Abstract
Background
After a change in Quebec's policy on drug coverage in August 1996, elderly patients' copayments for prescription drugs increased. We assessed the impact of this drug policy reform on prescribing patterns for essential cardiac medications, utilization of medical care and related health outcomes after acute myocardial infarction.
Methods
Patients at least 65 years of age who experienced acute myocardial infarction between 1994 and 1998 were identified through the Quebec discharge summary database. Drug claims databases were analyzed to determine rates of prescription of essential cardiac medications for cohorts of patients admitted before and after the policy reform. The impact on readmissions for cardiac-related complications, outpatient visits to physicians and emergency departments, and mortality rate was also assessed.
Results
The proportion of patients who received prescriptions for β-blockers, angiotensin-converting enzyme inhibitors and lipid-lowering drugs increased over time and, more specifically, did not appear to decline with the change in the drug policy. In addition, the policy reform did not appear to affect persistence of drug therapy (the proportion of time for which patients were covered by prescriptions over the year after discharge). There was no within-class shift from more to less expensive drugs. Use of cardiac procedures increased over time, but this increase was unrelated to the date of the policy reform. Finally, rates of readmission for complications, visits to individual physicians and to emergency departments, and mortality rate were unchanged. The findings did not vary with sex or socioeconomic status.
Interpretation
Prescriptions for essential cardiac medications and care related to acute myocardial infarction in elderly patients did not change with increases in out-of-pocket copayment, regardless of sex or socioeconomic status.
As the population ages, drug coverage for elderly patients has become an issue of increasing concern.1 Canada has had experience with drug coverage for elderly patients since the inception of universal health care in the late 1960s. Before August 1996, drug coverage in the province of Quebec was universal, with minimal or no copayment, for everyone 65 years of age or older and for welfare recipients. However, the government's goal to broaden coverage, combined with economic pressures, led to a change in policy whereby copayment was introduced for elderly people and welfare recipients who had previously had full coverage. In addition, people who cannot afford private insurance coverage for drug costs or who are not covered through employment can now obtain government insurance, if eligible on the basis of personal income.
Previous studies of the impact of cost-sharing for prescription drugs did not have information on the indications for the drugs studied.2 Our objective was to assess the impact of the change in Quebec's drug coverage on prescribing patterns for essential cardiac medications, utilization of medical care and related health outcomes after acute myocardial infarction.
Methods
Before Aug. 1, 1996, all prescription medications for elderly Quebec patients (65 years of age or older) were free, except for a $2 copayment per prescription (to a maximum of $100 per year). Low-income elderly patients (representing 5.7% of all elderly patients), as well as welfare recipients, had no copayments. Beginning Aug. 1, 1996, all elderly patients were required to pay a 25% coinsurance fee for prescription drugs. Annual ceilings for this copayment ($200, $500 or $750, depending on personal income) were also introduced. As of Jan. 1, 1997, an annual deductible was added to the program. The deductible varied from zero to $350 depending on personal income. Starting on July 1, 1997, the coinsurance and the deductible, which until then had been prorated quarterly, were prorated monthly to reduce the amount of any one payment. As a result, the maximum amount that a person pays for prescription drugs per month now ranges from $16.67 ($200 ceiling) to $62.50 ($750 ceiling).
This study was approved by the Research Ethics Board at the Montreal General Hospital, McGill University Health Centre. We identified patients 65 years of age or older who had been admitted to acute care hospitals in Quebec between Jan. 1, 1994, and Dec. 31, 1998, with a most responsible discharge diagnosis of acute myocardial infarction (code 410 of the International Classification of Diseases, 9th revision [ICD-9]).1 Patient data were obtained by linking the discharge database to physician and prescription claims databases by means of patients' encrypted Quebec medicare numbers. This linkage process has been previously validated.3 To increase the comparability of patients, we included only patients for whom the index diagnosis of myocardial infarction was the first such diagnosis since Jan. 1, 1988. Because we examined outpatient prescriptions, only patients who survived the initial admission were included in the analyses.
