CONTINUING MEDICAL EDUCATION.
LEARNING OBJECTIVES
At the end of the activity, participants will be able to:
Initiate basal insulin therapy without unnecessary delays for patients with type 2 diabetes (T2D) for whom insulin treatment is appropriate.
Review the clinical efficacy and safety data for new and emerging ultra-long-acting, once-weekly insulins.
Compare and contrast the potential benefits and risks of once-weekly insulins compared with traditional basal insulins.
KEY TAKEAWAYS
Basal insulin remains an essential and effective glucose-lowering treatment for many patients with T2D.
Ultra-long-acting, once-weekly insulins may soon be approved and provide an additional option for basal insulin therapy.
Once-weekly insulins may improve adherence and persistence, increase flexibility in administration time, and reduce glycemic variability compared with once-daily basal insulins.
Potential concerns with once-weekly insulins may include challenges with dose calculations and concerns about hypoglycemia, which may be resolved as clinicians become more familiar with these insulin formulations.
TARGET AUDIENCE
Family physicians and clinicians who wish to gain increased knowledge and greater competency regarding primary care management of diabetes.
DISCLOSURES
As a continuing medical education provider accredited by the Accreditation Council for Continuing Medical Education (ACCME), Primary Care Education Consortium requires any individual in a position to influence educational content to disclose any financial interest or other personal relationship with any commercial interest. This includes any entity producing, marketing, reselling, or distributing health care goods or services consumed by, or used on, patients. Mechanisms are in place to identify and mitigate any potential conflict of interest prior to the start of the activity. All relevant financial relationships have been mitigated. In addition, any discussion of off-label, experimental, or investigational use of drugs or devices will be disclosed by the faculty.
Dr. Shubrook serves as a consultant to Abbott Laboratories, Bayer, and Novo Nordisk, and is on the advisory boards of Eli Lilly, Madrigal Pharmaceuticals, and Nevro. Austin Ulrich has no disclosures to report.
SPONSORSHIP
This article is sponsored by Primary Care Education Consortium and the Primary Care Metabolic Group.
ACCREDITATION
The Primary Care Education Consortium is accredited by the ACCME to provide continuing medical education for physicians.
CREDIT DESIGNATION
Primary Care Education Consortium designates this enduring material for a maximum of 1.0 AMA PRA Category 1 credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
PAs AND NURSE PRACTITIONERS
AANP, ANCC, and AAPA accept certificates of participation from educational activities certified for AMA PRA Category 1 Credit™ from organizations accredited by ACCME.
CME is available from November 1, 2024 – October 31, 2025.
To receive credit: https://www.pcmg-us.org/survey/post/owiht2024

FACULTY
Jay H. Shubrook, DO, Professor and Diabetologist, Tuoro University California, College of Osteopathic Medicine, Vallejo, CA
ACKNOWLEDGMENT
Editorial support was provided by Austin Ulrich, PharmD, BCACP, Primary Care Education Consortium.
SUPPORTER
This article is supported by an educational grant from Novo Nordisk.
