Abstract
Gastric cancer (GC), a prevalent malignancy worldwide, encompasses a multitude of biological processes in its progression. Recently, ferroptosis, a novel mode of cell demise, has become a focal point in cancer research. The microenvironment of gastric cancer is composed of diverse cell populations, yet the specific gene expression profiles and their association with ferroptosis are not well understood. Our study employed single-cell RNA sequencing to thoroughly investigate the transcriptomic profiles and identify differential gene expression in gastric cancer, offering fresh insights into the cellular diversity and underlying molecular mechanisms of this disease. We discovered a set of significantly differentially expressed genes in GC, which may serve as valuable leads for future functional investigations. Subsequent analyses, including gene set intersection and functional enrichment, pinpointed genes implicated in ferroptosis and conducted comprehensive Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses to elucidate their biological roles. In the gene selection and model validation section, critical genes were identified using machine learning algorithms, constructing a model with high predictive accuracy. Besides, distorted immune landscapes were further identified in RBL using ssGSEA analysis such that the complex association of gene expression features and its interaction networks as well as infiltration by various types of immune cells can be more clearly understood. Correlation analysis with different immune cell subtypes showed CTSB as an important regulator in the distributions of cancer infiltrating cells. Single-cell RNA sequencing analysis was utilized to map the cellular composition and gene expression profiles of cells in the gastric cancer microenvironment, which provide critical information for elucidating cellular heterogeneity as well as tumor microenvironment regulation in GC. Moreover, the distribution of FTH1, ZFP36 and CIRBP at different expression levels show new research prospects for functional information of these promoters in tumor microenvironment. In summary, the present study augments our knowledge of molecular mechanisms underlying gastric tumorigenesisa and provide scientific basis for identifing new targets and biomarkers in therapeutic diagnosis.
Keywords: Gastric cancer, Single-cell RNA sequencing, Differentially expressed genes, Gene co-expression network, Immune microenvironment, Biomarkers
Introduction
Due to high incidence and mortality rates, gastric cancer remains a significant global health problem, especially in East Asia. Gastric cancer is a common malignancy in the world, at an early stage, the onset of gastric cancer is insidious, and patients with these symptoms are easy to ignore medical advice and do not access early diagnosis and treatment. The disease is often underestimated, and the diagnosis is often missed in the late stage, leading to missing the best treatment opportunity and poor prognosis [1]. Therefore, it is imperative to well comprehend the underlying molecular mechanisms involved in gastric cancer and to discover novel prognostic biomarkers and therapeutic targets that may improve therapeutic efficacy and the patients' quality of life. The development of bioinformatics and systems biology involved moving away from studies centered on individual biomarkers toward studies that incorporate the complexity and interactions of whole networks of molecules. This recent advancement, single-cell RNA sequencing, allows for interrogation of cellular states and behaviors in individual tumor cells and in the tumor microenvironment at an unprecedented level of resolution, revealing tumor heterogeneity. This heterogeneity is essential for tumor adaptation, evolution, and therapeutic resistance [2–4].
There are various forms of cell death, such as apoptosis, pyroptosis, ferroptosis, etc. [5]. Ferroptosis as a novel form of cell death, its molecular mechanisms and regulatory networks are gradually being elucidated [6, 7]. Increasing evidence suggests that ferroptosis not only participates in various physiological and pathological processes but also plays an important role in the initiation and progression of tumors. In some cases, ferroptosis can act as an anti-tumor mechanism by selectively eliminating malignant cells to inhibit tumor growth; while in other cases, tumor cells may evade this form of death by regulating the expression of iron death-related genes, promoting tumor invasion and metastasis.The study found that reducing the expression of HCP5 can induce ferroptosis and inhibit tumor growth, suggesting that HCP5 may be a potential target for gastric cancer treatment [8]. Based on a multi-cohort comprehensive analysis, researchers discovered that ABCC2 can cause disruptions in AA metabolism by exporting glutathione, mediating the induction of lipid peroxidation, and ultimately leading to ferroptosis in gastric cancer cells [9]. This highlights the potential of targeting multiple ferroptosis pathways for the combined treatment of gastric cancer, which could aid in developing new therapeutic strategies [10–12]. We not only identified differentially expressed genes in gastric cancer, but also discovered potential links between these genes and iron death. We then investigated the biological function and signaling pathways of these genes in gastric cancer using Gene Set Enrichment Analysis (GSEA) as well functional enrichment analysis. Moreover, we employed a lot of genes screening via machine learning algorithms and selected critical genes related with the prognosis in gastric cancer. These genes were extensively involved in the tumorigenesis of gastric cancer, which might be promising biomarkers and therapeutic targets. Furthermore, we built prediction models with these critical genes and they exhibited accuracy in other independent datasets.
