2017;9(3):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Birrell SN, Butler LM, Harris JM, Buchanan G, Tilley WD. Disruption of androgen receptor signaling by synthetic progestins may increase risk of developing breast cancer. FASEB J. 2007;21(10):2285–93. [DOI] [PubMed] [Google Scholar]
- 118.Wilson JD, Griffin JE, Leshin M, George FW. Role of gonadal hormones in development of the sexual phenotypes. Hum Genet. 1981;58(1):78–84. [DOI] [PubMed] [Google Scholar]
- 119.Birrell SN, Bentel JM, Hickey TE, Ricciardelli C, Weger MA, Horsfall DJ, et al. Androgens induce divergent proliferative responses in human breast cancer cell lines. J Steroid Biochem Mol Biol. 1995;52(5):459–67. [DOI] [PubMed] [Google Scholar]
- 120.Cops EJ, Bianco-Miotto T, Moore NL, Clarke CL, Birrell SN, Butler LM, et al. Antiproliferative actions of the synthetic androgen, mibolerone, in breast cancer cells are mediated by both androgen and progesterone receptors. J Steroid Biochem Mol Biol. 2008;110(3–5):236–43. [DOI] [PubMed] [Google Scholar]
- 121.Andò S, De Amicis F, Rago V, Carpino A, Maggiolini M, Panno ML, et al. Breast cancer: from estrogen to androgen receptor. Mol Cell Endocrinol. 2002;193(1–2):121–8. [DOI] [PubMed] [Google Scholar]
- 122.Giovannelli P, Di Donato M, Galasso G, Di Zazzo E, Bilancio A, Migliaccio A. The androgen receptor in breast cancer. Front Endocrinol. 2018;9. Available from: https://www.frontiersin.org/articles/10.3389/fendo.2018.00492. Cited 2023 Oct 22. [DOI] [PMC free article] [PubMed]
- 123.Micello D, Marando A, Sahnane N, Riva C, Capella C, Sessa F. Androgen receptor is frequently expressed in HER2-positive, ER/PR-negative breast cancers. Virchows Arch. 2010;457(4):467–76. [DOI] [PubMed] [Google Scholar]
- 124.Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, et al. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. 2006;25(28):3994–4008. [DOI] [PubMed] [Google Scholar]
- 125.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6(5):517–27. [DOI] [PubMed] [Google Scholar]
- 126.Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, et al. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann Oncol. 2011;22(8):1755–62. [DOI] [PubMed] [Google Scholar]
- 127.Ni M, Chen Y, Lim E, Wimberly H, Bailey ST, Imai Y, et al. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell. 2011;20(1):119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Naderi A, Hughes-Davies L. A functionally significant cross-talk between androgen receptor and ErbB2 pathways in estrogen receptor negative breast cancer. Neoplasia. 2008;10(6):542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kassam F, Enright K, Dent R, Dranitsaris G, Myers J, Flynn C, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9(1):29–33. [DOI] [PubMed] [Google Scholar]
- 130.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–34. [DOI] [PubMed] [Google Scholar]
- 131.Metzger-Filho O, Tutt A, de Azambuja E, Saini KS, Viale G, Loi S, et al. Dissecting the heterogeneity of triple-negative breast cancer. J Clin Oncol. 2012;30(15):1879–87. [DOI] [PubMed] [Google Scholar]
- 132.Jézéquel P, Loussouarn D, Guérin-Charbonnel C, Campion L, Vanier A, Gouraud W, et al. Gene-expression molecular subtyping of triple-negative breast cancer tumours: importance of immune response. Breast Cancer Res. 2015;17:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yu KD, Zhu R, Zhan M, Rodriguez AA, Yang W, Wong S, et al. Identification of prognosis-relevant subgroups in patients with chemoresistant triple negative breast cancer. Clin Cancer Res. 2013;19(10):2723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hu R, Dawood S, Holmes MD, Collins LC, Schnitt SJ, Cole K, et al. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. 2011;17(7):1867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barton VN, D’Amato NC, Gordon MA, Lind HT, Spoelstra NS, Babbs BL, et al. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther. 2015;14(3):769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, et al. Androgen receptor inhibits estrogen receptor-α activity and is prognostic in breast cancer. Cancer Res. 2009;69(15):6131–40. [DOI] [PubMed] [Google Scholar]
