Abstract
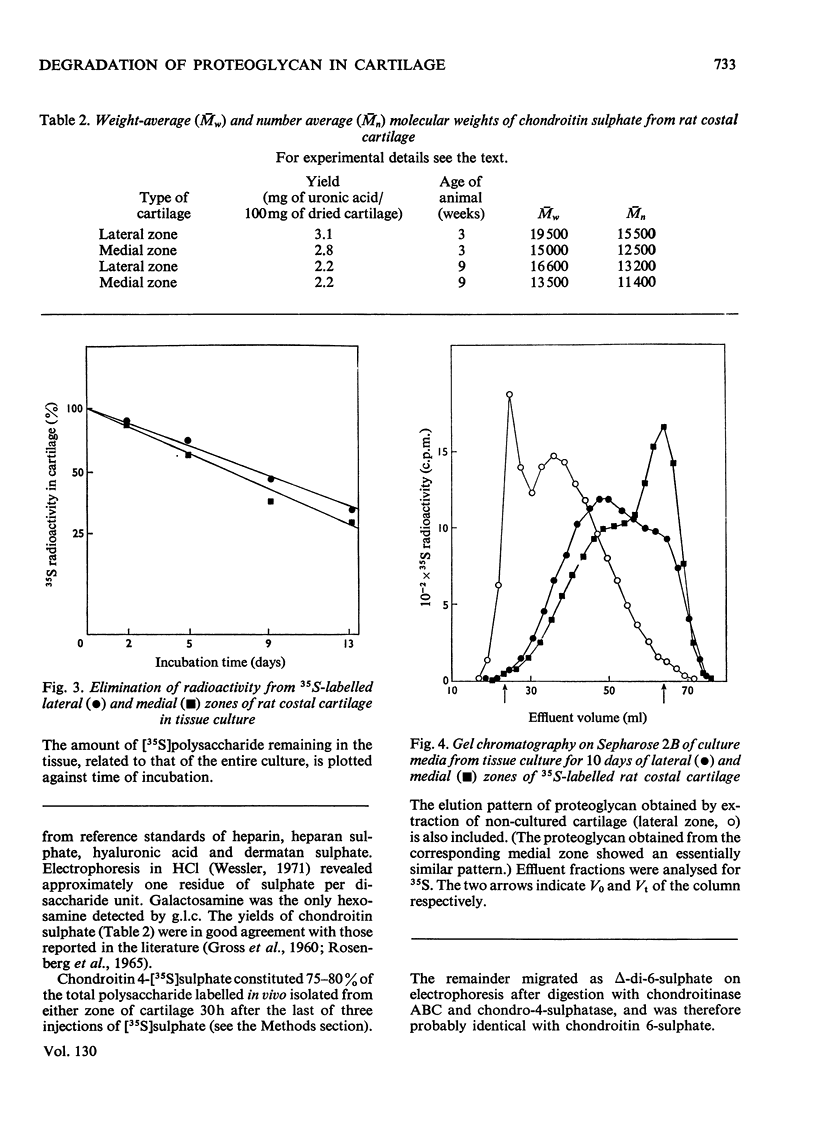
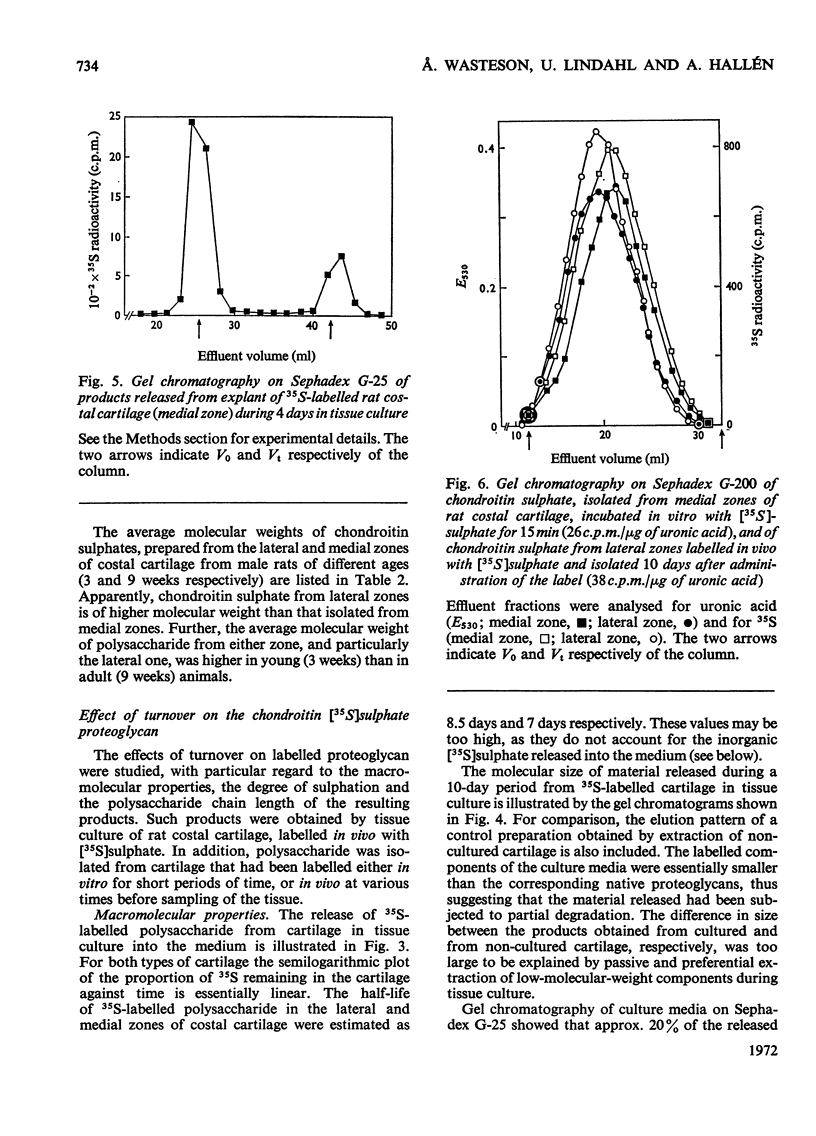
1. Chondroitin sulphate was isolated from different regions of rat costal cartilage after extensive proteolysis of the tissues. The molecular weight, determined by gel chromatography, of the polysaccharide obtained from an actively growing region (lateral zone) near the osteochondral junction was higher than that of the polysaccharide isolated from the remaining portion of the costal cartilage (medial zone). 2. In both types of cartilage the molecular weight of chondroitin sulphate, labelled with [35S]sulphate, remained unchanged in vivo over a period of 10 days, approximately corresponding to the half-life of the chondroitin sulphate proteoglycan. The molecular-weight distribution of chondroitin [35S]sulphate, labelled in vivo or in vitro, was invariably identical with that of the bulk polysaccharide from the same tissue. It is concluded that the observed regional variations in molecular-weight distribution were established at the time of polysaccharide biosynthesis. 3. In tissue culture more than half of the 35S-labelled polysaccharide–proteins of the two tissues was released into the medium within 10 days of incubation. The released materials were of smaller molecular size than were the corresponding native proteoglycans. In contrast, the molecular-weight distribution of the chondroitin [35S]sulphate (single polysaccharide chains) remained constant throughout the incubation period. 4. A portion (about 20%) of the total radioactive material released from 35S-labelled cartilage in tissue culture was identified as inorganic [35S]sulphate. No corresponding decrease in the degree of sulphation of the labelled polysaccharide could be detected. These findings suggest that a limited fraction of the proteoglycan molecules had been extensively desulphated. 