Abstract
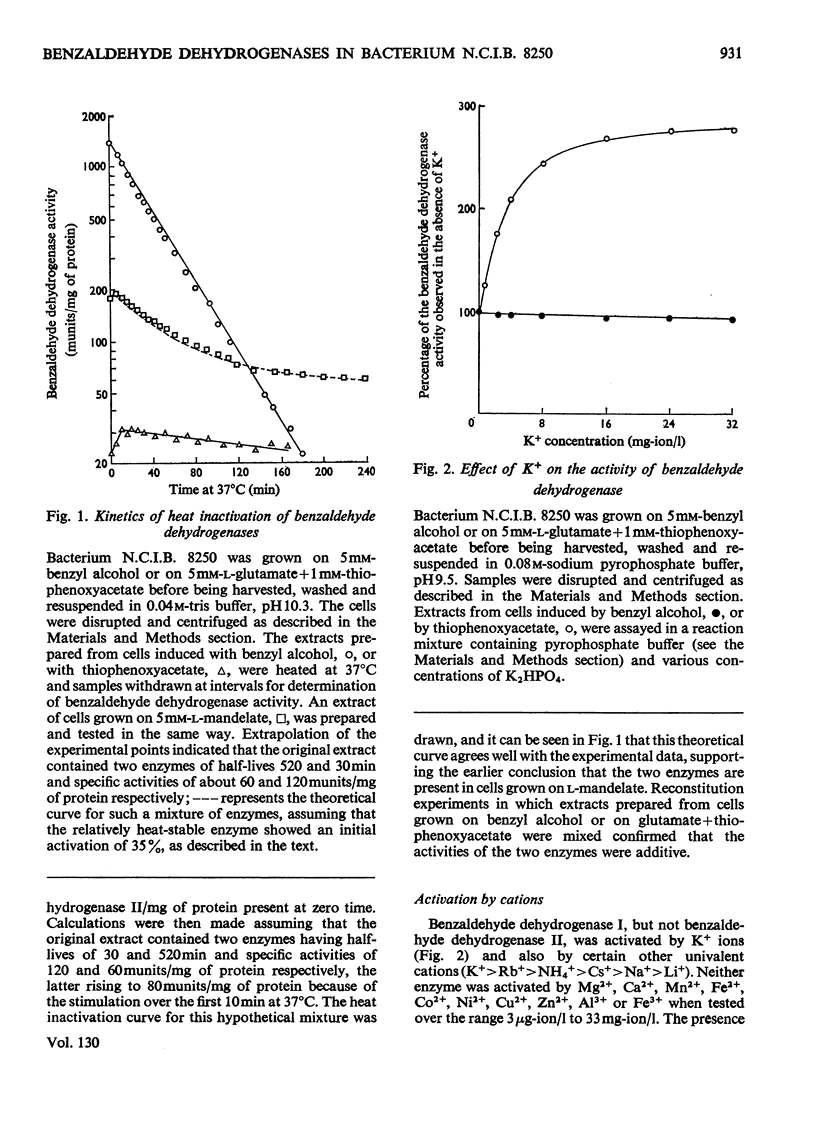
Evidence is presented for the existence in bacterium N.C.I.B. 8250 of two inducible NAD+-linked benzaldehyde dehydrogenases. They may be distinguished in crude extracts by their different thermal stabilities at high pH values, benzaldehyde dehydrogenase I being much more heat-stable than benzaldehyde dehydrogenase II. Only benzaldehyde dehydrogenase I is activated by K+ and certain other univalent cations. Gel-filtration experiments indicate that both enzymes have molecular weights of about 180000. Both enzymes are induced by growth on l-mandelate or phenylglyoxylate; only benzaldehyde dehydrogenase I is gratuitously induced by thiophenoxyacetate and only benzaldehyde dehydrogenase II is induced by benzyl alcohol, by benzaldehyde, and by a number of heterocyclic compounds which do not support growth. Mutants have been isolated that lack either benzaldehyde dehydrogenase II or benzyl alcohol dehydrogenase, or both of the enzymes. Results obtained in induction experiments with the wild-type bacterium N.C.I.B. 8250 and with the mutants show that benzaldehyde dehydrogenase II and benzyl alcohol dehydrogenase are co-ordinately regulated. Overall, the results suggest that benzaldehyde dehydrogenase I is associated with the metabolism of l-mandelate whereas benzaldehyde dehydrogenase II is associated with the metabolism of benzyl alcohol.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELES R. H., LEE H. A., Jr The dismutation of formaldehyde by liver alcohol dehydrogenase. J Biol Chem. 1960 May;235:1499–1503. [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cánovas J. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 1. General aspects. Eur J Biochem. 1967 May;1(3):289–300. doi: 10.1007/978-3-662-25813-2_40. [DOI] [PubMed] [Google Scholar]
- Fewson C. A. The growth and metabolic versatility of the gram-negative Bacterium NCIB 8250 ("Vibrio 01"). J Gen Microbiol. 1967 Feb;46(2):255–266. doi: 10.1099/00221287-46-2-255. [DOI] [PubMed] [Google Scholar]
- GUNSALUS C. F., STANIER R. Y., GUNSALUS I. C. The enzymatic conversion of mandelic acid to benzoic acid. III. Fractionation and properties of the soluble enzymes. J Bacteriol. 1953 Nov;66(5):548–553. doi: 10.1128/jb.66.5.548-553.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey N. L., Fewson C. A., Holms W. H. Apparatus for batch culture of micro-organisms. Lab Pract. 1968 Oct;17(10):1134–1136. [PubMed] [Google Scholar]
- Hegeman G. D., Rosenberg S. L. The evolution of bacterial enzyme systems. Annu Rev Microbiol. 1970;24:429–462. doi: 10.1146/annurev.mi.24.100170.002241. [DOI] [PubMed] [Google Scholar]
- Holms W. H., Bennett P. M. Regulation of isocitrate dehydrogenase activity in Escherichia coli on adaptation to acetate. J Gen Microbiol. 1971 Jan;65(1):57–68. doi: 10.1099/00221287-65-1-57. [DOI] [PubMed] [Google Scholar]
