Abstract
Background
Improvements in diagnostics and clinical care have allowed more women of childbearing age, suffering from neurological diseases, to safely have pregnancy, reducing peripartum complications. However, these patients remain at risk and are a constant challenge for anesthesiologists in the delivery room.
Methods
To assess the type of anesthesiologic management performed for delivery in obstetric patients with preexisting neurological disease and who reported significant neurological symptoms during pregnancy, a retrospective observational study was carried out between 1 October 2008 and 30 September 2021.
Results
Data from 49,202 pregnant women were assessed over 13 years; 239 pregnant women with a diagnosis of preexisting neurological disease and who reported significant neurological symptoms during pregnancy were identified (prevalence 0.49%). The main neurological disorders that affected pregnant women included vascular abnormalities and intracranial bleeding (N = 42, 17.6%), central nervous system tumors (N = 35, 14.6%), epilepsy and episodic and paroxysmal disorders of the central nervous system (N = 34, 14.2%), diseases of the neuromuscular junction and muscles (N = 26, 10.9%), demyelinating central nervous system diseases (N = 24, 10%). A total of 234 (97.9%) pregnant women with neurological disorders and significant neurological symptoms underwent cesarean section: 192 (80.3% of the total cesarean sections) were elective, 39 (16.3%) were urgent type 2 and 3; 3 (1.2%) were emergency cesarean sections. General anesthesia was administered to 73 patients (30.5%), while 166 patients (69.5%) were managed with neuraxial techniques. 2 patients who had had neuraxial block reported worsening neurological symptoms that required a change in medical therapy. Postoperative multiparameter monitoring was performed for less than 24 h in the recovery room for 226 patients (94.6%). 3 patients (1.2%) were observed with multiparameter monitoring in the post-anesthesia care unit (PACU) for more than 24 hours; 10 patients (4.2%) were moved to the postoperative intensive care unit (ICU). The median hospitalization duration was 4 days (with an interquartile difference of 3–6 days).
Conclusions
In our experience, when neuraxial anesthesia was feasible, it proved to be a safe option for pregnant patients with symptomatic neurological disease, resulting in uncommon maternal complications.
Keywords: Pregnancy, Neurological disease, Neuromuscular disease, Obstetric anesthesia, Cesarean section
Background
Improvements in diagnostics and clinical care have allowed more women of childbearing age suffering from neurological diseases to safely have pregnancy, reducing peripartum complications [1–3].
However, pregnant patients with neurological disease are at high risk and a constant challenge for obstetricians and anesthesiologists in the delivery room, as confirmed by international reports; in the 2023 MMBRACE-UK, neurological disorders were the third most common indirect cause of maternal death (defined as death of the mother due to a disease preexisting to pregnancy or arising during pregnancy) [4].
Anesthesiologic management is challenged by the association between pregnancy and preexisting neurological disease. Cardiovascular (increased heart rate and cardiac output, increased preload) and respiratory (decreased functional residual capacity and increased oxygen consumption) changes in pregnancy can exacerbate clinical conditions due to underlying neurological disease, especially in the case of neuromuscular disorder. Similarly, neurological disease can affect the development of pregnancy and change the clinical mode and clinical condition at the time of delivery. Consequently, patients are at high risk of peripartum complications that may even be life-threatening [5].
The current scientific literature about the anesthesiologic management of these patients is insufficient and represented mainly by case reports; some recommendations and case reports can guide obstetric anesthesiologists in the optimization of the patient before delivery and in choosing the type of anesthesia [6–15]; however, they do not seem to be completely exhaustive, particularly regarding the anesthesiologic management in the delivery room.
Most patients with preexisting neurological disease do not develop significant symptoms in pregnancy and perform vaginal delivery without increased anesthesiologic risk compared to the general population. Patients who develop worsening neurological symptoms represent a risk category for maternal complications in the peripartum period.
A detailed diagnosis and neurological evaluation before delivery are essential for assessing the risks and may aid in choosing adequate anesthesiologic management. Additionally, patients with neurological disease with significant clinical symptoms should be evaluated before the delivery, by obstetrician, anesthesiologist, and neurologist to determine neurological status, optimize medical therapy, determine the mode and timing of delivery, establish type of anesthesia and peripartum management.
Neurological diseases have a broad spectrum of presentations and each group of patients may present with specific complications affecting cardiovascular, respiratory, muscular or neurological function [6].
Several neuromuscular diseases are associated with cardiologic alterations (cardiomyopathies or alterations in the myocardial conduction system), which may remain unrecognized until more advanced states. The ability to increase cardiac output in response to stress during delivery may be limited in pregnant patients with neurological disease due to a reduced functional reserve [16, 17].