We used the provincial prescription claims database to determine prescription patterns for cardiac medications. This database accurately records drugs dispensed to elderly people.4 We investigated the use of β-blockers, angiotension-converting enzyme (ACE) inhibitors, lipid-lowering agents and acetylsalicylic acid (ASA) because these drugs have positive effects on death rates after myocardial infarction.5,6 We calculated the proportion of patients receiving prescriptions for any of these medications within 30 days after discharge. In addition, we calculated the persistence of drug therapy (the proportion of time for which a patient was covered by prescriptions over the year after discharge or until death if the patient died in the year after discharge) among patients for whom these drugs were prescribed within 30 days after discharge. Persistence (in days) was measured according to the claims database variable indicating duration of each prescription filled. Finally, we measured adherence of drug therapy at 1 year by determining the proportion of patients who filled at least one prescription during the period 305 to 365 days after discharge. This variable was calculated for patients who had received a discharge prescription and who were alive at 1 year after discharge from the initial admission.
We used the discharge database to identify admissions following the index myocardial infarction for diagnoses of recurrent myocardial infarction (ICD-9 code 410), unstable angina (ICD-9 codes 411 and 413) or congestive heart failure (ICD-9 code 428), all of which are potential complications of myocardial infarction that might have been affected by the change in drug policy. For each of these diagnoses, we ordered the related admissions chronologically. Patients who had been readmitted for elective procedures but had been assigned one of these admission diagnoses were excluded on the basis of previously defined criteria.7
To assess the vital status of the subjects, we used both the hospital discharge and physician claims databases. Vital status data were available for virtually all (99.8%) of the patients in our study. These data are based on several sources, including physician billing, in-hospital deaths, pensions and automobile insurance records.1
We also obtained data on outpatient visits to physicians and emergency departments from the physician claims database, which contains records for all inpatient and outpatient physician services, including diagnostic and therapeutic procedures. These outpatient services might be expected to increase as a result of the change in the drug coverage policy. The frequency of visits during the year after discharge was determined. All visits to emergency departments were included, irrespective of the reason for consultation. Outpatient physician visits included visits to family physicians, internists and cardiologists. However, only visits to medical offices, outpatient clinics and local community health centres were considered for the analyses.
For the statistical analysis, we constructed 6-month cohorts of patients admitted for a first acute myocardial infarction between Jan. 1, 1994, and Dec. 31, 1998. For each cohort, we calculated proportions of patients with discharge prescriptions, as well as persistence and adherence of drug therapy. These analyses were repeated for subgroups according to sex and socioeconomic status. The variables for socioeconomic status were created by linking patients' postal codes with data from the profile of forward sortation areas (FSAs) from the 1996 Census of Canada (each FSA corresponds to a census area, which is defined by the first 3 characters of the postal code). These variables were chosen because of their relevance to the key components of socioeconomic status (income, education and occupational status) and because previous studies have found them to be associated with health status.8,9 For our analyses, we created 4 approximately equal-sized socioeconomic status groups based on the quartile values for each variable. In addition, for each cohort, we calculated the cumulative incidence of readmissions for complications related to acute myocardial infarction and death within 30 days, 6 months and 1 year after discharge, as well as the frequency of outpatient visits to physicians and emergency departments over the first year after discharge.
Multivariate analyses were performed to confirm absence of confounding of changes over time. We performed 3 sets of logistic regression analyses for each medication studied, adjusting for several potential confounding variables: age, sex, comorbidities, length of stay, admission to a hospital with on-site cardiac catheterization facilities, specialty of the treating physician and socioeconomic status. In the first set, we estimated the association between admission for myocardial infarction after the date of the drug policy reform (Aug. 1, 1996) and receipt of a discharge prescription. In the second set, we investigated whether the change in drug policy led to changes in prescription rates even after adjustment for possible trends in cardiac drug prescriptions over time. In the third set, to assess whether the reform affected the pattern of changes in prescription rates over time, we examined the interaction between admission before and after the policy reform and trends over time. In all analyses, to account for multiple testing, we employed the Bonferroni correction, using an α level of 0.0125 to determine statistical significance.
