Abstract
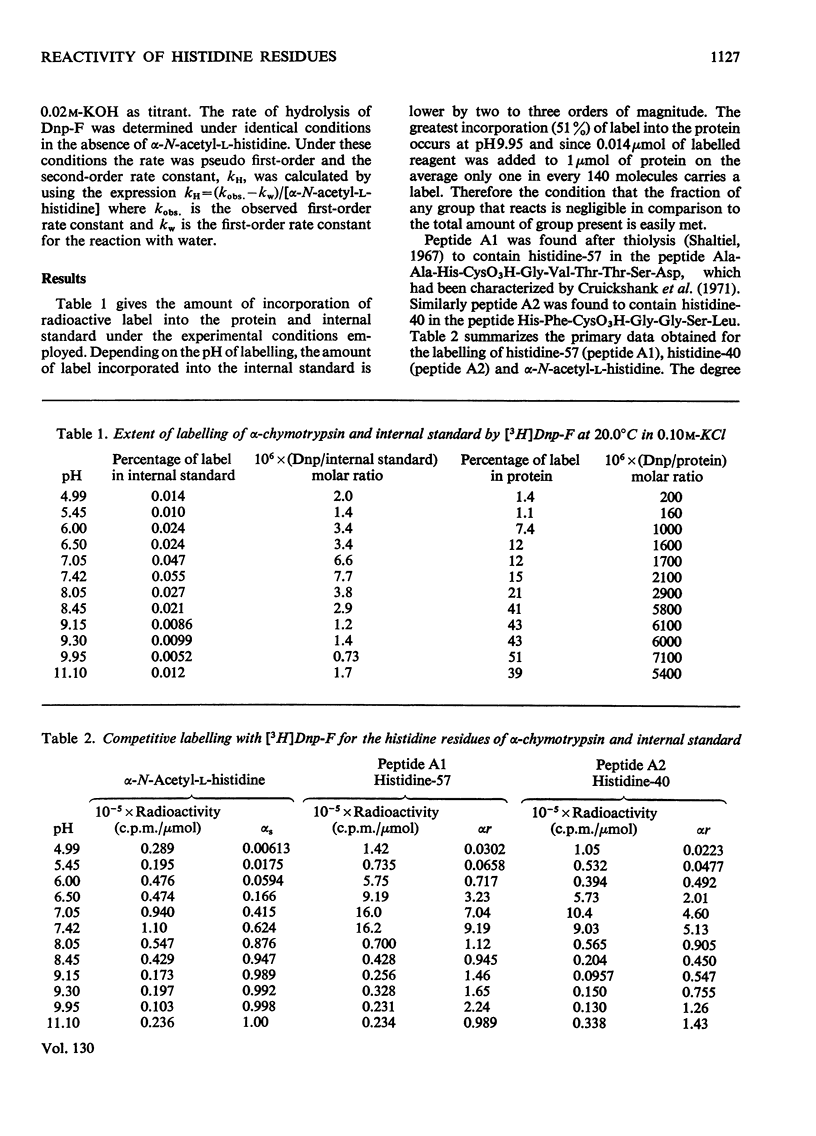
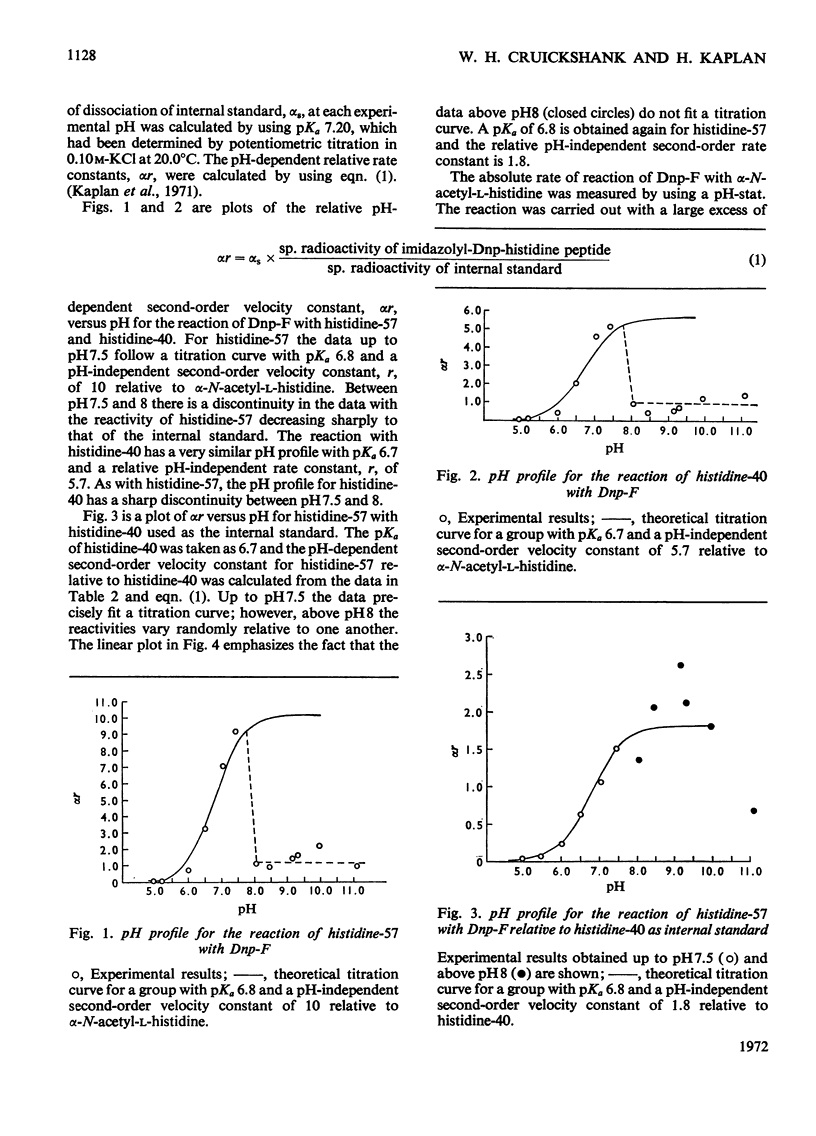
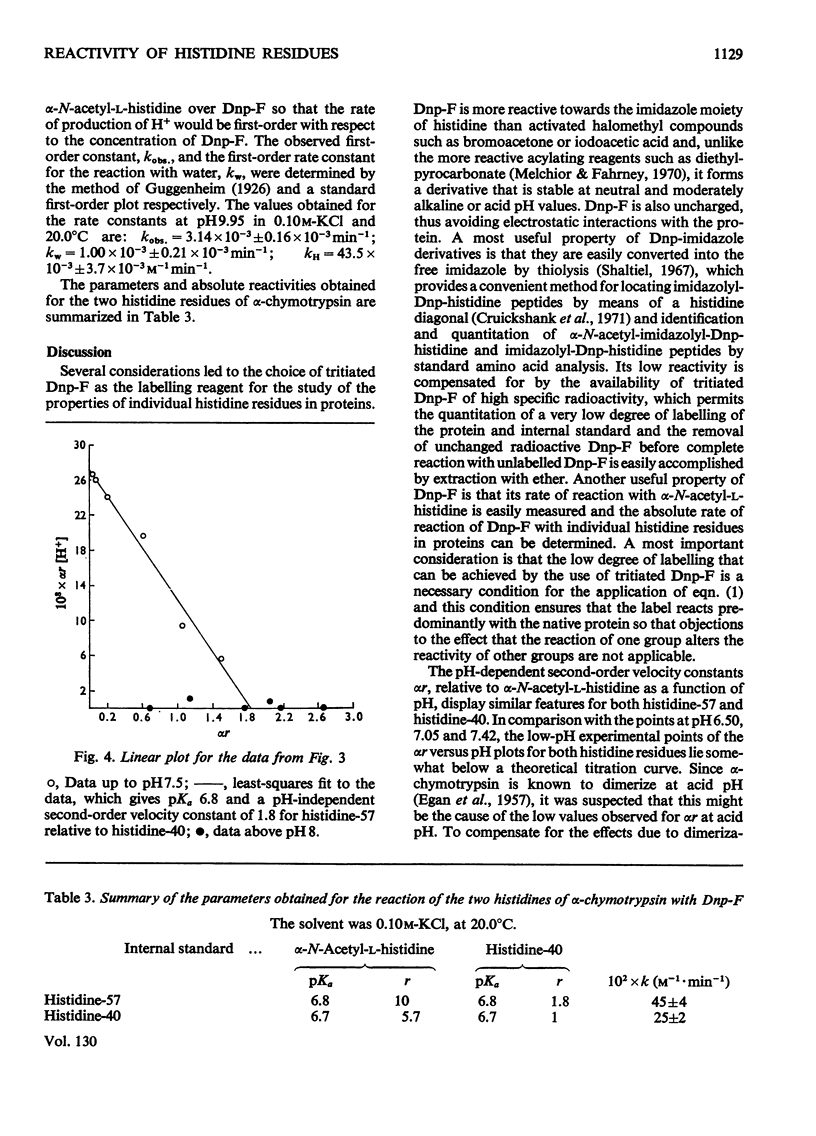
A competitive labelling method (Kaplan et al., 1971), using tritiated 1-fluoro-2,4-dinitrobenzene as the labelling reagent, is described for determining the ionization constants and reactivities of individual histidine residues in proteins. When this method was applied to the two histidines of α-chymotrypsin, histidine-57 was found to have pKa 6.8 and a reactivity ten times that of α-N-acetyl-l-histidine. Histidine-40 had pKa 6.7 and a reactivity approximately six times that of α-N-acetyl-l-histidine. Between pH7.5 and 8 the reactivities of both histidines decrease simultaneously to approximately that of α-N-acetyl-l-histidine. The high reactivities of the histidines are attributed to hydrogen bonding, which increases the nucleophilicity of the imidazole ring. The sharp decrease in reactivity between pH7.5 and 8 is attributed to a conformational change that disrupts the hydrogen bonding by these residues. The reactivity data support the proposal of a charge-relay mechanism involving histidine-57 (Blow et al., 1969), which makes serine-195 more nucleophilic but indicates that this system is fully operative only in the enzyme–substate complex.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDER M. L., KEZDY J. MECHANISM OF ACTION OF PROTEOLYTIC ENZYMES. Annu Rev Biochem. 1965;34:49–76. doi: 10.1146/annurev.bi.34.070165.000405. [DOI] [PubMed] [Google Scholar]