Additionally, in some disorders, general anesthesia can precipitate cardiovascular insufficiency; these patients are at high risk of experiencing cardiologic complications related to the negative inotropic effect of volatile and intravenous anesthetics, positive pressure ventilation, hypoxemia and acute anemia.
Respiratory involvement may vary significantly between different neurological diseases: a reduction in inspiratory muscle strength results in restrictive pulmonary impairment with a progressive decrease in force vital capacity; similarly, weakness of expiratory muscles leads to inadequate clearance of airway secretions. In these patients, hypoventilation may also be associated with impaired cough with a high risk of developing pulmonary complications.
Moreover, regarding anesthesiologic management, motor and sympathetic block caused by neuraxial anesthesia, if not properly titrated, may worsen respiratory function or lead to increased heart failure [18].
Methods
Data collection
To assess the type of anesthesiologic management performed for delivery in obstetric patients with preexisting neurological disease and who reported significant neurological symptoms during pregnancy, a retrospective observational study was carried out over 13 years. This article adheres to the applicable STROBE guidelines. The study received approval for data extraction and processing from the Policlinico A. Gemelli Ethics Committee, without ad hoc consent from the enrolled patients (protocol ID 3741).
This was a retrospective analysis of prospectively collected data enclosed in an electronic medical database that covered patient history, labor and delivery parameters, maternal and neonatal outcomes of all deliveries in the delivery room of the Fondazione Policlinico A. Gemelli IRCCS (Rome, Italy) between 1 October 2008 and 30 September 2021. All deliveries of parturients with preexisting neurological disease and who reported significant neurological symptoms during pregnancy were identified and included in the analysis. Data regarding admission records, medical records, labor and delivery records, anesthesiologic management, postpartum care and discharge codes from the International Classification of Diseases, (Ninth Revision, Clinical Modification) were extracted and analyzed.
The main clinical symptoms of neurological disorders that occurred during pregnancy and used to collect cases were seizure, headache, visual disturbances, perceptual deficits, disorientation, syncope, dysphagia, vomiting, skeletal muscle weakness or paralysis, trouble walking, loss of balance or coordination.
The main outcomes measured were type of neurological disease, mode of delivery, type of anesthesia, post-operative monitoring, worsening of neurological symptoms, neonatal outcomes, length of hospital stay.
Statistical analysis
Data in the manuscript are reported as mean and standard deviation or count and percentage or median and interquartile range as appropriate for continuous variables. Comparisons of continuous variable in the electronic database were tested with the Mann–Whitney test, having previously tested for normality with the Shapiro–Wilk test. Frequency comparisons were performed with the z-test for proportions.
All analyses were performed with SAS v. 9.4 (SAS Institute, Cary, NC, USA).
Results
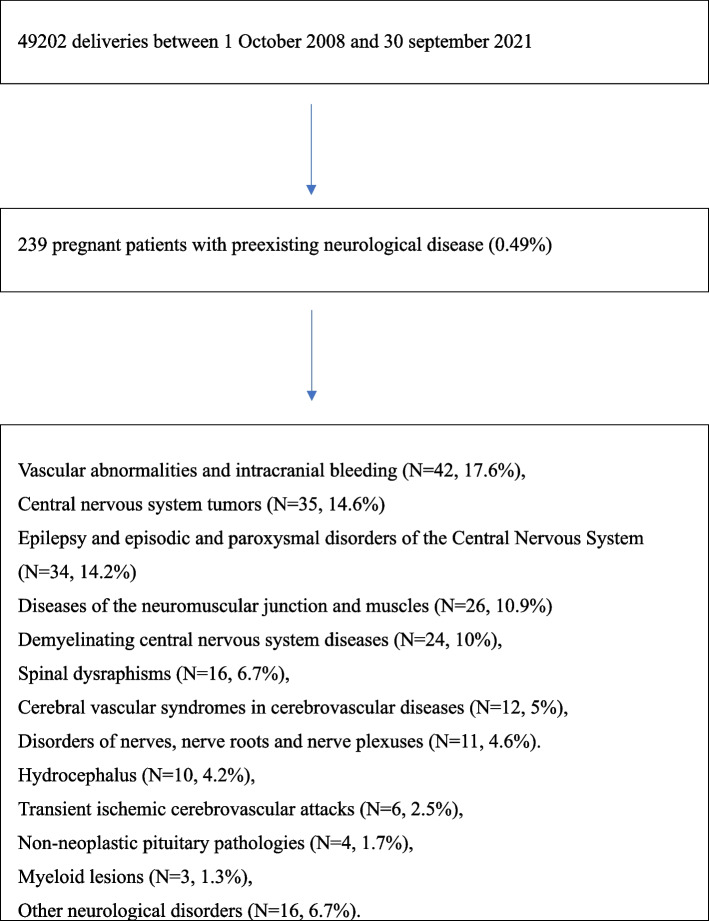
The data of 49,202 pregnant women who consecutively gave birth in the delivery room were assessed over 13 years: 239 pregnant women with a diagnosis of preexisting neurological disease and who reported significant neurological symptoms during pregnancy were identified (prevalence 0.49%). A Flowchart of the study is presented in Fig. 1.