INTRODUCTION
Type 2 diabetes (T2D) is a chronic and progressive disease characterized by impaired blood glucose control. It is increasingly recognized as a serious public health concern globally.1 In the US, an estimated 14.7% of adults have diabetes; of these, up to 95% have T2D.1–3 Patients with T2D often experience significant morbidity due to microvascular and macrovascular complications resulting from elevated blood glucose, and they can also have diminished functional capacity, lower quality of life, and premature death.4–6
Primary care clinicians (PCCs) treat at least 90% of patients in the US with T2D and are often the first clinicians to diagnose the disease.7,8 While some patients with T2D may see an endocrinologist, projections indicate current and future shortages for this medical specialty. Thus, PCCs play a critical role in the management of patients with T2D, which often involves the use of basal insulin.8,9
THE ROLE OF BASAL INSULIN IN T2D
Despite the emergence of newer agents to treat T2D, insulin is still an essential and effective glucose-lowering treatment for many patients.10 Over the years, insulin therapy has evolved with corresponding advances in molecular biology, chemistry, and technologies for drug delivery. According to current American Diabetes Association guidelines, basal insulin should be considered as the first injectable therapy when a patient with T2D has significant (blood glucose ≥300 mg/dL or glycated hemoglobin [HbA1c] >10%) or symptomatic hyperglycemia or signs of catabolism due to glucotoxicity.11
Many patients with T2D do not achieve glycemic control, and few achieve simultaneous control of associated cardiovascular risk factors (glucose, blood pressure, and lipids). Data extrapolated from the National Health and Nutrition Examination Survey (NHANES) 2013–2016 showed that 55.8% of people with T2D were at their target HbA1c level, while only 17.3% reached control of the composite of HbA1c, blood pressure, and blood lipids.12 Inclusion of body mass index (BMI) targets lowered the finding to <10%.12 Analysis of NHANES data from 2005 to 2016 showed that the cascade of diabetes care, defined as the composite of diabetes diagnosis, linkage to care, and achievement of individual and combined treatment targets, did not significantly improve over time.13 Of US adults diagnosed with diabetes, 23% met therapeutic targets from 2005–2008, 25% met targets from 2009–2012, and 23% met targets from 2013–2016.13
Recent advances in insulin development have been largely focused on improving ease and convenience for the patient and greater stability in glucose readings. This has led to newer formulations of basal insulins, such as ultra-long-acting, once-weekly insulins, which are nearing US Food and Drug Administration (FDA) approval. Once-weekly basal insulins are predicted to increase treatment adherence, decrease clinical inertia, and improve patient quality of life, provided that potential risks are properly addressed.10
An estimated 7.4 million Americans with diabetes use one or more form of insulin to manage their condition.14 The goals of insulin therapy are to replicate as closely as possible a normal glycemic profile without unacceptable weight gain or hypoglycemia. For patients with T2D, initiating insulin therapy should start with basal insulin with a preference for basal insulin analogs.11 However, acceptability of insulin is still low among patients with T2D, leading to reluctance to initiate and continue insulin therapy.15 Moreover, a large proportion of people interrupt or discontinue treatment shortly after initiation.16,17 According to 1 analysis, only 20% of people initiating basal insulin continued with insulin treatment within the year after initiation.16
Clinical inertia in reaching T2D treatment goals
A retrospective cohort study of more than 80,000 patients showed that median time to treatment intensification with insulin was longer than 7 years for those who were not meeting glycemic goals on oral antihyperglycemic medications alone.18 In another study, clinicians waited for an average of 9 years before insulin initiation, at which point the average HbA1c was 9.5% and diabetic complications had emerged. Even after insulin initiation, the clinicians did not intensify therapy adequately, and average HbA1c remained at 7.9% after 4 years.19
Clinical inertia is characterized by lack of treatment initiation or intensification resulting in failure to achieve glycemic goals and is a common reason for poor glycemic control.20 Unfortunately, clinical inertia is very prevalent in clinical settings in the US. One study showed that fewer than 50% of patients with T2D and a high HbA1c had their treatment appropriately intensified.21 Another study showed that clinical inertia was seen in more than 26% of patients who had an HbA1c of ≥7%, and more than 18% of patients with an HbA1c of ≥8%—with failure to intensify the medication regimen over a median 4.2 years of follow-up.22 Additionally, clinical inertia has resulted in inadequate glycemic control in 40% to 60% of patients with T2D.23,24 These findings are remarkable considering the focus of guidelines on the importance of glycemic control, indicating that increased attention is needed to achieve glycemic targets in patients with T2D.
CASE STUDY.
A 59-year-old woman with a 20-year history of T2D presents to her primary care clinic for a follow-up visit 3 months after starting basal insulin once daily, despite her hesitation to use an injectable medication. After about 1 month of titrating her basal insulin dose via twice-weekly phone appointments, she had reached a dose of 45 units of insulin glargine once daily (0.5 units/kg), with a fasting glucose level ranging from 130 mg/dL to 140 mg/dL over a week of measurements and no hypoglycemic episodes. Since then, she says that she has had a difficult time remembering to do her daily injections on her own. She also doesn’t like having to give herself an injection every day.