- 138.Elebro K, Borgquist S, Simonsson M, Markkula A, Jirström K, Ingvar C, et al. Combined androgen and estrogen receptor status in breast cancer: treatment prediction and prognosis in a population-based prospective cohort. Clin Cancer Res. 2015;21(16):3640–50. [DOI] [PubMed] [Google Scholar]
- 139.Ravaioli S, Tumedei MM, Foca F, Maltoni R, Rocca A, Massa I, et al. Androgen and oestrogen receptors as potential prognostic markers for patients with ductal carcinoma in situ treated with surgery and radiotherapy. Int J Exp Pathol. 2017;98(5):289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tumedei MM, Silvestrini R, Ravaioli S, Massa I, Maltoni R, Rocca A, et al. Role of androgen and estrogen receptors as prognostic and potential predictive markers of ductal carcinoma in situ of the breast. Int J Biol Markers. 2015;30(4):e425-428. [DOI] [PubMed] [Google Scholar]
- 141.Ravaioli S, Puccetti M, Tumedei MM, Silvestrini R, Bedei L, Bravaccini S. Are androgen and estrogen receptors in DCIS patients prognostic indicators of relapse independently of treatment? Appl Immunohistochem Mol Morphol. 2019;27(4):301–5. [DOI] [PubMed] [Google Scholar]
- 142.Kraby MR, Valla M, Opdahl S, Haugen OA, Sawicka JE, Engstrøm MJ, et al. The prognostic value of androgen receptors in breast cancer subtypes. Breast Cancer Res Treat. 2018;172(2):283–96. [DOI] [PubMed] [Google Scholar]
- 143.Cochrane DR, Bernales S, Jacobsen BM, Cittelly DM, Howe EN, D’Amato NC, et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014;16(1):R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lin FML, Pincerato KM, Bacchi CE, Baracat EC, Carvalho FM. Coordinated expression of oestrogen and androgen receptors in HER2-positive breast carcinomas: impact on proliferative activity. J Clin Pathol. 2012;65(1):64–8. [DOI] [PubMed] [Google Scholar]
- 145.Traina TA, Miller K, Yardley DA, Eakle J, Schwartzberg LS, O’Shaughnessy J, et al. Enzalutamide for the treatment of androgen receptor–expressing triple-negative breast cancer. J Clin Oncol. 2018;36(9):884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Anestis A, Karamouzis MV, Dalagiorgou G, Papavassiliou AG. Is androgen receptor targeting an emerging treatment strategy for triple negative breast cancer? Cancer Treat Rev. 2015;41(6):547–53. [DOI] [PubMed] [Google Scholar]
- 147.Anestis A, Sarantis P, Theocharis S, Zoi I, Tryfonopoulos D, Korogiannos A, et al. Estrogen receptor beta increases sensitivity to enzalutamide in androgen receptor-positive triple-negative breast cancer. J Cancer Res Clin Oncol. 2019;145(5):1221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. 2013;19(19). 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed]
- 149.Astvatsaturyan K, Yue Y, Walts AE, Bose S. Androgen receptor positive triple negative breast cancer: clinicopathologic, prognostic, and predictive features. PLoS One. 2018;13(6): e0197827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang J, Zhang C, Chen K, Tang H, Tang J, Song C, et al. ERβ1 inversely correlates with PTEN/PI3K/AKT pathway and predicts a favorable prognosis in triple-negative breast cancer. Breast Cancer Res Treat. 2015;152(2):255–69. [DOI] [PubMed] [Google Scholar]
- 151.Kono M, Fujii T, Lyons GR, Huo L, Bassett R, Gong Y, et al. Impact of androgen receptor expression in fluoxymesterone-treated estrogen receptor-positive metastatic breast cancer refractory to contemporary hormonal therapy. Breast Cancer Res Treat. 2016;160(1):101–9. [DOI] [PubMed] [Google Scholar]