Results
Between Jan. 1, 1994, and Dec. 31, 1998, a total of 23 698 patients 65 years of age or older were admitted to a Quebec hospital for a first acute myocardial infarction and were discharged alive. The following exclusion criteria were applied sequentially to these potential subjects: the main diagnosis of acute myocardial infarction was made after transfer from another hospital (346 patients); data were incomplete because of a change in medicare number (136 patients); the total length of stay was less than 3 days (since a short length of stay indicates that the diagnosis might not have been definitive) (323 patients); or the patient was discharged to a long-term care institution, a rehabilitation centre or an institution in another province (in this situation, prescription information could not be ascertained) (827 patients). The final study population was therefore 22 066 subjects, 93% of the original sample.
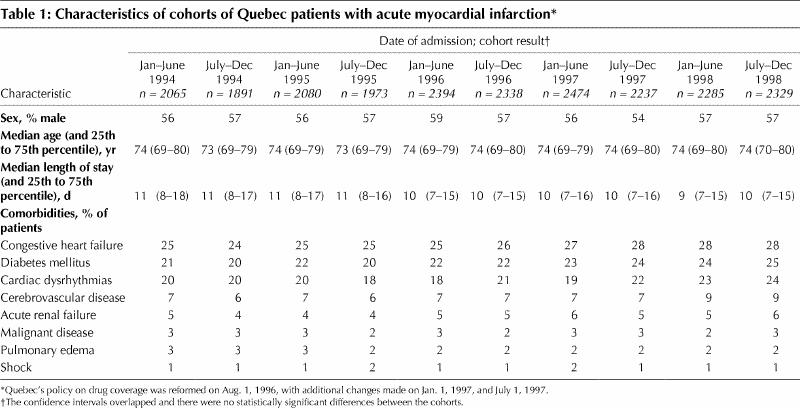
The patients were divided into 6-month cohorts on the basis of the date of the index myocardial infarction. Each cohort comprised about 2000 to 2300 patients. The demographic characteristics and comorbidities of patients who sustained a first myocardial infarction between Jan. 1, 1994, and July 31, 1996 (before the policy reform) did not differ from those of patients who experienced their first myocardial infarction on or after Aug. 1, 1996, the date of the first change in the drug coverage program (Table 1).
Table 1
Overall, there were no apparent declines related to the policy reform in the proportions of patients receiving prescriptions within 30 days after discharge for any of the cardiac medications that we studied (Table 2). Because most of the study patients filled their first prescriptions within 5 days after discharge, we believe that our analysis of prescriptions filled within 30 days after discharge captured most discharge prescriptions. However, after adjustment for potential confounding variables, we found that patients admitted after the policy reform were more likely to receive prescriptions for β-blockers (odds ratio [OR] 1.23, 95% confidence interval [CI] 1.16–1.30), ACE inhibitors (OR 1.26, 95% CI 1.19–1.33) and lipid-lowering agents (OR 2.57, 95% CI 2.38–2.78) and less likely to receive a prescription for ASA (OR 0.82, 95% CI 0.78–0.87). Rates of prescription for β-blockers, ACE inhibitors and lipid-lowering agents increased significantly over time (OR 1.07, 1.05 and 1.21 respectively; 95% CI 1.05–1.09, 1.03–1.07 and 1.18–1.24 respectively), whereas rates of prescription for ASA decreased significantly over time (OR 0.96, 95% CI 0.95–0.98). After adjustment for changing trends in prescription rates over time, there were no differences in prescription rates for ACE inhibitors, lipid-lowering agents or ASA for patients admitted before and after the policy reform (OR 0.98, 1.03 and 0.97 respectively; 95% CI 0.88–1.09, 0.89–1.19 and 0.87–1.09 respectively). It appeared that patients admitted after the policy reform were slightly less likely to receive a prescription for β-blockers (OR 0.89, 95% CI 0.80–0.99). However, this decrease was not statistically significant with a Bonferroni corrected α of 0.0125 (p = 0.03). Finally, in examining the interaction between admission before and after the policy reform and the time trends, we found that the rate of change in prescription rates for β-blockers and lipid-lowering agents was similar before and after the policy reform. Even though prescription rates for ACE inhibitors continued to increase over time, the rate of change in prescription rates for these agents was slightly lower after the reform. For ASA, the rate of decrease in prescriptions was slightly higher after the policy reform.