Her fasting blood glucose in clinic today is 195 mg/dL and her HbA1c is 9.6%, which is improved from her HbA1c of 10.3% measured 3 months ago. Her other antihyperglycemic medications include metformin 2000 mg daily and oral semaglutide 14 mg once daily. She states that she would like to try to work on her diet and exercise to get her HbA1c lower, toward her goal of <7%.
The patient in this case scenario originally responded well to basal insulin therapy, with fasting blood glucose values that indicated improvement in overall glucose control. However, after she was no longer under close follow-up, she began to miss doses and is at risk for clinical inertia, raising her chances of complications that can result from hyperglycemia. This patient may be a good candidate for receiving a once-weekly basal insulin and more support to improve her adherence and overall glucose control.
NEW AND EMERGING ONCE-WEEKLY BASAL INSULINS
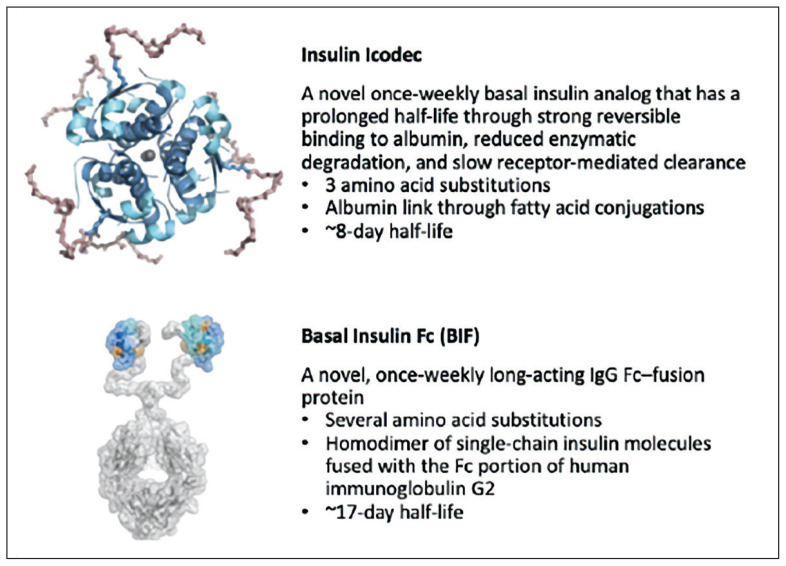
Innovative insulin formulations and delivery systems have resulted in an expansion of choices that include basal insulins, rapid-acting insulins, and intermediate-acting formulations.25 Now, once-weekly basal insulin formulations are in late-stage development, with insulin icodec receiving a recommendation for marketing approval in Europe and an FDA decision expected soon.26,27 Insulin icodec is a novel once-weekly basal insulin analog that has a prolonged half-life through strong reversible binding to albumin, reduced enzymatic degradation, and slow receptor-mediated clearance.28 Basal insulin Fc (BIF) is a novel, once-weekly, long-acting IgG Fc–fusion protein that is currently being assessed for diabetes treatment.29 While these once-weekly insulins are not yet clinically available, evidence supports their comparable efficacy and safety in patients with T2D10 (FIGURE 1).35
FIGURE 1.