- Katagiri M., Wheelis M. L. Comparison of the two isofunctional enol-lactone hydrolases from Acinetobacter calcoaceticus. J Bacteriol. 1971 May;106(2):369–374. doi: 10.1128/jb.106.2.369-374.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Enzymes of the mandelate pathway in Bacterium N.C.I.B. 8250. Biochem J. 1968 Apr;107(4):497–506. doi: 10.1042/bj1070497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Metabolism of mandelate and related compounds by bacterium NCIB 8250. J Gen Microbiol. 1968 Sep;53(2):259–273. doi: 10.1099/00221287-53-2-259. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS E. B. Pseudoallelism and gene evolution. Cold Spring Harb Symp Quant Biol. 1951;16:159–174. doi: 10.1101/sqb.1951.016.01.014. [DOI] [PubMed] [Google Scholar]
- Livingstone A., Fewson C. A. Regulation of the enzymes converting L-mandelate into benzoate in bacterium N.C.I.B. 8250. Biochem J. 1972 Dec;130(4):937–946. doi: 10.1042/bj1300937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEISTER A. Reduction of alpha gamma-diketo and alpha-keto acids catalyzed by muscle preparations and by crystalline lactic dehydrogenase. J Biol Chem. 1950 May;184(1):117–129. [PubMed] [Google Scholar]
- MONOD J., PAPPENHEIMER A. M., Jr, COHEN-BAZIRE G. La cinétique de la biosynthèse de la beta-galactosidase chez E. coli considérée comme fonction de la croissance. Biochim Biophys Acta. 1952 Dec;9(6):648–660. doi: 10.1016/0006-3002(52)90227-8. [DOI] [PubMed] [Google Scholar]
- Markert C. L. Lactate Dehydrogenase Isozymes: Dissociation and Recombination of Subunits. Science. 1963 Jun 21;140(3573):1329–1330. doi: 10.1126/science.140.3573.1329. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. Regulation of catabolic pathways in Pseudomonas. Bacteriol Rev. 1971 Jun;35(2):87–116. doi: 10.1128/br.35.2.87-116.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. II. Enzymes of the protocatechuate pathway. J Biol Chem. 1966 Aug 25;241(16):3787–3794. [PubMed] [Google Scholar]
- ROSETT T. COOLING DEVICE FOR USE WITH A SONIC OSCILLATOR. Appl Microbiol. 1965 Mar;13:254–256. doi: 10.1128/am.13.2.254-256.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. L. Regulation of the mandelate pathway in Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1257–1269. doi: 10.1128/jb.108.3.1257-1269.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachow C. S., Stevenson I. L., Day D. Purification and properties of nicotinamide adenine dinucleotide phosphate-specific benzaldehyde dehydrogenase from Pseudomonas. J Biol Chem. 1967 Nov 25;242(22):5294–5300. [PubMed] [Google Scholar]
- Stanier R. Y. The Oxidation of Aromatic Compounds by Fluorescent Pseudomonads. J Bacteriol. 1948 Apr;55(4):477–494. doi: 10.1128/jb.55.4.477-494.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson I. L., Mandelstam J. Induction and multi-sensitive end-product repression in two converging pathways degrading aromatic substances in Pseudomonas fluorescens. Biochem J. 1965 Aug;96(2):354–362. doi: 10.1042/bj0960354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhara K., Takemori S., Katagiri M. The purification and properties of benzylalcohol dehydrogenase from Pseudomonas sp. Arch Biochem Biophys. 1969 Mar;130(1):422–429. doi: 10.1016/0003-9861(69)90054-x. [DOI] [PubMed] [Google Scholar]


