Abstract
In severe osteoporosis, anabolic agents such as teriparatide (Forteo) are more commonly used. As a PTH analog, teripararide has a limited side effect profile with potential for mild hypercalcemia and dyspepsia. In this report we highlight another less common side effect, asymptomatic elevation in alkaline phosphatase.
Keywords: Alkaline phosphatase, Osteoblast, Teriparatide, Forteo, Parathyroid hormone, PTH, Osteoporosis
1. Introduction
First line therapy in osteoporosis often includes antiresorptive medications, of which bisphosphonates are selected due to its proven efficacy, favorable safety profile, and low cost.1 In patients with contraindications to antiresorptive options or in those with severe osteoporosis (T-score of ≤ − 2.5 plus fragility fracture or T-score of ≤ −3.0), anabolic therapy may be utilized first line.1
Among the anabolic agents, teriparatide is widely used due to its safety and efficacy. In previous studies comparing bisphosphonates and teriparatide in patients with severe osteoporosis, vertebral fractures occurred less frequently in patients taking teriparatide.2 Teriparatide is administered subcutaneously once daily for 1–2 years, after which patients are transitioned to antiresorptive maintenance therapy.3
Teriparatide is a recombinant form of parathyroid hormone and possesses anabolic and resorptive properties. The most common adverse effects are nausea, and hypercalcemia affecting up to 6% of males and 11% of females.4 The effect of teriparatide on raising alkaline phosphatase levels is less recognized. A randomized clinical trial evaluating teriparatide’s effect on markers of bone formation, namely bone-specific alkaline phosphatase and procollagen I carboxy-terminal propeptide (PICP), described such effects.5 A separate case report highlighted a case of teriparatide-treated patient with generalized bone pain and extreme elevation in alkaline phosphatase suggestive of osteoblast hyperactivation.6
2. Case
A 74-year-old female of Asian American origin presented with a history of Graves Disease, hypertension, and osteoporosis. She was seen in an ambulatory setting and noted to have asymptomatic elevation of her alkaline phosphatase level to 160 U/L (reference range 34–104 U/L). Other liver tests were normal and determination of alkaline phosphatase isoenzymes confirmed an isolated skeletal source.
Nuclear bone scanning was performed to assess for Paget Disease and demonstrated diffuse calvarium uptake (Fig. 1); however, plain radiographs of the skull were negative and no other areas of irregular bone activity were detected, arguing against Pagetic activity (Fig. 2). While anabolic osteoporosis therapy has been associated with rare occurrence of osteosarcoma, our patient did not have bone pain or any signs or symptoms of a focal malignant process of the bone.
Fig. 1.
Diffuse calvarium uptake in nuclear bone scan.
Fig. 2.
Skull radiographs negative for Pagetic activity.
Of note, she has a family history of primary biliary cirrhosis in two female relatives, yet isoenzyme differentiation supported a bone source of her elevated alkaline phosphatase and she had no other clinical features of liver disease.
The alkaline phosphatase was normal at 75 U/L the year prior to starting teriparatide 20 mcg daily for severe osteoporosis with T-score of −4.6 and history of lumbar compression fracture. Her other medications included amlodipine and past, but not current, use of methimazole. Temporally, the elevation of alkaline phosphatase levels ultimately coincided with her timeline of taking teriparatide and suggested this as the most probable cause. She continued teriparatide and although her alkaline phosphatase remained elevated, it was stable. She tolerated the medication well without adverse issues.
3. Discussion
Our case demonstrates a less common, but important effect of teriparatide, which is elevation in alkaline phosphatase. A prior randomized controlled trial in 2005 evaluated serological markers of bone turnover in patients on anabolic osteoporosis therapy and assessed correlation with bone structure. Bone specific alkaline phosphatase and procollagen I C-terminal propeptide (PICP) levels were measured and a statistically significant increase in both peaking at 12 months of teriparatide use was seen with a median increase of 74 U/L.5 Interestingly, the rise and peak of alkaline phosphatase levels did not directly align chronologically with bone density. Despite this, the increase in alkaline phosphatase heralded improvement in bone density and correlated positively with radiological improvement in osteoporosis. Javinani et al. described this rise in alkaline phosphatase in which a 22-year-old with systemic lupus erythematosus and glucocorticoid-induced osteoporosis was noted to have extreme elevation of alkaline phosphatase, up to 6480 U/L, about 7 months after teriparatide therapy.6 The case demonstrates extreme osteoblast hyperactivation which was suspected to be affected by the patient’s young age, autoimmune condition, and medication interaction.