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- Bradbury J. H., Wilairat P. Proton magnetic resonance spectroscopy of histidine residues in proteins. Biochem Biophys Res Commun. 1967 Oct 11;29(1):84–89. doi: 10.1016/0006-291x(67)90545-1. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM L. W. Proposed mechanism of action of hydrolytic enzymes. Science. 1957 Jun 7;125(3258):1145–1146. doi: 10.1126/science.125.3258.1145. [DOI] [PubMed] [Google Scholar]
- Cruickshank W. H., Radhakrishnan T. M., Kaplan H. A diagonal paper electrophoretic method for the selective isolation of histidyl peptides. Can J Biochem. 1971 Nov;49(11):1225–1232. doi: 10.1139/o71-176. [DOI] [PubMed] [Google Scholar]
- Dent C. E. The amino-aciduria in Fanconi syndrome. A study making extensive use of techniques based on paper partition chromatography. Biochem J. 1947;41(2):240–253. doi: 10.1042/bj0410240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGAN R., MICHEL H. O., SCHLUETER R., JANDORF B. J. Physicochemical investigation of the chymotrypsins. II. On the mechanism of dimerization of chymotrypsin. Arch Biochem Biophys. 1957 Feb;66(2):366–373. doi: 10.1016/s0003-9861(57)80011-3. [DOI] [PubMed] [Google Scholar]