Table 2
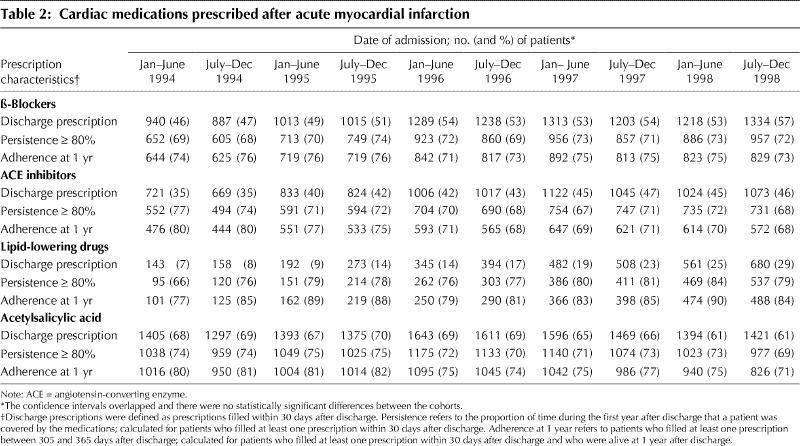
To investigate whether the policy reform affected long-term prescription rates for these medications in the year after myocardial infarction, we looked at medication persistence, the proportion of days within the first year after discharge that a patient was covered with a prescription for a particular drug type. Persistence for β-blockers, ACE inhibitors and ASA was at least 80% for about 70% of patients in each cohort (Table 2). Persistence for lipid- lowering drugs was at least 80% for a somewhat higher proportion of patients (about 80%). Again, the policy reform did not seem to have any effect on persistence for any of the agents examined.
Another effect of the policy reform might have been a shift from more expensive to less expensive drugs within a same class. However, we observed no such shift. For example, the most commonly used ACE inhibitors were enalapril, lisinopril, captopril and fosinopril. The proportion of patients on each of these agents did not change markedly around the time of the policy reform. The same was true for the other medications studied (data not shown).
To determine if the policy reform had more subtle impacts, we looked at the monthly variation in discharge prescriptions for patients who sustained acute myocardial infarction during a 2-month period before and within 1 month after the change. The mean monthly prescription rates were generally the same before and after the policy reform (Fig. 1), as were persistence (Fig. 1) and adherence (data not shown).
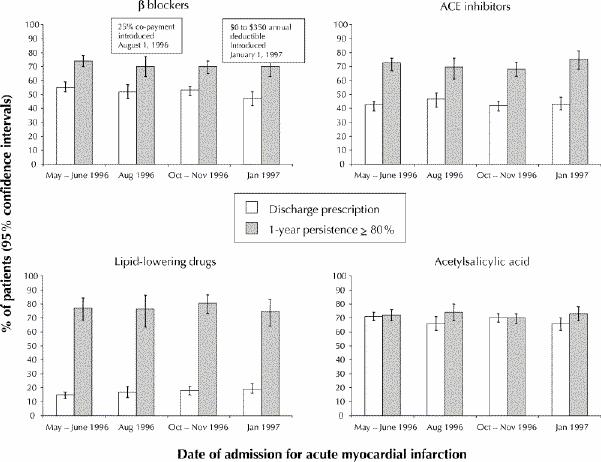
Fig. 1: Discharge prescriptions and persistence of drug therapy (proportion of time during the first year after discharge that a patient was covered by the medication in question), with 95% confidence intervals, for cardiac drugs prescribed for patients with acute myocardial infarction for a 2-month period before and a 1-month period after changes in Quebec's drug coverage policy. The major changes in the drug policy took place on Aug. 1, 1996, and Jan. 1, 1997. ACE = angiotensin-converting enzyme.
