Abstract
Sevelamer is a non-absorbable polymer used to treat hyperphosphatemia in individuals with end-stage renal disease (ESRD) undergoing hemodialysis. The deposition of sevelamer crystals in the gastrointestinal (GI) tract, especially in the colon, can cause mucosal inflammation, pseudopolyps, ulceration, ischemia, or necrosis. Owing to its rarity and lack of physician awareness, the actual incidence and prevalence of sevelamer-induced gastrointestinal mucosal injury (SIGMI) remain unknown. The current evidence is retrospective, in the form of observational studies. This systematic review of case reports provides an overview of SIGMI, with a focus on its etiology, signs and symptoms, pathogenesis, diagnosis, and management. Electronic databases, including PubMed, Embase, and Google Scholar, were searched for published case reports, case series, and abstracts from inception to August 2023. The search yielded 1239 articles that were filtered using the study design, English language, and human subjects. After screening for duplicates and irrelevant articles, only 28 articles were included in the final review. Melena and abdominal pain were the most common complaints. Sevelamer was discontinued in all patients, and 27 (75%) experienced clinical improvement or symptom resolution. Eight patients (22%) required colectomy due to colonic perforation, malignant obstruction, or extensive necrosis. SIGMI is a unique complication of sevelamer use in patients undergoing hemodialysis. Prompt diagnosis and management are crucial to prevent life-threatening complications.
Keywords: Sevelamer, Sevelamer crystals, Sevelamer-induced gastrointestinal mucosal injury, Sevelamer-induced colitis, Gastrointestinal mucosal injury, Phosphate binder, End-stage renal disease
1. Introduction
According to the 2017 US Renal Data Systems (USRDS) report, an estimated 30 million American adults have chronic kidney disease (CKD), with 500,000 patients receiving maintenance dialysis therapy.1 As kidney function declines, it loses its capacity to excrete excess phosphorus, leading to hyperphosphatemia, which predisposes patients to renal bone disease and organopathy.2,3 Phosphate binders, including calcium acetate, lanthanum carbonate, and sevelamer, facilitate the fecal excretion of phosphorus.3 Sevelamer was first approved for use in the US in 1998, and its demand continues to grow due to the increasing burden of CKD.4,5 Sevelamer is a non-absorbable polymer that is used to treat hyperphosphatemia in individuals with end-stage renal disease (ESRD) undergoing hemodialysis.4–9 Sevelamer binds dietary phosphate within the gastrointestinal (GI) tract, impeding its reabsorption.5–7 Generally, sevelamer is well tolerated; even so, nausea, vomiting, abdominal pain, diarrhea, excess flatulence, and constipation may occur.4–6,9
The deposition of sevelamer crystals in the colon can give rise tomucosal inflammation, pseudopolyps, ulceration, ischemia, necrosis, and luminal obstruction due to fecaliths.4,5,8–10 Sevelamer crystals can be identified in the fibropurulent necrotic debris of ulcerated tissue, displaying a characteristic “fish-scale” pattern on histopathology.9 Due to its rarity and lack of physician awareness, the actual incidence of SIGMI remain unknown, and current evidence is in the form of descriptive observational studies from post marketing analysis.5–7,9,11 In this targeted literature review, we provide an overview of SIGMI with a focus on its etiology, signs and symptoms, pathogenesis, diagnosis, and management.
2. Methods
SIGMI is a very rare clinical entity, and the current evidence is in the form of case reports. Electronic databases, including PubMed, Embase, and Google Scholar, were systematically searched for published case reports, case series, and abstracts from inception to August 2023 (Table 1). Major keyword terms included: “Sevelamer,” “Renvela,” “crystal-induced colitis,” “Renagel,” “colitis,” “lower gastrointestinal bleeding.” Articles were eligible for inclusion in the study if they were in English, available as full-text, and the case report featured a patient with GI symptoms due to sevelamer use. Non-English articles, articles on non-human subjects, articles with insufficient data, and articles on GI mucosal injury due to other medications were excluded. The results obtained from the search (1239 articles) were cross-referenced for the type of study and included based on the inclusion criteria. Twenty-eight articles met the inclusion criteria and were included in this descriptive review. Statistical analysis was descriptive in the form of means, proportions, ranges, and percentages.