Key considerations for new and emerging once-weekly insulins with late-stage trial data35
Source: Trevisan R, Conti M, Ciardullo S. Once-weekly insulins: a promising approach to reduce the treatment burden in people with diabetes. Diabetologia. Published online April 29, 2024. doi:10.1007/s00125-024-06158-9. Figure licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution, and reproduction. The license can be viewed at this link: https://creativecommons.org/licenses/by/4.0/legalcode
Both insulin icodec and BIF have been investigated in late-stage trials (TABLE 1), with overall positive results.30–35 Results of the phase 3 ONWARDS trials evaluating insulin icodec are summarized in TABLE 2.30–36 As a whole, the ONWARDS trials showed noninferiority of insulin icodec compared to basal insulin analogs (insulin glargine and insulin degludec) for HbA1c reduction.30–34 In ONWARDS 2 and ONWARDS 5, insulin icodec also demonstrated superiority compared to other basal insulins in reducing HbA1c from baseline.31,34 In ONWARDS 1 and ONWARDS 3, more patients receiving insulin icodec (10% more and 15% more, respectively) achieved target HbA1c without significant hypoglycemia.30,32 In all ONWARDS trials, the rates of hypoglycemia were similar between insulin icodec and other basal insulins.
TABLE 1.
Phase 3 trials evaluating the once-weekly insulins icodec and BIF in patients with T2D35
| Trial | Design | Patients with T2D | Comparator | Baseline treatment | Duration, wks |
|---|---|---|---|---|---|
| Insulin icodec | |||||
| ONWARDS 130 | Open label | Insulin-naïve N=984 |
Glargine U100 | Any noninsulin drugs | 78 |
| ONWARDS 231 | Open label | Insulin-treated N=526 |
Degludec | Basal insulins ± noninsulin glucose-lowering agents | 26 |
| ONWARDS 332 | Double blind | Insulin-naïve N=588 |
Degludec | Any noninsulin drugs | 26 |
| ONWARDS 433 | Open label | Insulin-treated N=582 |
Glargine U100 | Multiple daily insulin injections ± noninsulin drugs | 26 |
| ONWARDS 534 | Open label | Insulin-naïve N=1085 |
Glargine U100/300 and degludec | Any noninsulin drugs | 52 |
| BIF | |||||
| QWINT-1 NCT05662332 |
Open label | Insulin-naïve N=670 |
Glargine U100 | At least 1 glucose-lowering medication | 52 |
| QWINT-2 NCT05362058 |
Open label | Insulin-naïve N=912 |
Degludec | At least 1 glucose-lowering medication | 52 |
| QWINT-3 NCT05275400 |
Open label | Insulin-treated N=986 |
Degludec | Basal insulins ± up to 3 noninsulin drugs (except SUs) | 78 |
| QWINT-4 NCT05462756 |
Open label | Insulin-treated N=670 |
Glargine U100 | Multiple daily insulin injections | 26 |
Abbreviation: BIF, basal insulin Fc, T2D, type 2 diabetes.
Adapted from Trevisan R, Conti M, Ciardullo S. Once-weekly insulins: a promising approach to reduce the treatment burden in people with diabetes. Diabetologia. Published online April 29, 2024. doi:10.1007/s00125-024-06158-9
CC License: Figure licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution, and reproduction. The license can be viewed at this link: https://creativecommons.org/licenses/by/4.0/legalcode
TABLE 2.
Key results and hypoglycemic events of the phase 3 ONWARDS trials evaluating insulin icodec in patients with T2D30–36
| Trial | Main results | Hypoglycemic events |
|---|---|---|
| ONWARDS 130 | Icodec compared to glargine:
|
Icodec: 226 episodes in 61 patients (12.4%); 1 severe episode Glargine: 114 episodes in 66 patients (13.4%); 7 severe episodes |
| ONWARDS 231 | Icodec demonstrated noninferiority and superiority to degludec in reducing HbA1c from baseline | No significant differences in hypoglycemia rates |
| ONWARDS 332 | Icodec compared to degludec:
|
Icodec: 53 episodes in 26 patients (9%); 0 severe episode Degludec: 23 episodes in 17 patients (6%); 2 severe episodes |
| ONWARDS 433 | Icodec compared to glargine:
|
Icodec: 35 episodes in 22 patients (8%) Glargine: 33 episodes in 25 patients (9%) |
| ONWARDS 534 | Icodec in conjunction with the dosing guide app demonstrated noninferiority and superiority compared with basal insulin analogs in reducing mean HbA1c from baseline | No significant differences in hypoglycemia rates |
Abbreviations: HbA1c, glycated hemoglobin; TIR, time in range.