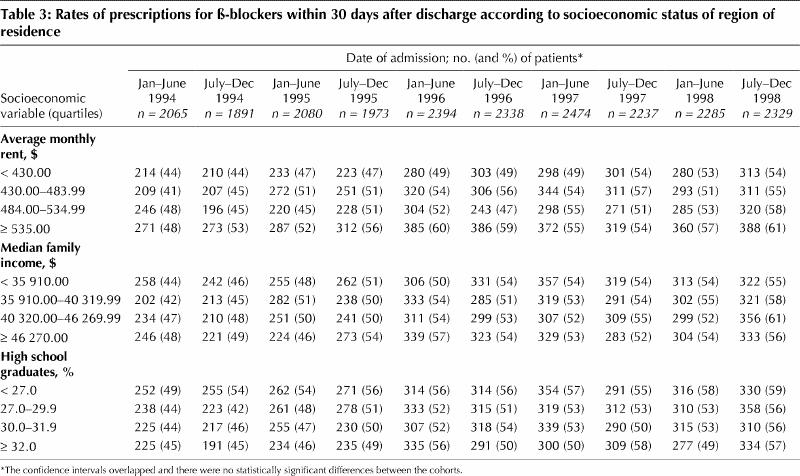
Finally, we investigated whether women and patients with low socioeconomic status were more affected by the policy reform. Within each subgroup, we found no differences in prescription patterns before and after the policy reform (results of analyses for β-blockers in Table 3; other data not shown).
Table 3
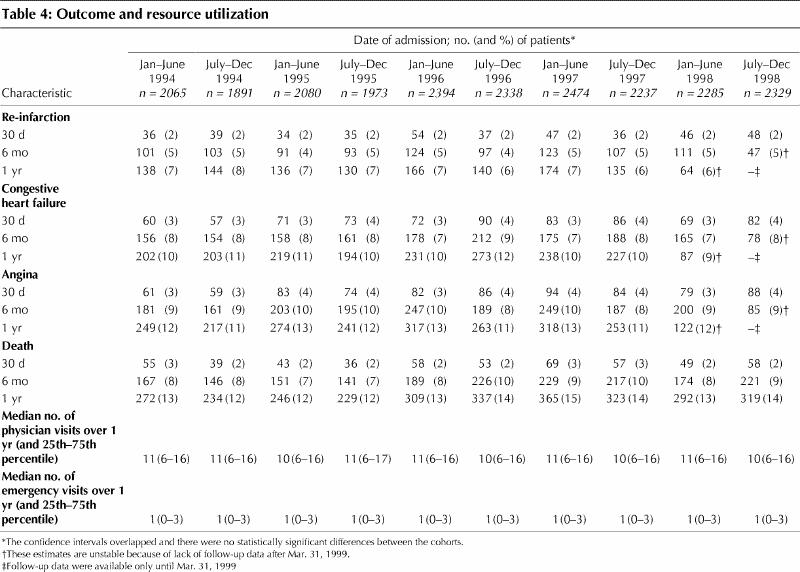
Cumulative incidence of readmission for cardiac complications and mortality rate did not change in association with the date of the policy reform (Table 4). Furthermore, we did not identify any change in the use of outpatient physician and emergency department services before and after the policy reform (Table 4).
Table 4
Interpretation
The major reform in drug coverage that took place in Quebec in 1996 allowed us to study the impact of cost-sharing on the care of elderly patients. We found that this policy reform had no effect on prescription rates, persistence of drug therapy, adherence of drug therapy, mortality rate, readmissions for complications related to acute myocardial infarction, or outpatient physician and emergency department visits. Furthermore, women and patients with low socioeconomic status did not appear to be differentially affected by the policy reform.