Table 1.
A summary of our search strategy.
| Items | Specification |
|---|---|
| Search date | Databases searched up to August 31st, 2023, |
| Electronic Databases | PubMed, Embase, Google Scholar |
| Search terms | Sevelamer, Renvela, Renagel, crystal-induced colitis, colitis, lower gastrointestinal bleeding |
| Timeframe | From inception to August 2023 |
| Inclusion and Exclusion criteria | Inclusion:
|
3. Results
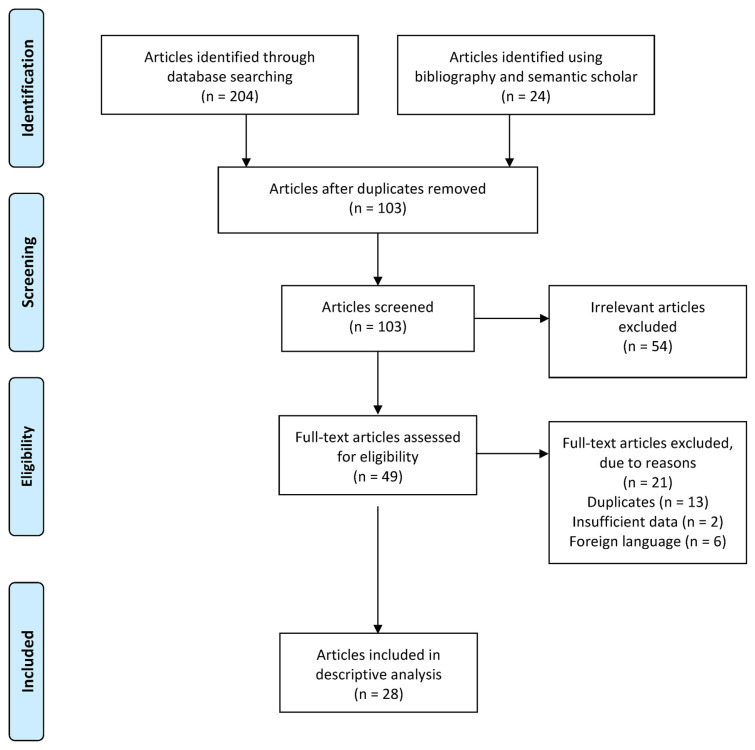
In total, our search stratagem yielded 1239 articles, which were filtered to 228 articles using the study type. Twenty-eight articles comprising 36 patients were included in the review, after screening for duplicates and irrelevant articles. The PRISMA flow diagram represents the search strategy and results (Fig. 1). There were six conference abstracts, three image articles, 17 case reports, and two case series. Of the 36 patients, 21 (58%) were female and 15 (42%) were male. The patients’ age ranged from 17 to 83 years, with an average age of 52.25 years. Eighteen patients (50%) presented with GI bleeding (melena, hematochezia, or both), 17 (47%) presented with abdominal pain, and seven patients (20%) presented with both abdominal pain and GI bleeding. Sevelamer was discontinued in all the patients, and 27 (75%) experienced clinical improvement or symptom resolution. One patient required sevelamer dose reduction, one required aggressive lavage, and one required prophylaxis with diphenoxylate/atropine. One patient required hemoclip placement and coil embolization because of persistent GI bleeding. Eight patients (22%) required colectomy due to colonic perforation, malignant obstruction, or extensive necrosis.
Fig. 1.
PRISMA flow diagram showcasing the search strategy and results.
4. Discussion
4.1. Epidemiology
Sevelamer is a complex molecule made of a backbone polyallylamine chloride cross-linked with epichlorohydrin, which is not absorbed in the GI tract.12,13 This structure allows the molecule to act as a non-specific anion binder when reduced by the acidic pH of the stomach.12,13 Sevelamer is not absorbed in the GI tract but instead binds phosphate, displacing it from previously bound bile acid, reducing its GI absorption.5,12,13 The only indication for sevelamer approved by the Food and Drug Administration (FDA) is the control of serum phosphorus in adults and children aged 6 years and older with chronic kidney disease (CKD) on dialysis.13 Sevelamer is also hypothesized to lower serum cholesterol, proinflammatory mediators, and uremic toxins, such as p-Cresol, which are inadequately eliminated via dialysis.13 Sevelamer carbonate is available as a tablet or powder, and the initial dose is 800 mg or 1600 mg administered orally three times a day with meals.14 Sevelamer hydrochloride (Renagel) on the other hand, exists only in tablet formulation but has the same dosing schedule as Renvela. In pediatric patients, sevelamer dosing is titrated based on the body surface area.