As of the time of this publication, all phase 3 QWINT trials evaluating BIF are still ongoing, with no results reported. As clinical data continue to emerge for once-weekly insulins, their potential role in clinical practice will be further clarified.
LOOKING TO THE FUTURE: ONCE-WEEKLY INSULINS IN CLINICAL PRACTICE
As once-weekly insulin formulations become available, PCCs will be at the forefront of providing practical strategies for the integration of these formulations into clinical practice. Indications for once-weekly insulin are likely to be similar to those for once-daily insulin, but treatment adherence and quality of life may be important considerations, especially for patients who frequently miss doses of antihyperglycemic medications. New insulin titration strategies will be needed because glucose-lowering will not achieve a steady state for several weeks after initial dosing. Clinicians will need to learn these approaches and educate patients on how to manage dosing and concomitant preprandial insulin.10 Though these strategies are not yet well defined, they will become clearer in the coming years with the potential approval of once-weekly insulin products in the US.
Potential candidates for receiving once-weekly insulins, if approved, include patients with T2D who are inadequately controlled on multiple glucose-lowering agents and require basal insulin therapy.37 Patients who prefer flexibility in dose timing and those who have difficulty with adherence to daily injections may also be good candidates, as they may benefit from a reduced injection burden and attenuated consequences of missing a dose.37
Compared to once-daily basal insulins, once-weekly basal insulins have a variety of potential benefits, including improved adherence and persistence, flexibility in time of administration, and reduced glycemic variability (FIGURE 2).37 Potential concerns include lack of familiarity, challenges with dose calculations, and hypoglycemia.37
FIGURE 2.
Comparison of once-daily and once-weekly basal insulins37
Abbreviations: IDeg, insulin degludec; IDet, insulin detemir; IGlar, insulin glargine; NPH, neutral protamine Hagedorn.
Figure licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution, and reproduction. The license can be viewed at this link: https://creativecommons.org/licenses/by/4.0/legalcode
Potential benefits of once-weekly insulins
T2D regimens are complex for a large proportion of affected individuals and include dietary management, physical exercise, receiving multiple antihyperglycemic medications, and blood glucose monitoring. Injection burden is a major barrier to insulin adherence as prescribed and results in one-third of those prescribed insulin not being adherent or persistent in treatment.38 Fewer injections may reduce this burden and improve the likelihood of treatment adherence.
Once-weekly insulins may provide an option to improve convenience, adherence, and quality of life as compared to once-daily basal insulins.28 Once-weekly insulins may provide more flexibility in dose timing and provide better glucose coverage in the case of missed doses.37 When once-weekly insulins reach steady state, a missed dose does not result in immediate loss of efficacy due to the agent’s long half-life. Additionally, due to the flatter pharmacokinetic profile of once-weekly insulins, a decrease in day-to-day glycemic variability is expected.
Potential concerns about once-weekly insulins
Because clinicians are less familiar with once-weekly insulins, they may have concerns such as worry over a “large dose” of insulin injected at once and hypoglycemia management.37 As such, there is a need for education about the pharmacokinetics of weekly insulins. As with any insulin, a potential safety concern with once-weekly insulins is the risk for hypoglycemia. A meta-analysis of 7 randomized trials found an increased risk for hypoglycemic events with insulin icodec compared to once-daily basal insulins (risk ratio 1.24; 95% CI, 1.02–1.50; P = .03) and a numerically decreased risk for severe hypoglycemia (risk ratio 0.81; 95% CI, 0.31–2.08).39
To manage hypoglycemia that occurs while a patient is receiving once-weekly insulin, the fundamental principles are similar to treating typical hypoglycemia episodes, as insulin icodec has a similar counter-regulatory hormone response and recovery compared with insulin glargine.37 Principles for treating hypoglycemia in patients with T2D typically include advising the patient to consume 15 g of glucose (or other fast-acting carbohydrate) for a blood glucose value of ≤70 mg/dL and recheck the blood glucose 15 minutes afterward.40 If blood glucose remains at or near 70 mg/dL (or less), or if glucose is not rising, an additional 15 g of fast-acting carbohydrates should be consumed, repeating the process until glucose rises. In the case of continual ongoing hypoglycemia, the patient should seek additional care.