Most previous studies that have looked at the impact of cost-sharing for prescription drugs have reported a decline in the use of medications. The impact tended to be greater for the use of nonessential medications than for essential drugs.2,10,11,12,13,14,15,16,17 Tamblyn and associates16 recently examined the impact of the reform of Quebec drug policy on elderly patients and welfare recipients affected by the changes. They found that use of both essential and nonessential drugs decreased after the policy reform. In addition, they found that the decline in the use of essential drugs was associated with an increase in serious adverse events and visits to the emergency department. However, this and other studies have been limited by the inability to identify patients with a clear-cut medical condition in need of specific essential prescription drugs. Furthermore, because the focus of the study by Tamblyn and associates16 was on the change in the total number of medications over time in a fixed cohort, the authors had to account for potential variation in indications for medications, changes in clinical status and the tendency for an increase in mean number of medications with age. In addition, the relation between drug use and outcome in the previous studies has been an indirect one, since both variables have been measured at the population level, rather than the level of individual patients.
Why did we not see any effects? First, we studied essential cardiac medications prescribed for a recent serious medical condition. Patients in this situation might have felt compelled to fill any prescription given, whatever the cost. Second, the amount of out-of-pocket spending associated with these essential medications was modest. The extent of cost-sharing might have to be more substantial than that imposed by the Quebec policy reform to affect the use of essential drugs by patients with serious medical conditions. Third, the absence of a limit on drug benefits and the presence of a limit on out-of-pocket spending probably contributed to the lack of effect.
It is possible that other aspects of patients' well-being were affected by the reform, for example, the filling of prescriptions for nonessential medications. Furthermore, although patients' worries engendered by the complexity of drug cost-sharing are real, they can be measured only with difficulty, and we did not attempt to gather such data. Finally, it is possible that patients purchased large amounts of cardiac medications in anticipation of the policy reform. However, such stockpiling would not be expected, since patients could not have predicted that they would experience myocardial infarction around the time of the policy reform. Also, our observations of cohorts of patients before and after the policy reform and our measurements of persistence and adherence failed to show any evidence of pre-reform stockpiling.
Our study was limited by the absence of a control group. It is possible that the rate of increase in prescription rates for these medications would have been greater had the policy reform not taken place. Nevertheless, even if the increase in prescription rates was slower than it would have been without the changes, it did not translate into adverse health effects. Furthermore, we did not observe any increase in adverse health effects, although it is possible that a decline might have occurred had the policy reform not taken place. A decline in mortality rate after myocardial infarction has been previously described for this population.1 However, the decline has slowed in the past 5 years (i.e., after the policy reform). Another limitation is that our prescription data related only to drugs that were purchased, not drugs that were actually taken. However, it is likely that essential drugs that are purchased repetitively are actually taken, especially when copayment is required. Finally it is possible that the slow decline in ASA prescriptions was related to increased over-the-counter purchase of this drug.
In the continuous efforts to develop programs that will provide some form of drug coverage, a major policy question is defining the kind and level of prescription drug benefit that will allow necessary drug therapy while containing costs. The drug policy reform implemented in Quebec in 1996 is an example of a moderate plan that increased but still limited out-of-pocket expenses for elderly patients. This policy reform did not affect the use of essential cardiac drugs and was without any apparent adverse health consequences related to acute myocardial infarction.
Acknowledgments
We gratefully acknowledge Dr. Michal Abrahamowicz for statistical advice. This work was supported jointly by operating grants to the Canadian Cardiovascular Outcomes Research Team from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada. Christine Beck was supported by a Canadian Cardiovascular Outcomes Research Team PhD fellowship, funded by the Canadian Institutes for Health Research and the Heart and Stroke Foundation of Canada.
Footnotes
This article has been peer reviewed.
Contributors: Drs. Louise Pilote and Mark Eisenberg were responsible for designing the study and writing the manuscript. Christine Beck, Hugues Richard and Dr. Pilote were responsible for the data analyses and interpretation.
Dr. Pilote is a research scholar of the Canadian Institutes of Health Research. Dr. Eisenberg is a research scholar of the Fonds de la recherche en santé du Québec.
Competing interests: None declared.