Sevelamer causes mild GI symptoms such as nausea, vomiting, abdominal pain, and constipation.2,4–6,13,15–23 Rare complications such as obstruction, mucosal injury, bleeding ulceration, necrosis, and perforation have also been reported.4,5,13,20 Despite its known association with GI injury, little research has been conducted on its mechanism.2 The current data is retrospective in the form of case reports and conference proceedings. In 2008, Madan et al.17 reported the first case of SIGMI in a patient who presented with rectal bleeding and was diagnosed with stercoral ulcers during colonoscopy. Since then, 27 additional cases have been reported (Table 2); however, prospective studies evaluating GI complications of sevelamer in patients with ESRD are still lacking.
Table 2.
Summary table of sevelamer-induced gastrointestinal mucosal injury cases published in the literature.
| Author(s) | Year | Patient | Presentation | Intervention/Outcome | Endoscopy | Pathology | Article |
| Prlic et al.1 | 2023 | 74F | Rectal bleeding, colonic perforation, shock | Total colectomy & ileostomy Sevelamer discontinued. | Large ulceration in the transverse colon | Mucosal injury with “fish-scale” crystals | Case report |
| Pant et al.2 | 2023 | 77F | SOB, fatigue, and weakness | Sevelamer discontinued | Ulcers in the cecum and ileocecal valve, | Active colitis with ulceration and granulation tissue, SC | Case report |
| Deshmukh et al.3 | 2022 | 66M | Hematochezia | IVF and PPIs. Sevelamer discontinued. Colitis resolved | Friable, erythematous mucosa and ulcerations in the colon | Necrotic debris, acute inflammation & ulceration “Fish-scale” crystals | Abstract |
| Yamada et al.4 | 2022 | 24F | Abdominal pain & melena | Sevelamer discontinued. Started on ferric citrate | Ulcerations in the cecum | Crystalline material consistent with sevelamer | Abstract |
| Hryzak et al.5 | 2022 | 67M | Abdominal pain, nausea, & vomiting | Hemi-colectomy due to bowel necrosis | Crystalline resin within lumen & stoma ulceration consistent with sevelamer crystals | Abstract | |
| Cockrell et al.6 | 2021 | 65F | Abdominal pain and hematochezia | Sigmoid colectomy and end colostomy due to large bowel obstruction | Multiple non-bleeding sigmoid diverticula | Pericolonic abscess and sevelamer crystals | Case report |
| Schoot et al.7 | 2021 | 67F | Rectal bleeding | Stopped Sevelamer. Rectal bleeding decreased/resolved | Multiple deep ulcers & diffuse edematous mucosa in sigmoid and rectum | Ulcerated mucosa | Case report |
| Al-Qaisi et al.8 | 2020 | 71M | Hematochezia | Sevelamer discontinued. Colitis resolved | Ulcerations, mucosal erythema & nodularity in rectum & rectosigmoid colon | Crypt distortion, fragments of resin consistent with sevelamer | Abstract |
| Lai et al.9 | 2020 | 47M | Crampy abdominal pain | Sevelamer switched to calcium carbonate. Symptoms improved | Circumferential ulceration in the colon | Ragged colonic mucosa with ulcerative debris and non-polarizing crystalline material | Case report |
| Keri et al.10 | 2019 | 35F | Abdominal pain, rectal bleeding, and hematochezia | Right hemicolectomy followed by ileocolic anastomosis | Patchy transmural ischemic necrosis with vascular fibrin thrombi and sevelamer crystals | Case report | |
| Uy et al.11 | 2018 | 33M | Hematochezia & periumbilical pain | Sevelamer switched to lanthanum carbonate. Hematochezia resolved | Non-bleeding ulcerated colonic mucosa | Sevelamer crystals | Case report |
| Nambiar et al.12 | 2018 | 56F | Rectal bleeding, syncope, and shortness of breath | Emergent hemicolectomy | Erythema and ulceration near hepatic flexure without active bleeding | Colonic mucosa with inflammation & ulceration. Clusters of “fish-scale” crystals | Case report |