- 152.Coss CC, Jones A, Dalton JT. Selective androgen receptor modulators as improved androgen therapy for advanced breast cancer. Steroids. 2014;90:94–100. [DOI] [PubMed] [Google Scholar]
- 153.Overmoyer B, Sanz-Altamira P, Taylor RP, Hancock ML, Dalton JT, Johnston MA, et al. Enobosarm: a targeted therapy for metastatic, androgen receptor positive, breast cancer. JCO. 2014;32(15_suppl):568–568. [Google Scholar]
- 154.Narayanan R, Ahn S, Cheney MD, Yepuru M, Miller DD, Steiner MS, et al. Selective androgen receptor modulators (SARMs) negatively regulate triple-negative breast cancer growth and epithelial:mesenchymal stem cell signaling. PLoS One. 2014;9(7): e103202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Harvell DME, Spoelstra NS, Singh M, McManaman JL, Finlayson C, Phang T, et al. Molecular signatures of neoadjuvant endocrine therapy for breast cancer: characteristics of response or intrinsic resistance. Breast Cancer Res Treat. 2008;112(3):475–88. [DOI] [PubMed] [Google Scholar]
- 156.De Amicis F, Thirugnansampanthan J, Cui Y, Selever J, Beyer A, Parra I, et al. Androgen receptor overexpression induces tamoxifen resistance in human breast cancer cells. Breast Cancer Res Treat. 2010;121(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang R, Han J, Liang X, Sun S, Jiang Y, Xia B, et al. Androgen receptor expression and bicalutamide antagonize androgen receptor inhibit β-catenin transcription complex in estrogen receptor-negative breast cancer. Cell Physiol Biochem. 2017;43(6):2212–25. [DOI] [PubMed] [Google Scholar]
- 158.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3(11):1427–38. [PubMed] [Google Scholar]
- 160.Robles AJ, Cai S, Cichewicz RH, Mooberry SL. Selective activity of deguelin identifies therapeutic targets for androgen receptor-positive breast cancer. Breast Cancer Res Treat. 2016;157(3):475–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hu DG, Hickey TE, Irvine C, Wijayakumara DD, Lu L, Tilley WD, et al. Identification of androgen receptor splice variant transcripts in breast cancer cell lines and human tissues. Horm Canc. 2014;5(2):61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hickey TE, Irvine CM, Dvinge H, Tarulli GA, Hanson AR, Ryan NK, et al. Expression of androgen receptor splice variants in clinical breast cancers. Oncotarget. 2015;6(42):44728–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ferguson DC, Mata DA, Tay TK, Traina TA, Gucalp A, Chandarlapaty S, et al. Androgen receptor splice variant-7 in breast cancer: clinical and pathologic correlations. Mod Pathol. 2022;35(3):396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Srivastava TP, Ajmeriya S, Goel I, Talukdar J, Srivastava A, Parshad R, et al. Prognostic role of androgen receptor splice variant 7 (AR-V7) in the pathogenesis of breast cancer. BMC Cancer. 2024;24(1):1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Armstrong CM, Gao AC. Current strategies for targeting the activity of androgen receptor variants. Asian J Urol. 2019;6(1):42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang T, Karsh LI, Nissenblatt MJ, Canfield SE. Androgen receptor splice variant, AR-V7, as a biomarker of resistance to androgen axis-targeted therapies in advanced prostate cancer. Clin Genitourin Cancer. 2020;18(1):1–10. [DOI] [PubMed] [Google Scholar]
- 167.Hirayama Y, Tam T, Jian K, Andersen RJ, Sadar MD. Combination therapy with androgen receptor N-terminal domain antagonist EPI-7170 and enzalutamide yields synergistic activity in AR-V7-positive prostate cancer. Mol Oncol. 2020;14(10):2455–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.