Correspondence to: Dr. Louise Pilote, Division of Clinical Epidemiology, Montreal General Hospital, 1650 Cedar Ave., Montreal QC H3G 1A4; fax 514 934-8293; louise.pilote@mcgill.ca
References
- 1.Pilote L, Lavoie F, Ho V, Eisenberg MJ. Changes in the treatment of acute myocardial infarction in Quebec, 1988–1995. CMAJ 2000;163:31-6. Available: www.cmaj.ca/cgi/content/full/163/1/31. [PMC free article] [PubMed]
- 2.Stuart B, Zacker C. Who bears the burden of Medicaid drug copayment policies? Health Aff (Millwood) 1999;18:201-12. [DOI] [PubMed]
- 3.Levy RA, Tamblyn R, Fitchett D, McLeod P, Hanley JA. Coding accuracy of hospital discharge for elderly survivors of myocardial infarction. Can J Cardiol 1999;15:1277-82. [PubMed]
- 4.Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims databases in Quebec. J Clin Epidemiol 1995;48:999-1009. [DOI] [PubMed]
- 5.Ryan TJ, Anderson JL, Antman EM, Braniff BA, Brooks NH, Califf RM, et al. ACC/AHA guidelines for the management of patients with acute myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction). J Am Coll Cardiol 1996;28:1328-428. [DOI] [PubMed]
- 6.Ryan TJ, Antman EM, Brooks NH, Califf RM, Hillis LD, Hiratzka LF, et al. 1999 update: ACC/AHA guidelines for the management of patients with acute myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction). J Am Coll Cardiol 1999; 34:890-911. [DOI] [PubMed]
- 7.Rodrigues E, Simpson E, Richard H, Pilote L. Regional variation in the management of acute myocardial infarction in the province of Quebec. Can J Cardiol. In press. [PubMed]
- 8.Adler NE, Boyce T, Chesney MA, Folkman SSL. Socioeconomic inequalities in health. JAMA 1993;269:3140-5. [PubMed]
- 9.Adler NE, Boyce T, Chesney MA. Socioeconomic status and health. Am Psychol 1994;49:15-24. [DOI] [PubMed]
- 10.Fortess EE, Soumerai SB, Ross-Degnan D. Utilization of essential medications by vulnerable older people after a drug benefit cap: importance of mental disorders, chronic pain, and practice setting. J Am Geriatr Soc 2001; 49: 793-7. [DOI] [PubMed]
- 11.Soumerai SB, Ross-Degnan D, Avorn J, McLaughlin TJ, Choodnovskiy I. Effects of Medicaid drug-payment limits on admission to hospitals and nursing homes. N Engl J Med 1991;325:1072-7. [DOI] [PubMed]
- 12.Soumerai SB, McLaughlin TJ, Ross-Degnan D, Casteris CS, Bollini P. Effects of limiting Medicaid drug-reimbursement benefits on the use of psychotropic agents and acute mental health services by patients with schizophrenia. N Engl J Med 1994;331:650-5. [DOI] [PubMed]
- 13.Johnson RE, Goodman MJ, Hornbrook MC, Eldredge MB. The effect of increased prescription drug cost-sharing on medical care utilization and expenses of elderly health maintenance organization members. Med Care 1997; 35: 1119-31. [DOI] [PubMed]
- 14.Blustein J. Drug coverage and drug purchases by medicare beneficiaries with hypertension. Health Aff(Millwood) 2000;19:219-30. [DOI] [PubMed]
- 15.Harris BL, Stergachis A, Ried LD. The effect of drug co-payments on utilization and cost of pharmaceuticals in a health maintenance organization. Med Care 1990;28:907-1017. [DOI] [PubMed]
- 16.Tamblyn R, Laprise R, Hanley JA, Abrahamowicz M, Scott S, Mayo N, et al. Adverse events associated with prescription drug cost-sharing among poor and elderly persons. JAMA 2001;285:421-9. [DOI] [PubMed]
- 17.Nelson AA Jr, Reeder CE, Dickson WM. The effect of a Medicaid drug copayment program on the utilization and cost of prescription services. Med Care 1984;22:724-36. [DOI] [PubMed]