| Okwara et al.13 | 2018 | 70M | Copious hematemesis, rectal bleeding, SOB, dizziness | Hemostatic clips Coil embolizations: distal R gastroepiploic artery, gastroduodenal artery, gastrohepatic trunk | EGD: ulcerated mass at pylorus | Sevelamer crystal-associated chronic, focally active gastritis | Case report/ Image article |
| Brahmbhatt et al.14 | 2017 | 40F | Diarrhea and LLQ abdominal pain | Sevelamer switched to calcium acetate. | Fungating, non-obstructing, circumferential, 6 cm mass in the proximal sigmoid colon | Necro-inflammatory debris with embedded crystalline fragments | Abstract |
| Bansal et al.15 | 2017 | 42F | LLQ abdominal pain & watery diarrhea | Sevelamer discontinued | 6 cm, fungating, oozing mass sigmoid colon | Necrotic debris and eosinophilic “fish-scale” crystals | Image article |
| Modi et al.16 | 2017 | 60F | Diffuse abdominal pain and watery diarrhea | Restarted on sevelamer with diphenoxylate/atropine, PRN | Solitary 14-mm ulcer with surrounding erythema in distal rectum | Colonic mucosal inflammation and crystal foreign material | Image article |
| Sy et al.17 | 2017 | 69M | Abdominal pain | Aggressive lavage | 35-mm mass lesion in the cecum | Benign inflamed colonic mucosa and sevelamer crystals | Image article |
| Yuste el.18 | 2017 | 51F | Hematochezia | Sevelamer switched to calcium carbonate | DRE: big anal fissure. Large ulcer in the ileocecal valve | Focal erosion with bacterial material mixed with sevelamer crystals | Case series |
| 53M | Painless rectal bleeding | PPIs. Sevelamer dose reduced | Pseudopolyps and inflammation of the colon | Chronic colitis with low inflammatory activity and sevelamer crystals | |||
| 76F | Lower GI bleeding and severe anemia | Symptoms resolved with the discontinuation of sevelamer | Chronic gastritis, diverticulosis, several gastric and colonic polyps | Superficial sevelamer crystals surrounded by mucus and detritus | |||
| Nandiraju et al.19 | 2016 | 67M | Symptomatic anemia, hematochezia, and melena | Symptoms resolved with the discontinuation of sevelamer | Large ulceration at the splenic flexure and indeterminate diffuse colitis and polyps | Rare surface crystals with “fish-scales” consistent with sevelamer crystals | Abstract |
| Desai et al.20 | 2016 | 45F | Intermittent abdominal cramping and hematochezia | Symptoms resolved with the discontinuation of sevelamer | Healing linear ulcerations at the flexures, transverse, and sigmoid colon Anal verge stricture, and inflamed and friable colonic mucosa |
Acute inflammation, ulceration, and granulation tissue associated with fragments of crystal material | Case report |
| Tieu et al.21 | 2016 | 74F | Constipation, abdominal discomfort, rectal pain & hematochezia | Sevelamer switched to calcium acetate. | Recto-sigmoid ulcers | Pill fragments consistent with sevelamer crystals | Case report |
| Kim et al.22 | 2016 | 17F | Acute-onset abdominal pain and emesis | Hemi-colectomy with colostomy. Sevelamer discontinued | 11-cm stricture and high-grade obstruction at the junction of the descending colon and sigmoid colon | Sevelamer crystals with adjacent ischemic change of the colonic mucosa | Case report |
| Yamaguchi et al.23 | 2016 | 66M | Abdominal pain | Hemi-colectomy with colostomy creation for colonic perforation | Sevelamer crystals | Case report | |
| Subramanian & Roorda24 | 2016 | 65F | Abdominal pain and rectal bleeding | Symptoms resolved with the discontinuation of sevelamer | Pseudomembrane and nodularity in rectosigmoid region | Sevelamer crystals in an area of ulceration | Case report |
| Hudacko et al.25 | 2015 | 83F | Mixed shock and abdominal distension | Subtotal colectomy for diffuse bowel necrosis and perforation of transverse colon | Mucosal ischemic injury with ulcers, transmural necrosis, and acute serositis Sevelamer crystals |
Case report | |
| Chintamaneni et al.26 | 2014 | 61F | Painless hematochezia and thrombosed AVF | Symptoms resolved with the discontinuation of sevelamer | 5-cm ulceration in sigmoid colon | Sevelamer crystals embedded in colonic mucosa | Case report |