In addition, in order to better investigate immune microenvironment changes of GC, we explored the correlations between key genes and immune cell infiltrations. Our findings suggest that the expression of some critical genes was significantly associated with tumor immune infiltrates, and might affect immunotherapeutic responses via regulating tumor microenvironment. This study has important implications for the design of new immunotherapy approaches. To summarize, by using the canceR single-cell dataset and multiple omic analysis method into this study not only presented us with cellular barcode, key gene expression pattern in cell cluster but also observed intricate interactions among those genes (or models) killed through iron death program et al. to characteristic immune microenvironment of GC tissue. These findings not only enrich our understanding of the molecular mechanisms of gastric cancer but also provide new perspectives and strategies for precision medicine in gastric cancer.
Methods
Data source
The data for this study is sourced from TCGA, accessible at http://cancergenome.nih.gov/. Single-cell expression data is obtained from all available gastric cancer collections in TISC [13, 14].
Acquisition of immune-related genes
ImmPort serves as a comprehensive resource for immunology data, facilitating data sharing and analysis. Through this platform, we identified 2,483 immune-related genes.
Differential gene expression in gastric cancer
To identify differentially expressed genes, we utilized the “limma” package in R to analyze mRNA samples from the TCGA database.
Weighted correlation network analysis(WGCNA)
To explore gene co-expression relationships and their association with phenotypes, we constructed a gene co-expression network using the “WGCNA” package in R. Abnormal samples were excluded based on the clustering tree. We retained the top 5,000 genes with a median absolute deviation (MAD) greater than 1. A similarity matrix was constructed by calculating the correlation coefficient between each gene pair [15–17].
GO/KEGG analysis
We performed Gene Ontology (GO) and KEGG enrichment analysis using the “clusterProfiler” R package to help us understand the underlying mechanisms of progression and pathogenesis [18–20].
Building prognostic models with machine learning
In gastric cancer research, we utilized the TCGA-STAD dataset for training machine learning models, and validated them on the GSE37023 dataset. Initially, potential feature genes were identified through differential expression analysis. Subsequently, data preprocessing and model training were performed using the caret package. Multiple machine learning algorithms, including logistic regression, random forest, support vector machine, and glmBoost, were employed to construct predictive models. During the validation phase, the generalization ability of the models was tested using the GSE37023 dataset, and ultimately, the glmBoost model, which demonstrated excellent performance in both training and validation, was selected. The pROC package was used to generate ROC curves for further assessment of the models’ diagnostic capabilities [21–24].
Single-cell RNA sequence
Single-cell RNA sequencing analysis [25–27] utilized the GSM7874174 dataset to unravel cell heterogeneity in the gastric cancer microenvironment. Data processing was performed using the Seurat package, starting with quality control to filter out low-quality cells. Principal component analysis (PCA) and t-distributed stochastic neighbor embedding (t-SNE) were conducted using the RunPCA and RunTSNE functions to identify distinct cell clusters. Cells with an abnormally high number of detected genes are often filtered out, as this may indicate contamination or a non-single-cell state. Typically, cells with fewer than 200 or more than 2500 detected genes are excluded. Similar to gene detection counts, the total number of UMIs (Unique Molecular Identifiers) reflects cell activity and integrity. A low UMI count may indicate insufficient cell contents, while a high count could suggest cell aggregation or double capture. A high proportion of mitochondrial gene expression is usually associated with cell stress or death. The PercentageFeatureSet function is used to calculate the proportion of mitochondrial gene expression, and cells with mitochondrial gene expression exceeding 5% are excluded.. Cell type annotation was based on known marker gene expressions, and clustering analysis was performed using the FindClusters function. Differential gene expression analysis employing the FindMarkers function identified expression patterns of feature genes in various cell clusters. Finally, the generated t-SNE plots and heatmaps helped uncover cell distribution and functional characteristics in the cancer microenvironment, providing foundational data and an analytical framework for subsequent biological studies.