Patient case revisited
Revisiting the case scenario, use of a once-weekly insulin would likely help the patient be more adherent to her regimen, because she prefers to avoid daily injections. With improved adherence to her basal insulin regimen, her blood glucose would likely improve, lowering her risk for cardiometabolic complications associated with hyperglycemia.
Advice when talking to patients:
Assess interest in once-weekly insulin injections
Discuss the timing action curves for ultra-long-acting insulin
Remind patients that the dose given will have a long 7-day time of action
Base titrations on prescribing instructions and the patient's blood glucose values
CONCLUSION
PCCs manage most patients with T2D in the US, including many who receive or are appropriate candidates for basal insulin therapy. Despite insulin’s long history in treating diabetes, clinical inertia routinely occurs due to a variety of factors, resulting in treatment delays and suboptimal glucose management. New and emerging once-weekly insulins offer additional approaches for basal insulin therapy in T2D that may reduce clinical inertia and improve treatment adherence and patient outcomes.
REFERENCES
URLs must be entered manually, rather than copied and pasted.
- 1.Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.About type 2 diabetes. Centers for Disease Control and Prevention; [Accessed August 3, 2024]. Published May 14, 2024. https://www.cdc.gov/diabetes/about/about-type-2-diabetes.html . [Google Scholar]
- 3.National Diabetes Statistics Report. Centers for Disease Control and Prevention; [Accessed August 3, 2024]. Published May 15, 2024. https://www.cdc.gov/diabetes/php/data-research/index.html . [Google Scholar]
- 4.American Diabetes Association. Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(Suppl 1):S179–S218. doi: 10.2337/dc24-S010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramtahal R, Khan C, Maharaj-Khan K, et al. Prevalence of self-reported sleep duration and sleep habits in type 2 diabetes patients in South Trinidad. J Epidemiol Glob Health. 2015;5(4 Suppl 1):S35–43. doi: 10.1016/j.jegh.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Professional Practice Committee. 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(Suppl 1):S219–S230. doi: 10.2337/dc24-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson JA. The increasing role of primary care physicians in caring for patients with type 2 diabetes mellitus. Mayo Clin Proc. 2010;85(12 Suppl):S3–4. doi: 10.4065/mcp.2010.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrivastav M, Gibson W, Shrivastav R, et al. Type 2 diabetes management in primary care: the role of retrospective, professional continuous glucose monitoring. Diabetes Spectr. 2018;31(3):279–287. doi: 10.2337/ds17-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vigersky RA, Fish L, Hogan P, et al. The clinical endocrinology workforce: current status and future projections of supply and demand. J Clin Endocrinol Metab. 2014;99(9):3112–3121. doi: 10.1210/jc.2014-2257. [DOI] [PubMed] [Google Scholar]
- 10.Rosenstock J, Del Prato S. Basal weekly insulins: the way of the future! Metabolism. 2022;126:154924. doi: 10.1016/j.metabol.2021.154924. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(Suppl 1):S158–S178. doi: 10.2337/dc24-S009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andary R, Fan W, Wong ND. Control of cardiovascular risk factors among US adults with type 2 diabetes with and without cardiovascular disease. Am J Cardiol. 2019;124(4):522–527. doi: 10.1016/j.amjcard.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 13.Kazemian P, Shebl FM, McCann N, Walensky RP, Wexler DJ. Evaluation of the cascade of diabetes care in the United States, 2005–2016. JAMA Intern Med. 2019;179(10):1376–1385. doi: 10.1001/jamainternmed.2019.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cefalu WT, Dawes DE, Gavlak G, et al. Insulin access and affordability working group: conclusions and recommendations. Diabetes Care. 2018;41(6):1299–1311. doi: 10.2337/dci18-0019. [DOI] [PubMed] [Google Scholar]