| Swanson et al.27 | 2013 | 59F | Anuric, dyspepsia, | Small bowel resection Symptoms resolved with the discontinuation of sevelamer |
Unknown | Small bowel ischemia, necrosis & sevelamer crystals | Case series |
| 68M | Screening colonoscopy | Unknown | Normal colon | Inflammatory polyps with acute inflammation, sevelamer crystals | |||
| 38M | Screening colonoscopy | Unknown | Colon polyps | Acute colitis & sevelamer crystals | |||
| 49F | Dyspepsia | Symptoms resolved with the discontinuation of sevelamer | Diffuse peptic changes | Extensive esophageal ulceration, sevelamer crystals | |||
| 53M | Screening colonoscopy | Unknown | Colon polyp | Mucosal prolapse, sevelamer crystals | |||
| 66M | Rectal bleeding | Symptoms resolved with the discontinuation of sevelamer | Hyperpigmented mucosa in duodenum, antrum, body. Colon polyps | Fragments of tubular adenoma | |||
| 81M | Dysphagia, odynophagia, vomiting | Symptoms resolved with the discontinuation of sevelamer | Extensive esophageal ulcerations and eroded mucosa, plaque-like white exudates | Extensive ulceration | |||
| Madan et al.28 | 2008 | 62F | Rectal bleeding | Symptoms resolved with the discontinuation of sevelamer | Stercoral ulcers in rectum | Denuded mucosa with acute and chronic inflammation | Case report |
4.2. Pathogenesis
There is a paucity of data on SIGMI and the mechanism of injury is not completely understood. The spectrum of mucosal injury ranges from acute inflammation, tissue ischemia and necrosis, pseudopolyps, and ulceration to fecaliths.5,15,20,24 Sevelamer crystal deposits are thought to be directly cytotoxic to mucosal cells, but this effect is yet to be verified.15 It is postulated that these crystals can aggregate intraluminally and precipitate into a mass leading to bowel obstruction or perforation.2,19 Although the etiopathogenesis of SIGMI remains unclear, temporality, dose-response relationship, and biological plausibility have been established in reported observational studies that further strengthen the causation hypothesis.5,15
4.3. Clinical manifestations
GI bleeding is the most commonly reported complaint, presenting as frank blood per rectum, hematochezia, or melena.2,5,17 In our review of published cases of SIGMI, hematochezia was the most common complaint, followed by abdominal pain and diarrhea.2,4,7,18,19,22,25,26 Sevelamer crystals can be deposited at unique sites such as the esophagus and stomach, resulting in esophageal ulceration, vomiting, hematemesis, dysphagia, odynophagia, gastric ulceration, and abdominal distension.15,20,22,23,27 Unique patient presentations, such as symptomatic anemia, shock, and syncope, have also been observed.13,15,22,23
4.4. Diagnosis
Owing to its rarity and non-specific presentation, SIGMI may be mistaken for ischemic colitis, infectious colitis, inflammatory bowel disease, or other medication-induced colitis. A temporal relationship has been observed between sevelamer use and the onset of GI symptoms. In patients presenting with GI bleeding, bloodwork may be significant for acute blood loss anemia or leukocytosis due to inflammation. Routine imaging studies may be nonspecific; however, intraluminal hemorrhage, colonic dilation, and fat stranding have been observed.17 Endoscopy with biopsy can be both diagnostic and therapeutic. Colonoscopic findings include colonic ulcers, friable and erythematous mucosa, pseudopolyps, masses, anal fissures, anal strictures, pseudomembranes and nodularity, and colonic polyps (Fig. 2).5–7,9,11,15,16,20,24,26–28 Gastritis, esophageal ulcers, gastric polyps, and ulcerated pylorus have been observed on upper endoscopy.15,20,22,29 Some patients had normal endoscopic evaluations despite having melena, hematochezia, or signs of obstruction.4,15,23
Fig. 2.