Fig. 1.
Flowchart of the study. Data are expressed as count (percentage)
The neurological disorders that affected pregnant patients were vascular abnormalities and intracranial bleeding (N = 42, 17.6%), central nervous system (CNS) tumors (N = 35, 14.6%), epilepsy and episodic and paroxysmal disorders of the CNS (N = 34, 14.2%), diseases of the neuromuscular junction and muscles (N = 26, 10.9%), demyelinating CNS disease (N = 24, 10%), spinal dysraphisms (N = 16, 6.7%), cerebral vascular syndromes in cerebrovascular diseases (N = 12, 5%), disorders of nerves, nerve roots and nerve plexuses (N = 11, 4.6%), hydrocephalus (N = 10, 4.2%), transient ischemic cerebrovascular attacks (N = 6, 2.5%), non-neoplastic pituitary pathologies (N = 4, 1.7%), myeloid lesions (N = 3, 1.3%), other neurological disorders (N = 16, 6.7%).
The demographic and clinical data of pregnant patients enrolled in the study are presented in Table 1.
Table 1.
Demographic and clinical data
| Age, years | 33.25 ± 5.68 |
| Height, cm | 164,88 ± 5,06 |
| Weight, kg | 76.37 ± 14.89 |
| BMI | 28.29 ± 5.06 |
| Gestational age, weeks | 38.07 ± 1.76 |
| Pregnancy, n. of patients | |
| Nulliparous | 132 (55.23%) |
| Multiparous | 107 (44.76%) |
| Other diseases, n. of patients | |
| Gestational diabetes mellitus | 8 (3.3%) |
| Hypertensive disorders | 3 (1.2%) |
| Mode of delivery, n. of patients | |
| Vaginal delivery | 5 (2.1%) |
| Cesarean Section | 234 (97,9%) |
| Cesarean section, urgency grade 4 | 192 (82%) |
| Cesarean section, urgency grade 2,3 | 39 (16.7%) |
| Cesarean section, urgency grade | 3 (1.3%) |
| Type of Anesthesia, n. of patients | |
| Subarachnoid | 138 (57,7%) |
| Epidural | 22 (9.2%) |
| Combined (spinal-epidural) | 6 (2.5%) |
| General | 73 (30.5%) |
| Neonatal outcomes | |
| Apgar at 1 min | 8.39 ± 1.17 |
| Apgar at 5 min | 9.35 ± 0.72 |
| Weight, g | 3017,8 ± 467,7 |
Data are expressed as mean ± standard deviation or count (percentage) as appropriate
234 (97.9%) pregnant women with neurological disorders underwent cesarean section (CS): in 211 patients (88.2%) CS was carried out for indication primarily related to the neurological disorder, while in 23 patients (9.6%) CS was indicated for obstetric reasons (CS was due to lack of progress in labor in 15 patients and abnormalities in the fetal heartbeat were reported in 8 patients).
192 CSs were elective (80.3% of the total CSs), 39 were urgent type 2 and 3 (16.3%); 3 (1.2%) were emergency CSs (Table 1).
General anesthesia was performed in 73 patients (31.2% of CSs), while the remaining 161 patients (68.8% of CSs) were managed with neuraxial techniques. 52 patients (71% of patients under general anesthesia) who were managed with general anesthesia underwent videolaringoscopy intubation. Neuromuscular block was reversed with sugammadex in most cases of general anesthesia (85%) and no cases of residual curarization have been described. In the case of general anesthesia, halogenated agents were averted in all patients with myopathies to avoid rhabdomyolysis.
Regarding the type of neuraxial block performed for management of delivery (both cesarean and vaginal), subarachnoid anesthesia was applied to 138 patients (57,7%), epidural anesthesia was applied to 22 patients (9.2%), combined spinal/epidural was applied to 6 patients (2.5%) (Table 1); specifically, five epidurals were performed in patients who gave birth vaginally.
Patients who underwent neuraxial anesthesia were mainly affected by vascular abnormalities and intracranial bleeding (N = 37, 22.3%), epilepsy and episodic and paroxysmal disorders of the CNS (N = 30, 18.1%), diseases of the neuromuscular junction and muscles (N = 22, 13.2%), CNS tumors (N = 18, 10.8%), demyelinating CNS disease (N = 18, 8.4%); no patients with hydrocephalus underwent neuraxial block (Table 2). Among the 22 patients with diseases of the neuromuscular junction and muscles undergoing neuraxial block, 10 exhibited severe scoliosis, leading to the use of an ultrasound-assisted technique instead of the traditional landmark-based approach.