5. It is suggested that the initial phase of degradation involves proteolytic cleavage of the proteoglycan, but the constituent polysaccharide chains remain intact. The partially degraded proteoglycan may be eliminated from the cartilage by diffusion into the circulatory system. An additional degradative process, which may occur intracellularly, includes desulphation of the polysaccharide, probably in conjunction with a more extensive breakdown of the polymer.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. Y. The degradation of cartilage matrix by an intracellular protease. Biochem J. 1964 Dec;93(3):611–618. doi: 10.1042/bj0930611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- BOLLET A. J., BONNER W. M., Jr, NANCE J. L. THE PRESENCE OF HYALURONIDASE IN VARIOUS MAMMALIAN TISSUES. J Biol Chem. 1963 Nov;238:3522–3527. [PubMed] [Google Scholar]
- BOSTROM H. On the metabolism of the sulfate group of chondroitinsulfuric acid. J Biol Chem. 1952 May;196(2):477–481. [PubMed] [Google Scholar]
- Balazs E. A., Berntsen K. O., Karossa J., Swann D. A. An automated method for the determination of hexuronic acids. Anal Biochem. 1965 Sep;12(3):547–558. doi: 10.1016/0003-2697(65)90221-6. [DOI] [PubMed] [Google Scholar]
- Calatroni A., Donnelly P. V., Di Ferrante N. The glycosaminoglycans of human plasma. J Clin Invest. 