Endoscopic image showing the descending colon (A) and sigmoid colon with continuous non-bleeding ulcerated mucosa. Used with permission from Uy et al.7
The histopathology of SIGMI varies from active colitis, ulceration and granulation tissue, crypt distortion, eosinophilia, necrotic debris, inflammatory polyps, to crystals.4,5,9,11,13,15,16,18,20,24 Examination of the sevelamer crystals will reveal a non-polarizable “fish-scale” pattern that stains pink centrally and yellow/orange peripherally with hematoxylin and eosin (H&E) stain (Fig. 3).5,7 Polystyrene sulfonate and cholestyramine are known to cause medication-induced colitis and must be considered in the differential diagnosis of SIGMI.20 Polystyrene sulfonate crystals share a similar architectural pattern with sevelamer crystals; however, they will stain purple with H&E stain.5,13,20 Cholestyramine has a unique structure from sevelamer and stains orange when exposed to an H&E stain, which further distinguishes it from sevelamer crystals.
Fig. 3.
Colonic mucosa with non-specific minimal reactive changes, and broad, curved, irregularly shaped “fish scales” sevelamer crystals without tissue necrosis on H & E (100x). Used with permission from Uy et al.7
4.5. Treatment
The management of SIGMI remains a challenge due to the limited understanding of its etiopathogenesis, and the absence of established prevention strategies.5 It is crucial for clinicians to recognize the symptoms associated with SIGMI, including hematochezia, abdominal pain, colonic ulceration, and luminal obstruction, to avoid further complications.7 In our review of 36 patients with SIGMI, we observed that discontinuing the offending agent resulted in symptom resolution in most patients. Although the clinical improvement was immediate in some patients, it took weeks for others to experience relief. The healing time was most likely determined by the degree of GI mucosal injury. In some cases, patients received supportive management with intravenous fluids, blood transfusion, and proton pump inhibitors.5,6,13,18,28,30 Some patients required dose reduction, whereas others required adjunctive treatment with diphenoxylate/atropine.16,20,29 Switching patients to alternative phosphate binders has also been effective. Clinically unstable patients with colonic perforation, malignant obstruction, or extensive necrosis required exploratory laparotomy with colectomy.2,15,19,24,25,27 Although follow-up endoscopy was performed in some cases, the benefit of relook endoscopy remains unclear. Further research is warranted to understand the etiopathogenesis of SIGMI to guide management and guidelines. Suspected or confirmed cases on SIGMI can reported on MedWatch, a U.S. Food and Drug Administration’s (FDA) safety information and adverse event reporting program. This program is designed to allow healthcare professionals and consumers to report adverse events, product quality problems, therapeutic inequivalence/failures, and product use errors associated with FDA-regulated products, including drugs, biologics, medical devices, dietary supplements, and cosmetics.31
5. Conclusion
SIGMI is a very rare clinical entity that results from the deposition of sevelamer crystals in the GI tract. Sevelamer causes mild gastrointestinal symptoms such as nausea, vomiting, diarrhea, and constipation. However, the deposition of sevelamer crystals in the GI tract may lead tomucosal injury, ulceration, tissue ischemia or necrosis, pseudopolyps, and bowel obstruction. SIGMI is often missed in clinical practice because of its rarity and the lack of physician awareness. This literature review provides an overview of SIGMI, focusing on its etiology, signs and symptoms, pathogenesis, diagnosis, and management.