Table 2.
Type of anesthesia and peripartum care
| Neurological disease | N | Neuraxial anesthesia | General anesthesia | Vaginal delivery | Cesarean section | Progression of neurological disease (Medical therapy) | Progression of neurological disease (Surgery) | Post partum: Recovery room < 24 h | Postpartum: Post-anesthesia care unit (PACU) > 24 h | Postpartum: intensive care unit (ICU) |
|---|---|---|---|---|---|---|---|---|---|---|
| Vascular abnormalities and intracranial bleeding | 42 | 37 | 5 | 0 | 42 | 1 | 0 | 41 | 0 | 1 |
| Central Nervous System Tumors | 35 | 18 | 17 | 0 | 35 | 0 | 2 | 33 | 0 | 2 |
| Epilepsy and episodic and paroxysmal disorders of the Central Nervous System | 34 | 30 | 4 | 0 | 34 | 3 | 0 | 31 | 1 | 2 |
| Diseases of the neuromuscular junction and of the muscles | 26 | 22 | 4 | 3 | 23 | 2 | 0 | 23 | 1 | 2 |
| Demyelinating CNS disease | 24 | 18 | 6 | 0 | 24 | 3 | 0 | 23 | 1 | 0 |
| Spinal dysraphisms | 16 | 4 | 12 | 1 | 15 | 0 | 0 | 16 | 0 | 0 |
| Cerebral vascular syndromes in cerebrovascular diseases | 12 | 8 | 4 | 1 | 11 | 0 | 0 | 11 | 0 | 1 |
| Disorders of nerves, nerve roots and nerve plexuses | 11 | 9 | 2 | 0 | 11 | 1 | 0 | 10 | 0 | 1 |
| Hydrocephalus | 10 | 0 | 10 | 0 | 10 | 1 | 0 | 10 | 0 | 0 |
| Transient ischemic cerebrovascular attacks | 6 | 6 | 0 | 0 | 6 | 0 | 0 | 6 | 0 | 0 |
| Non-neoplastic pituitary pathologies | 4 | 2 | 2 | 0 | 4 | 0 | 0 | 4 | 0 | 0 |
| Myeloid lesions | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 3 | 0 | 0 |
| Other neurological disorders | 16 | 12 | 4 | 0 | 16 | 1 | 0 | 15 | 0 | 1 |
| TOTAL | 239 | 166 (69.5%) | 73 (30.5%) | 5 (2.1%) | 234 (97.9%) | 12 (5.0%) | 2 (0.8%) | 226 (94.6%) | 3 (1,2%) | 10 (4,2%) |
Data are expressed as count (percentage)
The peripartum period was investigated as a source of clinical worsening for symptoms related to underlying neurological disorders: 12 patients (5%) experienced clinical worsening that required a new neurological evaluation and underwent a change of the ongoing medical therapy; moreover 2 patients (0.8%), underwent postpartum surgery to remove brain tumor (Table 2).
Deterioration of clinical symptoms due to underlying neurological disease evolved in pulmonary complications (respiratory failure, bronchial secretion retention) in 8 patients, worsening of epileptic seizures in 3 patients, myasthenic crisis in 1 patient. Specifically, 2 patients (1.2%) who had had neuraxial block reported worsening epileptic symptoms after cesarean section that required a change in medical therapy.
No obstetric complications were recorded after delivery (hemorrhage, uterine atony).
Referring to complications directly related to anesthesia, one case of orthostatic headache was reported in a patient with multiple sclerosis who underwent subarachnoid anesthesia and completely regressed with supine decubitus for 48 h.
Postoperative multiparameter monitoring was performed for less than 24 h in the recovery room for 226 patients (94.6%). 3 patients (1.2%) were observed with multiparameter monitoring in the post-anesthesia care unit (PACU) for more than 24 h; 10 patients (4.2%) were moved to the postoperative intensive care unit (ICU).
The median hospitalization duration was 4 days (with interquartile difference of 3–6 days) (Table 3).
Table 3.
Postpartum care
| Multiparameter post-operative monitoring, n. of patients | |
| Recovery room (< 24 h) | 226 (94.6%) |
| Post-anesthesia care unit (PACU) (> 24 h) | 3 (1.2%) |
| Postoperative intensive care unit (ICU) | 10 (4.2%) |
| Worsening of neurological symptoms, n. of patients | |
| None | 211(88.3%) |
| Clinical worsening that required variation of the ongoing medical therapy | 12 (5.0%) |
| Clinical worsening that required surgery | 2 (0.8%) |
| Lenght of hospital stay, days | 4 (IQR 3–6) |
Data are expressed as count (percentage) or value (interquartile range) as appropriate
About neonatal outcomes, the average Apgar score recorded at 1 min was 8.39 ± 1.17 and at 5 min 9.35 ± 0.72 (Table 1).