- Edsall J. T., Flory P. J., Kendrew J. C., Liquori A. M., Némethy G., Ramachandran G. N., Scheraga H. A. A proposal of standard conventions and nomenclature for the description of polypeptide conformations. J Mol Biol. 1966 Jan;15(1):399–407. doi: 10.1016/s0022-2836(66)80240-1. [DOI] [PubMed] [Google Scholar]
- Freer S. T., Kraut J., Robertus J. D., Wright H. T., Xuong N. H. Chymotrypsinogen: 2.5-angstrom crystal structure, comparison with alpha-chymotrypsin, and implications for zymogen activation. Biochemistry. 1970 Apr 28;9(9):1997–2009. doi: 10.1021/bi00811a022. [DOI] [PubMed] [Google Scholar]
- HAMMOND B. R., GUTFREUND H. Two steps in the reaction of chymotrypsin with acetyl-L-phenylalanine ethyl ester. Biochem J. 1955 Oct;61(2):187–189. doi: 10.1042/bj0610187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Hartley B. S. Homologies in serine proteinases. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):77–87. doi: 10.1098/rstb.1970.0010. [DOI] [PubMed] [Google Scholar]
- Henkart P. The chemistry and identification of im-dinitrophenyl histidine. J Biol Chem. 1971 Apr 25;246(8):2711–2713. [PubMed] [Google Scholar]
- Kaplan H. Properties of the isoleucyl amino-terminus of alpha-chymotrypsin. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1042–1049. doi: 10.1016/0006-291x(71)90009-x. [DOI] [PubMed] [Google Scholar]
- Kaplan H., Stevenson K. J., Hartley B. S. Competitive labelling, a method for determining the reactivity of individual groups in proteins. The amino groups of porcine elastase. Biochem J. 1971 Sep;124(2):289–299. doi: 