Footnotes
Disclosure: A portion of this manuscript was presented as an abstract at Digestive Disease Week, 2024, in Washington, DC. Bathobakae, Lefika, Saif Yasin, Phenyo Phuu, Rammy Bashir, Jessica Escobar, Ruhin Yuridullah, Gabriel Melki, Mohamed Elagami, Yana Cavanagh, and Walid Baddoura. “Mo1352 SEVELAMER-INDUCED COLITIS: A CONCISE REVIEW FOR CLINICIANS.” Gastroenterology 166, no. 5 (2024): S-1036.
Ethics statement: Our institution does not require ethical approval for review articles.
Conflict of interest: No conflicts of interest to declare.
Funding: No funding was obtained for the writing or submission of this manuscript.
Data availability statement
The authors declare that data supporting the findings of this study are available within the article.
References
- 1. Saran R, Robinson B, Abbott KC, et al. US renal data system 2017 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3):A7. doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prlic MF, Jelakovic M, Brinar M, et al. Case report: sevelamer-associated colitis—a cause of pseudotumor formation with colon perforation and life-threatening bleeding. Front Med. 2023;10(7) doi: 10.3389/fmed.2023.1097469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Provider Synergies. Phosphate binders review FDA-approved indications. Mason, Ohio: 2009. [Accessed October 19, 2023]. https://www.medicaid.nv.gov/Downloads/provider/NVRx_DCR_20090625_Phosphate_Binders.pdf . [Google Scholar]
- 4. Yamaguchi T, Ohyama S, Furukawa H, et al. Sigmoid colon diverticula perforation associated with sevelamer hydrochloride administration: a case report. Ann Med Surg. 2016;10:57–60. doi: 10.1016/j.amsu.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chintamaneni P, Das R, Kuan S-F, Kermanshahi TR, Hashash JG. Hematochezia associated with sevalamer-induced mucosal injury. ACG Case Reports J. 2014;1(3):145–147. doi: 10.14309/crj.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bansal V, Aggarwal P, Mittal A, Vachhani M, Aggarwal P, Aggarwal N. Colonic mass secondary to sevelamer-associated mucosal injury. ACG Case Reports J. 2017;4(1):e92. doi: 10.14309/crj.2017.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uy PP, Vinsard DG, Hafeez S. Sevelamer-associated rectosigmoid ulcers in an end-stage renal disease patient. ACG Case Reports J. 2018;5(11):e831–e833. doi: 10.14309/crj.2018.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brahmbhatt S, Cruise M, Simon J. Sevelamer induced diffuse crystal colitis: a case report of a rarely eported side effect. Am J Kidney Dis. 2017;69(4):A31. doi: 10.1053/j.ajkd.2017.02.065. [DOI] [Google Scholar]
- 9. Tieu C, Moreira RK, Song LMWK, Majumder S, Papadakis KA, Hogan MC. A case report of sevelamer-associated recto-sigmoid ulcers. BMC Gastroenterol. 2016;16(1):10–13. doi: 10.1186/s12876-016-0441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qiu L, Volk E, Mais DD. Histopathologic patterns of colitis in patients with impaired renal function. Am J Clin Pathol. 2020;153(3):380–386. doi: 10.1093/ajcp/aqz176. [DOI] [PubMed] [Google Scholar]
- 11.Nandiraju D, Amberker D, Rampure R. American journal of kidney diseases. A78. Bethesda; Maryland: 2016. Sevelamer induced gastrointestinal bleeding; p. 67. [Google Scholar]
- 12. Grinfeld J, Inaba A, Hutchison A. Update and critical appraisal of sevelamer in the management of chronic renal failure. Res Rep Urol. 2010;2:161–170. doi: 10.2147/rru.s7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pant P, LaBel D, Hernandez Garcilazo N, Sharma S, Rayamajhi S. Colitis associated with sevelamer carbonate: a case report. Ann Intern Med Clin Cases. 2023;2(7):2–5. doi: 10.7326/aimcc.2022.0924. [DOI] [Google Scholar]
- 14.Genzyme Corporation. Renvela (sevelamer carbonate) label. 2014. [Accessed October 19, 2023]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022127s011lbl.pdf .