Statistical analysis
All statistical analyses were conducted utilizing R software. The decision to employ either the t-test or the Mann–Whitney U test was determined by the normality of the data distribution. A p-value of less than 0.05 was generally considered statistically significant.
Results
The gene expression patterns and differentially expressed genes in stomach cancer samples
Figure 1A is an expression heatmap, showing the differences in gene expression among samples, with colors ranging from blue (low expression) to red (high expression), revealing similar expression patterns in sample clustering. Figure 1B is a volcano plot, with the horizontal axis representing the log2 fold change (logFC) and the vertical axis representing the − log10 adjusted p-value.
Fig. 1.
The gene expression patterns and differentially expressed genes in stomach cancer samples. Sample A stomach cancer expression heatmap B Differential expression gene volcano plot
The gene intersection and functional enrichment analysis in gastric cancer research
Figure 2 shows the gene intersection and functional enrichment analysis in gastric cancer research, providing a deep understanding of the molecular mechanisms of the disease. In Fig. 2A, the Venn diagram of differentially expressed genes and the FerrDB gene set demonstrates the intersection between differentially expressed genes (DEGs) and iron death-related genes in the FerrDB database, identifying 115 common genes, indicating that these genes may play important roles in the iron death process in gastric cancer. Figure 2B presents enrichment analysis results through Gene Ontology (GO) analysis, revealing significantly enriched biological processes, cellular components, and molecular functions, particularly emphasizing cell responses to metal ions and oxidative stress, pathways that may be related to cancer progression and drug resistance. Figure 2C visualizes the complex relationship between genes and their enriched GO categories in a chord diagram, aiding in the identification of key gene roles in different biological processes. In Fig. 2D, the network structure of cellular components is shown, highlighting the gene enrichment in key locations such as the cell membrane and extracellular matrix. Figure 2E displays a bubble chart of molecular function enrichment analysis, revealing significantly enriched molecular functions such as oxidoreductase activity and iron binding, further supporting the potential role of iron death in gastric cancer. Lastly, in Fig. 2F, a bar graph of KEGG pathway analysis emphasizes the importance of iron death, bladder cancer, and other related signaling pathways, which could provide targets for new treatment strategies. This comprehensive analysis offers a new perspective and direction for understanding the molecular mechanisms and potential therapeutic targets of gastric cancer.
Fig. 2.
The gene intersection and functional enrichment analysis in gastric cancer research. A Venn diagram of differentially expressed genes and the FerrDB gene set; B GO enrichment analysis results; C Chord diagram of BP genes and categories; D Network structure of cellular components shown in CC E Bubble chart of molecular function enrichment analysis in MF; F Bar graph of KEGG pathway analysis
The results of weighted gene co-expression network analysis (WGCNA) of gastric cancer samples, revealing the relationship between gene modules and clinical features
In Fig. 3A, the sample clustering tree and trait heatmap display the clustering results of the samples and indicate the distribution of the control group and treatment group, revealing the similarities and differences between the samples. Figure 3B shows the gene clustering tree and module colors, displaying the clustering results of the genes and identifying multiple color-coded co-expression modules. Figures 3C, D evaluate scale independence and mean connectivity under different soft thresholds, determining the optimal parameters for network construction. Figure 3E shows the module-trait relationships through a heatmap that illustrates the correlation of each module with clinical features, where the magenta and pink modules are significantly associated with the treatment group, suggesting these modules may play an important role in disease progression. Figure 3F displays the distribution of gene significance within each module. Figures 3G, H, I respectively demonstrate the relationships between module members and gene significance in the magenta, pink, and turquoise modules, revealing the potential biological significance of these modules in gastric cancer. These analyses provide important information for understanding the molecular mechanisms of gastric cancer and identifying potential therapeutic targets.
Fig. 3.