10.1042/bj1240289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior W. B., Jr, Fahrney D. Ethoxyformylation of proteins. Reaction of ethoxyformic anhydride with alpha-chymotrypsin, pepsin, and pancreatic ribonuclease at pH 4. Biochemistry. 1970 Jan 20;9(2):251–258. doi: 10.1021/bi00804a010. [DOI] [PubMed] [Google Scholar]
- ONG E. B., SHAW E., SCHOELLMANN G. THE IDENTIFICATION OF THE HISTIDINE RESIDUE AT THE ACTIVE CENTER OF CHYMOTRYPSIN. J Biol Chem. 1965 Feb;240:694–698. [PubMed] [Google Scholar]
- Rajender S., Lumry R., Han M. Studies of the chymotrypsinogen family of proteins. XV. pH and temperature dependence of the -chymotryptic hydrolysis of N-acetyl-L-tryptophan ethyl ester. J Phys Chem. 1971 May 13;75(10):1375–1386. [PubMed] [Google Scholar]
- Shaltiel S. Thiolysis of some dinitrophenyl derivatives of amino acids. Biochem Biophys Res Commun. 1967 Oct 26;29(2):178–183. doi: 10.1016/0006-291x(67)90583-9. [DOI] [PubMed] [Google Scholar]
- Sigler P. B., Blow D. M., Matthews B. W., Henderson R. Structure of crystalline -chymotrypsin. II. A preliminary report including a hypothesis for the activation mechanism. J Mol Biol. 1968 Jul 14;35(1):143–164. doi: 10.1016/s0022-2836(68)80043-9. [DOI] [PubMed] [Google Scholar]
- Stevenson K. J., Smillie L. B. The inhibition of chymotrypsin A4 with a homologous series of chloromethyl ketone reagents. Can J Biochem. 1970 Mar;48(3):364–375. doi: 10.1139/o70-059. [DOI] [PubMed] [Google Scholar]