- 15.Brod M, Kongsø JH, Lessard S, Christensen TL. Psychological insulin resistance: patient beliefs and implications for diabetes management. Qual Life Res. 2009;18(1):23–32. doi: 10.1007/s11136-008-9419-1. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Nieves M, Kabul S, Desai U, et al. Basal insulin persistence, associated factors, and outcomes after treatment initiation among people with type 2 diabetes mellitus in the US. Curr Med Res Opin. 2016;32(4):669–680. doi: 10.1185/03007995.2015.1135789. [DOI] [PubMed] [Google Scholar]
- 17.Idris I, Gulati K, Perez-Nieves M, et al. Associated factors that influenced persistence with basal analog insulin therapy among people with type 2 diabetes: an exploratory analysis from a UK real-world sample. Prim Care Diabetes. 2019;13(2):106–112. doi: 10.1016/j.pcd.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36(11):3411–3417. doi: 10.2337/dc13-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris SB, Kapor J, Lank CN, Willan AR, Houston T. Clinical inertia in patients with T2DM requiring insulin in family practice. Can Fam Physician. 2010;56(12):e418–424. [PMC free article] [PubMed] [Google Scholar]
- 20.Khunti S, Khunti K, Seidu S. Therapeutic inertia in type 2 diabetes: prevalence, causes, consequences and methods to overcome inertia. Ther Adv Endocrinol Metab. 2019;10:2042018819844694. doi: 10.1177/2042018819844694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah BR, Hux JE, Laupacis A, Zinman B, van Walraven C. Clinical inertia in response to inadequate glycemic control: do specialists differ from primary care physicians? Diabetes Care. 2005;28(3):600–606. doi: 10.2337/diacare.28.3.600. [DOI] [PubMed] [Google Scholar]
- 22.Mata-Cases M, Franch-Nadal J, Real J, et al. Therapeutic inertia in patients treated with two or more antidiabetics in primary care: factors predicting intensification of treatment. Diabetes Obes Metab. 2018;20(1):103–112. doi: 10.1111/dom.13045. [DOI] [PubMed] [Google Scholar]
- 23.Blonde L, Aschner P, Bailey C, et al. Gaps and barriers in the control of blood glucose in people with type 2 diabetes. Diab Vasc Dis Res. 2017;14(3):172–183. doi: 10.1177/1479164116679775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pantalone KM, Misra-Hebert AD, Hobbs TM, et al. Clinical inertia in type 2 diabetes management: evidence from a large, real-world data set. Diabetes Care. 2018;41(7):e113–e114. doi: 10.2337/dc18-0116. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch IB, Juneja R, Beals JM, Antalis CJ, Wright EE. The evolution of insulin and how it informs therapy and treatment choices. Endocr Rev. 2020;41(5):733–755. doi: 10.1210/endrev/bnaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novo Nordisk ’s once-weekly basal insulin icodec recommended for marketing approval in Europe. Reuters; [Accessed March 27, 2024]. Published March 21, 2024. https://www.reuters.com/business/healthcare-pharmaceuticals/novo-nordisksonce-weekly-basal-insulin-icodec-recommended-marketing-approval-2024-03-21/ [Google Scholar]
- 27.Bundgaard C. FDA extends review time for weekly Novo Nordisk insulin. [Accessed March 27, 2024]. Published February 5, 2024. https://medwatch.com/News/Pharma___Biotech/article16817544.ece .