1969 Feb;48(2):332–343. doi: 10.1172/JCI105989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J., Weston P. D. Cathepsin D. Characteristics of immunoinhibition and the confirmation of a role in cartilage breakdown. Biochem J. 1971 Jun;123(1):1–13. doi: 10.1042/bj1230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Fratantoni J. C., Hall C. W., Neufeld E. F. The defect in Hurler's and Hunter's syndromes: faulty degradation of mucopolysaccharide. Proc Natl Acad Sci U S A. 1968 Jun;60(2):699–706. doi: 10.1073/pnas.60.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J. I., MATHEWS M. B., DORFMAN A. Sodium chondroitin sulfate-protein complexes of cartilage. II. Metabolism. J Biol Chem. 1960 Oct;235:2889–2892. [PubMed] [Google Scholar]
- HUNG P. P., MARKS C. L., TARDREW P. L. THE BIOSYNTHESIS AND METABOLISM OF ERYTHROMYCINS BY STREPTOMYCES ERYTHREUS. J Biol Chem. 1965 Mar;240:1322–1326. [PubMed] [Google Scholar]
- Hallén A. Chromatography of acidic glycosaminoglycans on DEAE-cellulose. J Chromatogr. 1972 Aug 23;71(1):83–91. doi: 10.1016/s0021-9673(01)85691-0. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The effect of temperature on the biosynthesis of chondroitin 4-sulphate in cartilage slices in vitro. FEBS Lett. 1970 Sep 6;9(3):145–148. doi: 10.1016/0014-5793(70)80339-8. [DOI] [PubMed] [Google Scholar]
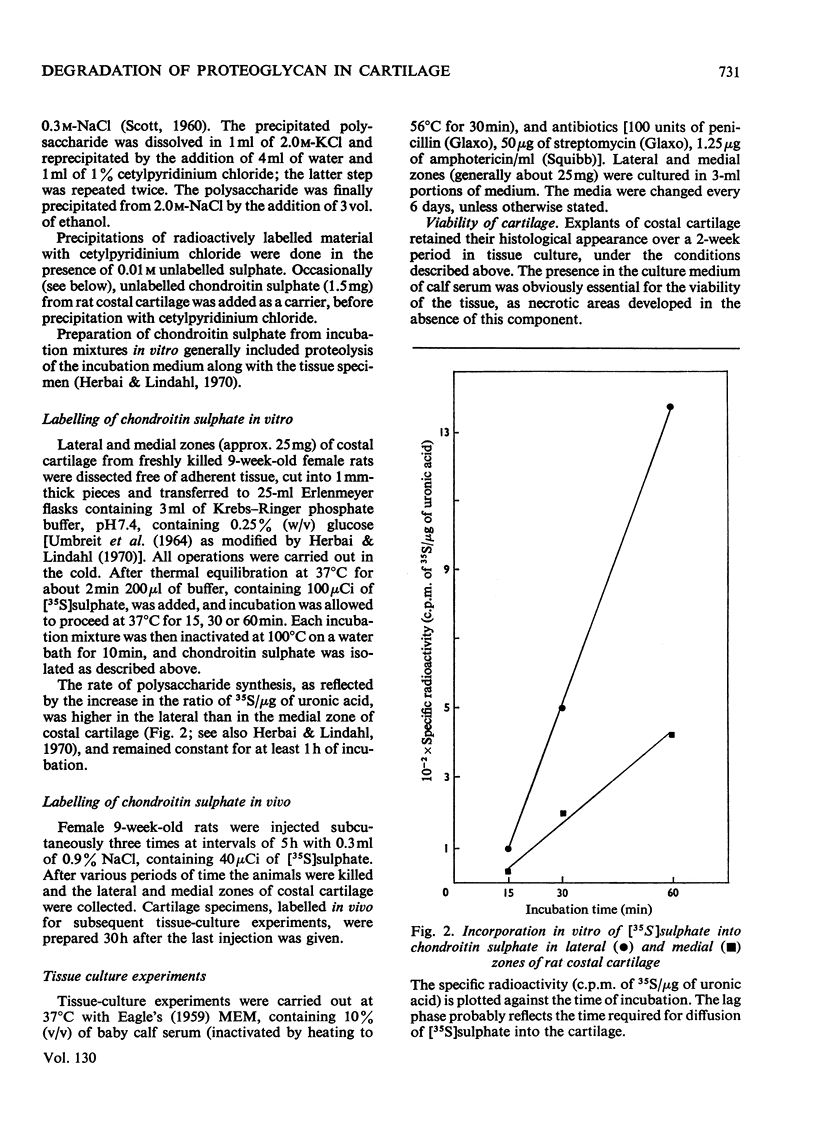
- Herbai G., Lindahl U. Regional differences in the incorporation rates of 3H-acetate and 35S-sulfate into chondroitin sulfate of mouse costal cartilage in vitro. Acta Physiol Scand. 1970 Dec;80(4):502–509. doi: 10.1111/j.1748-1716.1970.tb04817.x. [DOI] [PubMed] [Google Scholar]
- Hinton R. H., Dobrota M. A simple gradient maker for use with zonal rotors. Anal Biochem. 1969 Jul;30(1):99–110. doi: 10.1016/0003-2697(69)90377-7. [DOI] [PubMed] [Google Scholar]