The weighted gene co-expression network analysis (WGCNA) results of gastric cancer samples. A sample clustering tree with trait heatmap, B gene clustering tree with module colors, C and D scale independence and average connectivity, E module-trait relationship, F distribution of gene significance in each module, G, H, and I relationship between module members and gene significance
The gene selection and model validation in gastric cancer research
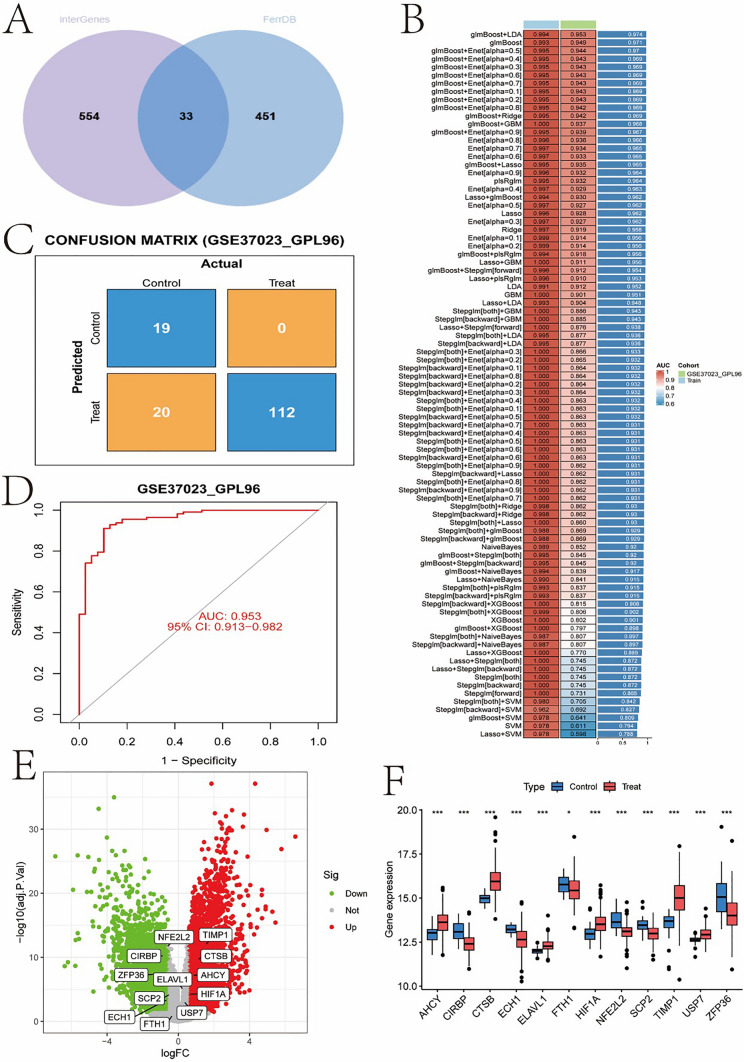
Figure 4 presents the results of gene selection and model validation in gastric cancer research. Figure 4A shows a Venn diagram of differentially expressed genes and characteristic genes, demonstrating an overlap of 33 intersecting genes identified from differential expression analysis and feature gene selection, indicating that these genes may have important biological significance in gastric cancer. Figure 4B lists the performance evaluation of various machine learning models in terms of AUC values on training and validation sets, showcasing the performance of different models in distinguishing control and treatment groups, with the glmBoost model performing the best. Figure 4C displays a confusion matrix showing the classification results on the validation set GSE37023_GPL96, demonstrating high predictive accuracy. Figure 4D further evaluates the diagnostic ability of the model through ROC curve analysis, achieving an AUC of 0.953, indicating good discriminative ability of the model. Figure 4E highlighting some significantly upregulated and downregulated key genes such as TIMP1 and NFE2L2, which may play crucial roles in the development of gastric cancer. Figure 4F compares the expression levels of these key genes in control and treatment groups using a boxplot, revealing significantly upregulated expression of multiple genes in the treatment group.
Fig. 4.
The gene selection and model validation in gastric cancer research. A Venn diagram of differentially expressed genes and feature genes, B evaluation of machine learning model performance, C confusion matrix, D ROC curve, E volcano plot, F boxplot of key gene expression
The expression patterns of key genes, network relationships, and their association with immune cell infiltration in gastric cancer research
In Fig. 5A, the scatter plot matrix of key genes reveals the correlations between these genes, providing a deeper understanding of gene expression patterns. Figure 5B evaluates the diagnostic abilities of various genes in distinguishing between the control group and the treatment group through ROC curves, with CTSB having the highest AUC value of 0.908, indicating its potential value as a biomarker in gastric cancer. In Fig. 5C, the interaction network of key genes demonstrates the interactions between genes, highlighting important genes such as ELAVL1 and HIF1A with central centrality in the network, which may play a crucial role in regulating the progression of gastric cancer. Immunocyte infiltration analysis in Fig. 5D shows the relative changes in the proportions of different immune cells between the control and treatment groups, indicating significant changes in certain immune cells in the treatment group, such as activated dendritic cells. Gene expression boxplots and immune cell correlation heatmaps in Figs. 5E, F further analyze the correlation between immune cell subtypes and gene expression, revealing a significant correlation between CTSB expression and activated and resting dendritic cells. Figures 5G, H display the relationship between CTSB expression and dendritic cells through scatter plots, illustrating the relationship between CTSB expression and the activation and resting states of dendritic cells.