- 28.Bajaj HS, Bergenstal RM, Christoffersen A, et al. Switching to once-weekly insulin icodec versus once-daily insulin glargine U100 in type 2 diabetes inadequately controlled on daily basal insulin: a phase 2 randomized controlled trial. Diabetes Care. 2021;44(7):1586–1594. doi: 10.2337/dc20-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frias J, Chien J, Zhang Q, et al. Safety and efficacy of once-weekly basal insulin Fc in people with type 2 diabetes previously treated with basal insulin: a multicentre, open-label, randomised, phase 2 study. Lancet Diabetes Endocrinol. 2023;11(3)(22):158–168. 00388–6. doi: 10.1016/S2213-8587. [DOI] [PubMed] [Google Scholar]
- 30.Rosenstock J, Bain SC, Gowda A, et al. Weekly icodec versus daily glargine U100 in type 2 diabetes without previous insulin. N Engl J Med. 2023;389(4):297–308. doi: 10.1056/NEJMoa2303208. [DOI] [PubMed] [Google Scholar]
- 31.Philis-Tsimikas A, Asong M, Franek E, et al. Switching to once-weekly insulin icodec versus once-daily insulin degludec in individuals with basal insulin-treated type 2 diabetes (ONWARDS 2): a phase 3a, randomised, open label, multicentre, treat-to-target trial. Lancet Diabetes Endocrinol. 2023;11(6)(23):414–425. 00093–1. doi: 10.1016/S2213-8587. [DOI] [PubMed] [Google Scholar]
- 32.Lingvay I, Asong M, Desouza C, et al. Once-weekly insulin icodec vs once-daily insulin degludec in adults with insulin-naive type 2 diabetes: the ONWARDS 3 randomized clinical trial. JAMA. 2023;330(3):228. doi: 10.1001/jama.2023.11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathieu C, Ásbjörnsdóttir B, Bajaj HS, et al. Switching to once-weekly insulin icodec versus once-daily insulin glargine U100 in individuals with basal-bolus insulin-treated type 2 diabetes (ONWARDS 4): a phase 3a, randomised, open-label, multicentre, treat-to-target, non-inferiority trial. Lancet. 2023;401(10392)(23):1929–1940. 00520–2. doi: 10.1016/S0140-6736. [DOI] [PubMed] [Google Scholar]
- 34.Bajaj HS, Aberle J, Davies M, et al. Once-weekly insulin icodec with dosing guide app versus once-daily basal insulin analogues in insulin-naive type 2 diabetes (ONWARDS 5): a randomized trial. Ann Intern Med. 2023;176(11):1476–1485. doi: 10.7326/M23-1288. [DOI] [PubMed] [Google Scholar]
- 35.Trevisan R, Conti M, Ciardullo S. Once-weekly insulins: a promising approach to reduce the treatment burden in people with diabetes. Diabetologia. doi: 10.1007/s00125-024-06158-9. Published online April 29, 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Argano C, Priola L, Manno F, Corrao S. What is the role of basal weekly insulin in clinical practice? The state of the art. Biomedicines. 2024;12(4):900. doi: 10.3390/biomedicines12040900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenstock J, Juneja R, Beals JM, Moyers JS, Ilag L, McCrimmon RJ. The basis for weekly insulin therapy: evolving evidence with insulin icodec and insulin efsitora alfa. Endocr Rev. 2024;45(3):379–413. doi: 10.1210/endrev/bnad037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vijan S, Hayward RA, Ronis DL, Hofer TP. Brief report: the burden of diabetes therapy: implications for the design of effective patient-centered treatment regimens. J Gen Intern Med. 2005;20(5):479–482. doi: 10.1111/j.1525-1497.2005.0117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukhopadhyay P, Chatterjee P, Pandit K, Sanyal D, Ghosh S. Once-weekly insulin icodec as compared to once-daily basal insulins: a meta-analysis. Endocr Pract. 2024;30(2):128–134. doi: 10.1016/j.eprac.2023.11.004. [DOI] [PubMed] [Google Scholar]
- 40.American Diabetes Association Professional Practice Committee. 6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(Suppl 1):S111–S125. doi: 10.2337/dc24-S006. [DOI] [PMC free article] [PubMed] [Google Scholar]