- Hjertquist S. O., Wasteson A. The molecular weight of chondroitin sulphate from human articular cartilage. Effect of age and of osteoarthritis. Calcif Tissue Res. 1972;10(1):31–37. doi: 10.1007/BF02012533. [DOI] [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- Kleine T. O., Hilz H. Untersuchungen zur Biosynthese der Chondroitinsulfat-Proteine. I. Charakterisierung der Protein-Polysaccharide aus Kalberrippenknorpel und ihre In-vitro-Markierung mit zweiwertig 35S-Sulfat. Hoppe Seylers Z Physiol Chem. 1968 Aug;349(8):1027–1036. [PubMed] [Google Scholar]
- LOEWI G. Changes in the ground substance of ageing cartilage. J Pathol Bacteriol. 1953 Apr;65(2):381–388. doi: 10.1002/path.1700650211. [DOI] [PubMed] [Google Scholar]
- LUCY J. A., DINGLE J. T., FELL H. B. Studies on the mode of action of excess of vitamin A. 2. A possible role of intracellular proteases in the degradation of cartilage matrix. Biochem J. 1961 Jun;79:500–508. doi: 10.1042/bj0790500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U. Attempted isolation of a heparin proteoglycan from bovine liver capsule. Biochem J. 1970 Jan;116(1):27–34. doi: 10.1042/bj1160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I. Biosynthesis of glycosaminoglycans (mucopolysaccharides) in leukemic myeloid cells. Biochim Biophys Acta. 1968 Oct 15;165(3):324–334. doi: 10.1016/0304-4165(68)90210-9. [DOI] [PubMed] [Google Scholar]
- Platt D., Dorn M. Nachweis, Reinigung und Eigenschaften der Glycosaminoglycano-Hydrolasen im menschlichen hyalinen Knorpel. Clin Chim Acta. 1968 Sep;21(3):333–345. doi: 10.1016/0009-8981(68)90064-8. [DOI] [PubMed] [Google Scholar]
- Radhakrishnamurthy B., Dalferes E. R., Jr, Berenson G. S. Determination of hexosamines by gas-liquid chromatography. Anal Biochem. 1966 Dec;17(3):545–550. doi: 10.1016/0003-2697(66)90190-4. [DOI] [PubMed] [Google Scholar]