Fig. 5.
The expression patterns of key genes, their network relationships, and their correlation with immune cell infiltration in gastric cancer research. A scatter plot matrix of key genes, B ROC curve of key genes, C Interaction network of key genes, D Analysis of immune cell infiltration, E and F Box plots of gene expression and heatmap of immune cell correlation, G and H Correlation between CTSB expression and dendritic cells
The correlation chart of CTSB gene expression and various immune cell subtypes.
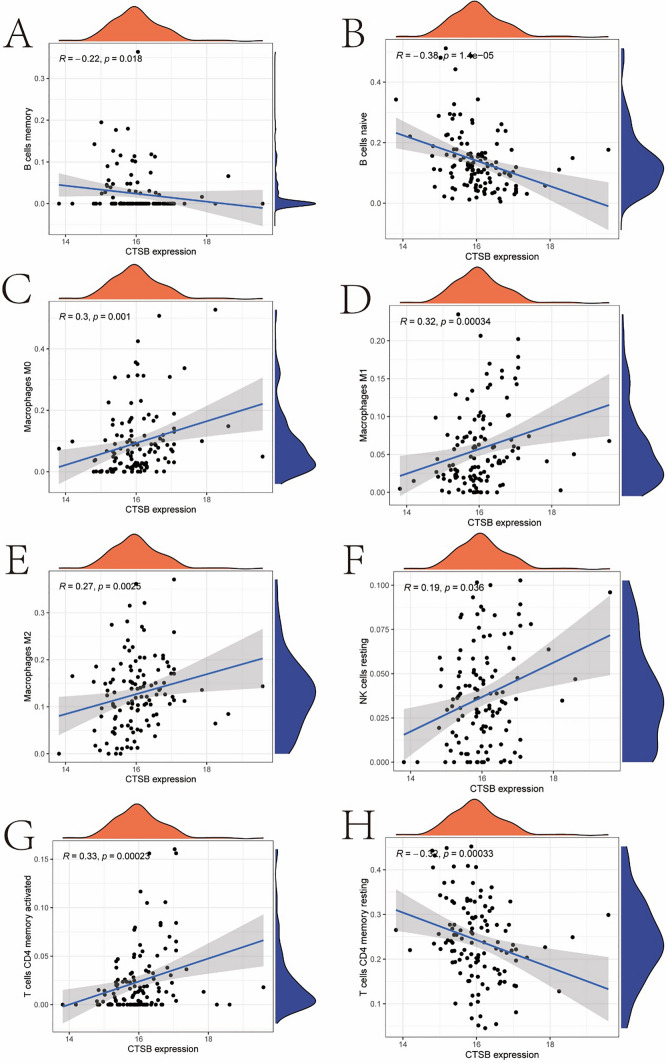
Figure 6 demonstrates the correlation of CTSB gene expression with various immune cell subtypes. Figure 6A shows a negative correlation between CTSB expression and memory B cells, while Fig. 6B reveals a stronger negative correlation with naive B cells, suggesting that CTSB may play an inhibitory role in B cell differentiation or function. Figures 6C, D demonstrate the positive correlation of CTSB with M0 and M1 macrophages, indicating its potential promoting role in macrophage activation. Figure 6E shows a positive correlation of CTSB with M2 macrophages, implying its complex role in immune regulation. Figure 6F indicates a positive correlation of CTSB with resting natural killer cells, which may impact their activation state. Figures 6G, H respectively show the correlation of CTSB with activated and resting CD4 memory T cells, suggesting that CTSB may have dual roles in T cell memory formation and maintenance. These results reveal the multiple impacts of CTSB in the immune microenvironment, providing important clues for understanding its potential role in gastric cancer.
Fig. 6.