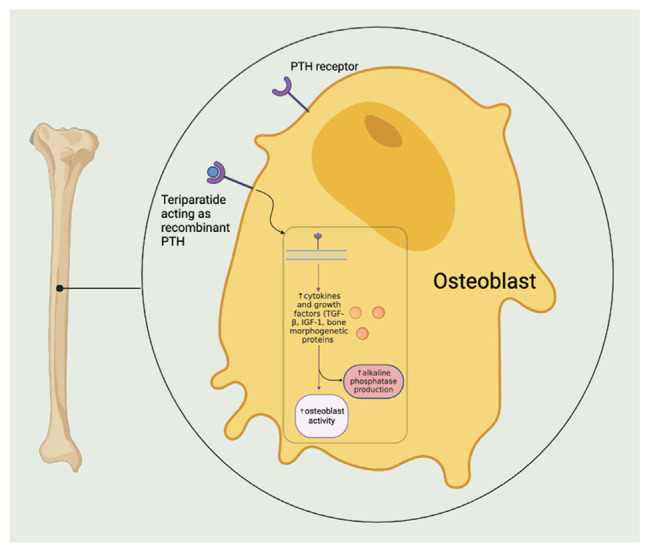
As suggested by the latter case, the mechanism of how teriparatide increases alkaline phosphatase is multidimensional, the primary component being stimulation of osteoblast activity. PTH analogs increase the production of cytokines and growth factors, including transforming growth factor β (TGF-β), insulin-like growth factor 1 (IGF-1), and bone morphogenetic proteins, which are involved in bone remodeling and promote alkaline phosphatase production in osteoblasts7 (Fig. 3). Other anabolic therapies for osteoporosis, such as abaloparatide and romosozumab have also been shown to increase alkaline phosphatase. Abaloparatide, being another peptide that selectively activates the PTH receptor, works similarly to teriparitide, and Romosozumab functions as monoclonal antibody that inhibits sclerostin, a protein that suppresses bone formation. By inhibiting sclerostin, romosozumab enhances bone formation.8 Bisphosphonates on the other hand work by inhibiting bone resorption. They are more likely to suppress markers of bone turnover, including alkaline phosphatase.9
Fig. 3.
Cascade of alkaline phosphatase production in osteoblasts triggered by teriparitide.10
A previously feared complication of teriparatide was its potential carcinogenicity, which led to the implementation of a black box warning by the FDA in 2002. However, after further investigation and additional data showing no increased risk of osteosarcoma in humans, the FDA removed this black box warning in 2020. The other most prominent disease to consider in these patients is Paget Disease which itself can present with isolated elevations of alkaline phosphatase. Although this condition may present with bone pain, enlarged skull, and hearing loss, often times it is asymptomatic. Patients on teriparatide may have fractures due to their underlying osteoporosis, however this may also be a presentation of Paget’s disease due to the abnormal bone remodeling. For this reason, proper attention with nuclear bone scans should be given in patients with isolated elevations in alkaline phosphatase with a history of fractures. Finally, a multitude of other processes can present with elevations in alkaline phosphatase, however enzyme assays to differentiate the source combined with clinical presentation can facilitate differentiation (Table 1).
Table 1.
Causes of isolated raised alkaline phosphatase.