Furthermore, data regarding the general obstetric population without clinical symptoms related to a neurological disease were analyzed in the same study period. The number of patients was N = 48,934 (99.51%). The mode of delivery was cesarean section in 19,144 patients (39.1%) and vaginal delivery in 29,819 patients (60.9%). Regarding anesthesia technique, subarachnoid anesthesia was performed in 16,402 patients (33.5%), epidural in 16,500 patients (33.7%), combined spinal-epidural in 587 patients (1.2%), general anesthesia in 1028 patients (2.1%).
In the general obstetric population, postoperative multiparameter monitoring was performed for less than 24 h in the recovery room for 48,596 patients (99.3%); 263 patients (0.5%) were observed with multiparameter monitoring in the post-anesthesia care unit (PACU) for more than 24 h; 104 patients (0.2%) were moved to the postoperative intensive care unit (ICU).
Discussion
The onset of neurological symptoms, due to a neurological disease, during pregnancy is uncommon as reported in this large retrospective study. Pregnant patients with preexisting neurological disorders and significant neurological symptoms during pregnancy constituted 0.49% of all deliveries over 13 years in our tertiary center. These data on the obstetric population seem to be in line with previous literature data on the general population. Although uncommon, this condition must be well known to the obstetric anesthesiologist because of the multiplicity of symptoms for each neurological disease and possible even fatal complications that can occur during and after delivery.
The literature specifically related to pregnant women with neurological disease in the delivery room remains scarce and consists mostly of case reports and series [6–15].
The most common neurological conditions that caused symptoms during pregnancy in our patients were as follows: vascular abnormalities and intracranial bleeding (18%), CNS tumors (15%), epilepsy and episodic and paroxysmal disorders of the CNS (14%), diseases of the neuromuscular junction and of the muscles (10%) and multiple sclerosis (10%). All patients enrolled in the study were evaluated before the delivery, as per institutional protocol, by obstetrician, anesthesiologist, and neurologist to determine neurological status and establish peripartum management.
Planned CS was the most frequent mode of delivery in our study population (80%); in many patients, underlying neurological disorders contraindicated the performance of vaginal delivery. Expulsive efforts can increase PIC by as much as 70 cmH2O above baseline, and this could even lead to life-threatening consequences. These data are in line with other reports: previous authors reported cesarean section in 77% of pregnant women with neuromuscular disorders [18].
The anesthesiologic technique of choice was general anesthesia in only 73 patients (30%), while most of the patients (70%) were managed with neuraxial blocks; specifically, subarachnoid anesthesia was performed in 58% of pregnant patients, epidural anesthesia in 9% and combined spinal-epidural anesthesia in 2%.
Compared to the general obstetric population in the same center, patients in the study group underwent a higher percentage of cesarean sections (97.9% vs. 39.1%), mostly because they had experienced neurological symptoms due to underlying neurological disorder, and the majority of cesareans were scheduled earlier. It should be pointed out that patients with cardiovascular, respiratory, neurological or obstetric disease are referred to our institution, so the cesarean section rate may be increased in these patients, while the cesarean section rate in our institution is 11% in patients with physiological pregnancy (Robson Ten Group Classification System, Group 1).
In patients with symptomatic neurological disease, general anesthesia was required more frequently (30.5%) than in the general obstetric population due to contraindications to neuraxial blockade (endocranial hypertension, space-occupying mass, maternal or iatrogenic coagulopathy). However, for our patients without contraindications to regional anesthesia, neuraxial block was performed successfully (69.5%).
Moreover, we observed the progression of neurological symptoms in only 6% of the enrolled patients; 12 patients (5%) experienced clinical worsening that required a change in ongoing medical therapy, while 2 patients (0.8%) required surgery after the delivery to remove a brain tumor. Specifically, the progression of neurological disease was also uncommon in patients undergoing neuraxial anesthesia: 2 patients who had had neuraxial block reported worsening epileptic symptoms after delivery that required a change in medical therapy.
Consequently, when neuraxial anesthesia was feasible, it proved to be a safe option for pregnant patients with symptomatic neurological disease, avoiding potential airway management challenges and resulting in uncommon maternal complications, even in the presence of neurological disease.
In our experience, neuraxial block allowed us to avoid the intubation issues specific to the pregnant population and, with adequate titration of the level of sensory and motor blockade, worsening of respiratory function and maintenance of hemodynamic stability during delivery.