- Rokosova B., Bentley J. P. The incorporation of ( 14 C) glucose into chondroitin sulfate of differing chain length. Biochim Biophys Acta. 1972 Mar 30;264(1):98–102. doi: 10.1016/0304-4165(72)90120-1. [DOI] [PubMed] [Google Scholar]
- Rosenberg L., Johnson B., Schubert M. Proteinpolysaccharides from human articular and costal cartilage. J Clin Invest. 1965 Oct;44(10):1647–1656. doi: 10.1172/JCI105271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
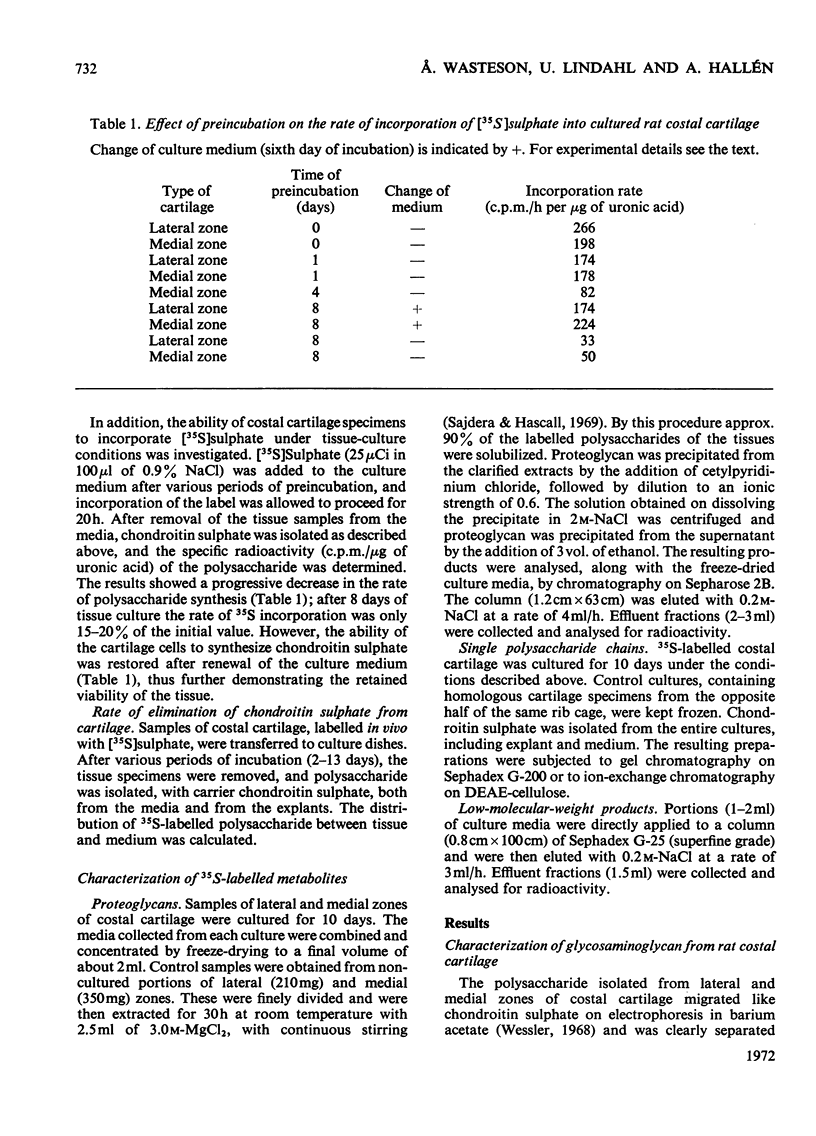
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Wasteson A., Lindahl U. The distribution of sulphate residues in the chondroitin sulphate chain. Biochem J. 1971 Dec;125(3):903–908. doi: 10.1042/bj1250903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A. Properties of fractionated chondroitin sulphate from ox nasal septa. Biochem J. 1971 May;122(4):477–485. doi: 10.1042/bj1220477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A., Wessler E. Molecular size and xylose content of urinary glycosaminoglycans. Biochim Biophys Acta. 1971 Oct;252(1):13–17. doi: 10.1016/0304-4165(71)90087-0. [DOI] [PubMed] [Google Scholar]
- Wessler E. Analytical and preparative separation of acidic glycosaminoglycans by electrophoresis in barium acetate. Anal Biochem. 1968 Dec;26(3):439–444. doi: 10.1016/0003-2697(68)90205-4. [DOI] [PubMed] [Google Scholar]
- Wessler E. Electrophoresis of acidic glycosaminoglycans in hydrochloric acid: a micro method for sulfate determination. Anal Biochem. 1971 May;41(1):67–69. doi: 10.1016/0003-2697(71)90192-8. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]