- 15. Swanson BJ, Limketkai BN, Liu TC, et al. Sevelamer crystals in the gastrointestinal tract (GIT): a new entity associated with mucosal injury. Am J Surg Pathol. 2013;37(11):1686–1693. doi: 10.1097/PAS.0b013e3182999d8d. [DOI] [PubMed] [Google Scholar]
- 16. Modi RM, Swanson B, Duggirala V. Long-standing diarrhea associated with sevelamer crystalopathy in colonic mucosa. Clin Gastroenterol Hepatol. 2017;15(2):A26–A27. doi: 10.1016/j.cgh.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 17. Madan P, Bhayana S, Chandra P, Hughes JI. Lower gastrointestinal bleeding: association with Sevelamer use. World J Gastroenterol. 2008;14(16):2615–2616. doi: 10.3748/wjg.14.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoot TS, Römkens TEH, Hoogeveen EK.Lower gastrointestinal bleeding in a patient receiving sevelamer: case report. SAGE Open Med Case Reports. 2021. p. 9. [DOI] [PMC free article] [PubMed]
- 19. Cockrell HC, Cottrell-Cumber S, Brown K, Murphy JG. Sevelamer crystals - an unusual cause of large bowel obstruction. J Surg Case Rep. 2021;228(6):1–2. doi: 10.1093/jscr/rjab228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yuste C, Mérida E, Hernandez E, et al. Gastrointestinal complications induced by sevelamer crystals. Clin Kidney J. 2017;10(4):539–544. doi: 10.1093/ckj/sfx013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okwara C, Choi C, Park JY. Sevelamer-induced colitis presenting as a pseudotumor. Clin Gastroenterol Hepatol. 2015;13(7):A39–A40. doi: 10.1016/j.cgh.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 22. Okwara CJ, Gulati R, Rustagi T, Birg A, Hanson J, McCarthy D. Upper gastrointestinal bleeding of unusual causation. Dig Dis Sci. 2018;63(10):2541–2546. doi: 10.1007/s10620-018-5260-8. [DOI] [PubMed] [Google Scholar]
- 23. Hudacko R, Kaye P. Sevelamer-associated ischemic colitis with perforation. Gastroenterol Insights. 2015;6(1):6–7. doi: 10.4081/gi.2015.6116. [DOI] [Google Scholar]
- 24. Nambiar S, Pillai UK, Devasahayam J, Oliver T, Karippot A. Colonic mucosal ulceration and gastrointestinal bleeding associated with sevelamer crystal deposition in a patient with end stage renal disease. Case Reports Nephrol. 2018;2018:1–3. doi: 10.1155/2018/4708068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keri KC, Veitla V, Samji NS. Ischemic colitis in association with sevelamer crystals. Indian J Nephrol. 2019;29(3):191–193. doi: 10.4103/ijn.IJN_80_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Subramanian CR, Roorda A. GI mucosal injury and bleeding: association with sevelamer crystals. J Gastrointest Dig Syst. 2016;6(2):6–7. doi: 10.4172/2161-069x.1000409. [DOI] [Google Scholar]
- 27. Kim J, Olson K, Butani L. Sevelamer crystals in the mucosa of the gastrointestinal tract in a teenager with end-stage renal disease. Pediatr Nephrol. 2016;31(2):339–341. doi: 10.1007/s00467-015-3269-1. [DOI] [PubMed] [Google Scholar]
- 28. Lai T, Frugoli A, Barrows B, Salehpour M. Sevelamer carbonate crystal-induced colitis. Case Rep Gastrointest Med. 2020;2020:1–4. doi: 10.1155/2020/4646732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sy S, Siddiki H, Horsley-Silva J, Byrne T, Lam-Himlin D. Sevelamer resin bezoar presenting as a cecal mass. Gastrointest Endosc. 2017;86(6):1186–1187. doi: 10.1016/j.gie.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 30. Desai M, Reiprich A, Khov N, Yang Z, Mathew A, Levenick J. Crystal-associated colitis with ulceration leading to hematochezia and abdominal pain. Case Rep Gastroenterol. 2016;10(2):332–337. doi: 10.1159/000446575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mihalko WM, Seth Greenwald A, Lemons J, Kirkpatrick J. Reporting and notification of adverse events in orthopaedics. J Am Acad Orthop Surg. 2010;18(4):193–198. doi: 10.5435/00124635-201004000-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.