Nevertheless, general anesthesia was performed in cases of absolute contraindications to neuraxial techniques or in the presence of obstetrical emergencies requiring rapid delivery. Among pregnant patients, many had predictive indices of difficult intubation (Mallampati class III and IV or an intercisor gap < 4 cm was reported in approximately 80% of enrolled patients), and 71% of patients under general anesthesia underwent videolaringoscopy intubation. These findings are not different from those already reported in the literature and confirm an increase in airway management difficulty in the general obstetric population [4].
Taking a look at specific neurological diseases in our study population, pregnant women suffering from cerebrovascular disease do not have absolute contraindications to neuraxial techniques; if active bleeding is present, caused by an aneurysm or an arteriovenous malformation, intracranial hypertension can occur and general anesthesia must be evaluated.
In the case of brain tumors, a brain-occupying space process presumes an imbalance in liquor homeostasis, contraindicating neuraxial techniques. On the other hand, a previous neurosurgical intervention involving the resolution of liquor hypertension does not contraindicate neuraxial techniques [6].
We reported worsening epileptic symptoms in two patients after cesarean section with neuraxial anesthesia. However, the literature suggests that neuraxial techniques can generally be used for these patients, except for those with active manifestations of epilepsy [6]. It is important to note that epileptic pregnant patients may be more susceptible to the seizure-inducing of some anesthetics [19].
Neuraxial anesthesia was administered to most patients with neuromuscular junction disease, as avoiding neuromuscular blocking agents was essential for preventing perioperative pulmonary complications. Since severe scoliosis may occur in patients with neuromuscular disorders, the use of ultrasound should be considered to enhance the efficacy and safety of neuraxial blocks [16].
Similarly, current clinical evidence does not support the knowledge that central neuraxial anesthesia negatively affects the course of multiple sclerosis, consequently, neuraxial block may be a safe option; nevertheless, in pregnant patients affected by multiple sclerosis with progressive and active neurological symptoms, general anesthesia should be considered on a case-by-case basis [6].
The wide use of neuraxial blocks in our patients has also made it possible to decrease the consequences associated with general anesthesia. Indeed, cardiac dysfunction and cardiomyopathy may be associated with several neurological diseases, especially in neuromuscular disorders. Pregnant women with significant cardiac dysfunction have a limited ability to increase cardiac output in response to stress and are at risk for perioperative cardiac side effects of volatile and intravenous anesthetics, positive pressure ventilation, hypoxemia and anemia [16, 20, 21]. Volatile anesthetics can also induce arrhythmias as a consequence of increased myocardial sensitivity to catecholamines and inhibition of related voltage-dependent K + channels; in patients with neuromuscular disorders, except for patients with neuromuscular junction diseases, succinylcholine can trigger severe reactions such as hyperkalemia and malignant hyperthermia; similarly halogenated agents must be avoided in all patients with muscle diseases, except for patients with mitochondrial myopathies [16, 22].
Moreover, neuromuscular pregnant patients with respiratory involvement may be affected by right ventricular modifications because of pulmonary hypertension. Pregnant patients with symptoms of cardiac dysfunction should undergo a careful assessment of heart function and a search for arrhythmias and conduction defects before the delivery [6]. Patients enrolled in our study who were diagnosed with cardiac dysfunction, were continuously monitored during cesarean section with invasive arterial pressure monitoring and, more recently, with noninvasive hemodynamic monitoring systems.
Furthermore, neurological diseases may lead to hypoventilation and ineffective cough, in addition to a reduction in residual functional capacity due to physiologic pregnancy, which increases the risk for pulmonary complications. Current recommendations for pregnant patients with neuromuscular diseases include multidisciplinary evaluation before the delivery, pulmonary assessment to estimate the risks of pulmonary complications and the need for specific management, including noninvasive ventilation combined with mechanical insufflation-exsufflation. Regarding anesthetic management for cesarean section, in patients with neurological disease and decreased pulmonary function, regional should be preferred to general anesthesia to minimize pulmonary complications. Interestingly, Racca and other authors proposed a protocol for identifying women affected by neuromuscular disorders with pulmonary risk factors and preventing pulmonary complications by applying noninvasive ventilation with mechanical insufflation-exsufflation in the peripartum period [23].
Most of the patients studied were monitored postoperatively for less than 24 h in the recovery room; this finding is not dissimilar to the general obstetric population (94.6% vs. 99.3%). An increase in patients requiring postoperative intensive care was found in neurological patients compared with the general obstetric population (4.2% vs. 0.2%).
An analysis of the overall length of stay revealed that the median length of stay was 4 days, with an interquartile range between 3 and 6 days: this finding did not diverge much from the median length of hospitalization in patients without neurological disease in our institution.