| Cause | Examples |
|---|---|
| Bone Diseases | Paget’s disease, Osteomalacia, Fractures, Bone metastases |
| Liver Diseases | Primary biliary cirrhosis, Cholestasis, Liver metastases |
| Pregnancy | Third trimester of pregnancy |
| Thyroid Disorders | Hyperthyroidism, Hypothyroidism |
| Kidney Diseases | Chronic kidney disease, Renal osteodystrophy |
| Certain Medications | Antiepileptics, Antipsychotics, Antibiotics |
| Other Causes | Sarcoidosis, Amyloidosis |
In this case, the decision not to discontinue teriparatide was based on the patient’s clinical context. She was previously on Boniva (ibandronate) for five years for osteoporosis, which she stopped in January 2021 after improvement in bone density. Despite this, she developed an L1 compression fracture, with a DEXA scan now showing a T-score of −4.6 in January 2023. Forteo, an anabolic agent, is known to be particularly effective in increasing bone density, especially in patients with severe osteoporosis who have not responded adequately to previous bisphosphonate therapy.4 After treatment with Forteo a repeat DEXA scan in January 2024 showed substantial improvement in her hip and lumbar spine, with T-score improving into the osteopenia range of −1.7. Given this positive response, it was evident that Forteo was crucial for her bone health. With the significant risks associated with undertreating severe osteoporosis, including the potential for further fractures and worsening bone density, the benefits of continuing Forteo outweighed the risk, especially considering her alkaline phosphatase levels, although elevated, remained stable and did not pose immediate harm, allowing for continued monitoring without interrupting therapy.
4. Conclusion
Our case report indicates that teriparatide may elicit a small rise in alkaline phosphatase, although this effect appears inconsequential and produced no worrisome symptoms. Future studies are needed to clarify whether enzyme levels recover to normal range after treatment and to monitor for pathological downstream consequences in patients experiencing this enzyme elevation in treatment. It is important to entertain a broad differential for isolated elevations of this enzyme in those treated with teriparatide. Overall, teriparatide remains a widely used anabolic agent for osteoporosis due to its efficacy in improving bone density and reducing fracture rates, and favorable adverse effect profile.
Footnotes
Ethical section: This case report did not require formal ethical approval as it does not involve any experimental interventions or research involving human subjects beyond standard clinical care. The patient described in this report provided informed consent for the publication of her clinical information and imaging data. All identifying information has been anonymized to protect the patient’s privacy in accordance with applicable guidelines and regulations.
Conflicts of interest: All authors declare that they have no conflicts of interest.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
References
- 1. Watts Nelson B, et al. American association of clinical endocrinologists/American college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2020 update. Endocr Pract. 2021;27(4):379–380. doi: 10.1016/j.eprac.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 2. Kendler David L, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391(10117):230–240. doi: 10.1016/s0140-6736(17)32137-2. [DOI] [PubMed] [Google Scholar]
- 3.Food and Drug Administration. Forteo - Food and Drug Administration. Food and Drug Adminstration (.Gov); Aug, 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2020/021318s053lbl.pdf . [Google Scholar]
- 4.Harold Rosen. In: Overview of the management of osteoporosis in postmenopausal women. Clifford Rosen, Kenneth Schmader., editors. UpToDate; 2023. www.uptodate.com/contents/teriparatide-including-biosimilars-available-in-canada-drug-information?search=osteoporosis&topicRef=2064&source=see_link#F15255018 . [Google Scholar]
- 5. Harald Dobnig, et al. Early changes in biochemical markers of bone formation correlate with improvements in bone structure during teriparatide therapy. J Clin Endocrinol Metabol. 2005;90(7):3970–3977. doi: 10.1210/jc.2003-1703. [DOI] [PubMed] [Google Scholar]
- 6. Javinani Ali, et al. Extremely elevated serum alkaline phosphatase level upon treatment with teriparatide: a case report. J Med Case Rep. 2020;14(1) doi: 10.1186/s13256-020-02416-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Siddiqui Jawed A, Partridge Nicola C. Physiological bone remodeling: systemic regulation and growth factor involvement. Physiology. 2016;31(3):233–245. doi: 10.1152/physiol.00061.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yik Lim Sian, Marcy Bolster. Profile of romosozumab and its potential in the management of osteoporosis. Drug Des Dev Ther. 2017;11:1221–1231. doi: 10.2147/dddt.s127568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vaisman Diego N, et al. Bone-specific alkaline phosphatase activity is inhibited by bisphosphonates: role of divalent cations. Biol Trace Elem Res. 2005;104(2):131–140. doi: 10.1385/bter:104:2:131. [DOI] [PubMed] [Google Scholar]
- 10. Hasan N. Cascade of alkaline phosphatase production in osteoblasts triggered by teriparitide Created with BioRendercom. 2024 [Google Scholar]