Interestingly, neonatal Apgar scores were also comparable to those in the general obstetric population. Neonatal outcomes are in line with previous data reported by Picone in patients with myasthenia gravis [24]. These findings can be explained by the widespread use of neuraxial anesthesia to minimize drug transfer to the fetus reducing neonatal complications.
Current evidence recommends a multidisciplinary approach involving neurologists (and neurosurgeons if appropriate), anesthesiologists and obstetricians to optimize the functional reserve before delivery and to tailor the anesthetic plan for each patient [25, 26].
It is worth noting that this retrospective study compiles cases over an extended period, whereas existing literature on anesthesia management in obstetric patients with neurological diseases is largely limited to single case reports or series. Unfortunately, establishing more statistically significant correlations was not possible due to the small sample collected and the distribution of measured variables. For instance, we could not draw specific associations between specific neurological disease presentations and the type of anesthesiologic management. This is an important limitation of this retrospective registry-based study. Another study limitation is the single-center design, even though the data were collected over a long period from an obstetric pathology referral center.
Conclusions
In our experience, when neuraxial anesthesia was feasible, it proved to be a safe option for pregnant patients with symptomatic neurological disease, resulting in uncommon maternal complications.
Acknowledgements
Not applicable.
Abbreviations
- CNS
Central nervous system
- CS
Cesarean section
- PACU
Post-anesthesia care unit
- ICU
Intensive care unit
Authors’ contributions
SC, BAZ, MS, SDM, PPG, LF, GLG, EC, FVDM, GM, AL, GD analyzed and interpreted patient’s data and then wrote the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
Datasets and clinical data used and/or reported in the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This observational retrospective study received approval for data extraction and processing from the Policlinico A. Gemelli Ethics Committee, without ad hoc consent from the enrolled patients (protocol ID 3741).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.van den Bersselaar LR, Heytens L, Silva HCA, Reimann J, Tasca G, Díaz-Cambronero Ó, Løkken N, Hellblom A, Hopkins PM, Rueffert H, Bastian B, Vilchez JJ, Gillies R, Johannsen S, Veyckemans F, Muenster T, Klein A, Litman R, Jungbluth H, Riazi S, Voermans NC, Snoeck MMJ. European Neuromuscular Centre consensus statement on anaesthesia in patients with neuromuscular disorders. Eur J Neurol. 2022;29(12):3486–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guidon AC, Massey EW. Neuromuscular disorders in pregnancy. Neurol Clin. 2012;30(3):889–911. [DOI] [PubMed] [Google Scholar]
- 3.Johnson N, Sermer M, Lausman A. Obstetric outcomes of women with intracranial neoplasms. Int J Gynaecol Obstet. 2009;105(1):56–9. [DOI] [PubMed] [Google Scholar]
- 4.MBRRACE-UK Saving Lives, Improving Mothers’ Care report for 2023. https://www.npeu.ox.ac.uk/assets/downloads/mbrrace-uk/reports/maternal-report-2023/MBRRACE-UK_Maternal_Compiled_Report_2023.pdf. [DOI] [PubMed]
- 5.Toscano M, Thornburg LL. Neurological diseases in pregnancy. Curr Opin Obstet Gynecol. 2019;31(2):97–109. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins AN, Alshaeri T, Akst SA, Berger JS. Neurologic disease with pregnancy and considerations for the obstetric anesthesiologist. Semin Perinatol. 2014;38(6):359–69. [DOI] [PubMed] [Google Scholar]
- 7.Vukusic S, Hutchinson M, Hours M, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127(Pt6):1353–60. [DOI] [PubMed] [Google Scholar]
- 8.Shehata HA, Okosun H. Neurological disorders in pregnancy. Curr Opin Obstet Gynecol. 2004;16:117–22. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson CB, Thompson RC. The clinical management of intracranial neoplasm in pregnancy. Clin Obstet Gynecol. 2005;48:24–37. [DOI] [PubMed] [Google Scholar]
- 10.Ferrero S, Pretta S, Nicoletti A, Petrera P, Ragni N. Myasthenia gravis: management issues during pregnancy. Eur JObstet Gynecol ReprodBiol. 2005;121(2):129–38. [DOI] [PubMed] [Google Scholar]
- 11.Almeida C, Coutinho E, Moreira D, Santos E, Aguiar J. Myasthenia gravis and pregnancy: anaesthetic management–a series of cases. Eur JAnaesthesiol. 2010;27(11):985–90. [DOI] [PubMed] [Google Scholar]
- 12.Maruotti GM, Anfora R, Scanni E, et al. Anesthetic management of a parturient with spinal muscular atrophy type II. J Clin Anesth. 2012;24(7):573–7. [DOI] [PubMed] [Google Scholar]
- 13.Draisci G, Sbaraglia F, Pinto R, Zanfini BA, Frassanito L, Catarci S. Does Huntington’s disease enhance cephalad spread during neuraxial anesthesia for cesarean section? J Clin Anesth. 2012;24(6):516–7. [DOI] [PubMed] [Google Scholar]
- 14.Elsheikh BH, Zhang X, Swoboda KJ, Chelnick S, Reyna SP, Kolb SJ, Kissel JT. Pregnancy and delivery in women with spinal muscular atrophy. Int J Neurosci. 2017;127(11):953–7. [DOI] [PubMed] [Google Scholar]
- 15.Kock-Cordeiro DBM, Brusse E, van den Biggelaar RJM, Eggink AJ, van der Marel CD. Combined spinal-epidural anesthesia with non-invasive ventilation during cesarean delivery of a woman with a recent diagnosis of amyotrophic lateral sclerosis. Int J Obstet Anesth. 2018;36:108–10. [DOI] [PubMed] [Google Scholar]
- 16.Racca F, Mongini T, Wolfler A, Vianello A, Cutrera R, Del Sorbo L, Capello EC, Gregoretti C, Massa R, De Luca D, Conti G, Tegazzin V, Toscano A, Ranieri VM. Recommendations for anesthesia and perioperative management of patients with neuromuscular disorders. Minerva Anestesiol. 2013;79(4):419–33. [PubMed] [Google Scholar]
- 17.Soma-Pillay P, Nelson-Piercy C, Tolppanen H, et al. Physiological changes in pregnancy. Cardiovasc J Afr. 2016;27(2):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Racca F, Longhitano Y, Zanza C, Balzanelli MG, Draisci G, Stoia PA, Gollo E, Maio M, Grattarola C, Astuto M, Ciccarelli A, Racca G, Romenskaya T, Giordano B, Serraino A, Sansone VAM, Gregoretti C, Conti G, Piccolella F, Vaschetto R. Peri-partum respiratory management of pregnant women with neuro-muscular disorders: a prospective observational study (IT-NEUMA-Pregn study). BMC Anesthesiol. 2023;23(1):342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voss LJ, Sleigh JW, Barnard JP, Kirsch HE. The howling cortex: seizures and general anesthetic drugs. Anesth Analg. 2008;107(5):1689–703. [DOI] [PubMed] [Google Scholar]
- 20.Birnkrant DJ, Panitch HB, Benditt JO, Boitano LJ, Carter ER, Cwik VA, Finder JD, Iannaccone ST, Jacobson LE, Kohn GL, Motoyama EK, Moxley RT, Schroth MK, Sharma GD, Sussman MD. American College of Chest Physicians consensus statement on the respiratory and related management of patients with Duchenne muscular dystrophy undergoing anesthesia or sedation. Chest. 2007;132(6):1977–86. [DOI] [PubMed] [Google Scholar]
- 21.Islander G. Anesthesia and spinal muscle atrophy. Paediatr Anaesth. 2013;23(9):804–16. [DOI] [PubMed] [Google Scholar]
- 22.Levitan R. Safety of succinilcoline in myasthenia gravis in pregnancy. Semin Neurol. 2004;24:95–100. [DOI] [PubMed] [Google Scholar]
- 23.Racca F, Longhitano Y, Zanza C, Draisci G, Stoia PA, Gollo E, Maio M, Grattarola C, Astuto M, Vaschetto R, Sansone VAM, Conti G, Gregoretti C. Peri-Partum respiratory management in neuro-muscular disorders (IT-NEUMA-Pregn study): a proposal by an italian panel and a call for an international collaboration. Pulmonology. 2024;30(3):210–3. [DOI] [PubMed] [Google Scholar]
- 24.Picone O, Audibert F, Gajdos P, Fernandez H. Myasthénie et grossesse: à propos de 13 grossesses [Myasthenia gravis and pregnancy. Report on 13 cases]. J Gynecol Obstet Biol Reprod (Paris). 2003;32(7):654–9. [PubMed] [Google Scholar]
- 25.Kuczkowski KM. Labor analgesia for the parturient with neurological disease: what does an obstetrician need to know? Arch Gynecol Obstet. 2006;274:41–6. [DOI] [PubMed] [Google Scholar]
- 26.Norwood F, Rudnik-Schöneborn S. 179th ENMC international workshop: pregnancy in women with neuromuscular disorders 5–7 November 2010, Naarden, The Netherlands. Neuromuscul Disord. 2012;22(2):183–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets and clinical data used and/or reported in the current study are available from the corresponding author on reasonable request.