The correlation chart of CTSB gene expression and various immune cell subtypes A shows a negative correlation between CTSB expression and memory B cells, while Fig. 6B reveals a stronger negative correlation with naive B cells. Figures. 6C, D demonstrate the positive correlation of CTSB with M0 and M1 macrophages, Fig. 6E shows a positive correlation of CTSB with M2 macrophages, Fig. 6F indicates a positive correlation of CTSB with resting natural killer cells, and Figs. 6G, H respectively show the correlation of CTSB with activated and resting CD4 memory T cells
Correlation between the CTSB gene and various immune cell subtypes
Figure 7 shows the correlation between the CTSB gene and various immune cell subtypes. In Fig. 7A, the correlation heat map and network graph reveal the interactions between CTSB and different immune cell types, with the colors and line thickness representing the direction and strength of the correlation. The results demonstrate a significant correlation between CTSB and various immune cells, particularly macrophages and T cell subtypes. Figure 7B further quantifies these correlations through a bar graph of correlation coefficients, highlighting the strong positive correlation between CTSB and activated CD4 memory T cells, M1 and M2 macrophages, and negative correlation with naive B cells and memory B cells. These findings suggest that CTSB may play a crucial role in regulating the activity and function of immune cells, especially in influencing the immune responses of T cells and macrophages. This provides important insights for further research on the role of CTSB in the immune microenvironment of gastric cancer.
Fig. 7.
Correlation between the CTSB gene and various immune cell subtypes. A correlation heat map and network graph B correlation coefficient bar graph
Cell type and gene expression features in the single-cell RNA sequencing data
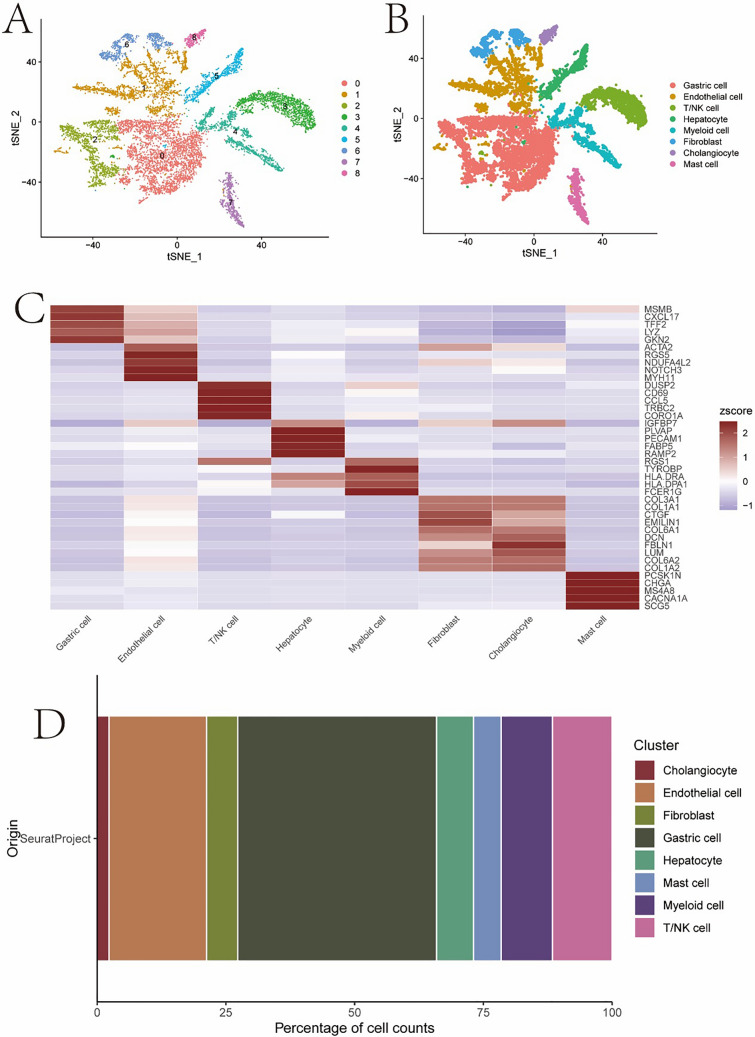
Figure 8A, B Colouring by the annotated cell types clearly shows how different cell populations cluster in the various samples. Heat map in the panel (Fig. 8C, D) depicts differential expression of certain genes among groups and reveals gene signature as well as potential function for each cell subtype %of proportions of the types of cells that make up a sample, useful to know what is actually represented by cell populations present on tissue. A comprehensive analysis of the localization and features of cell types across samples identifies key cellular subpopulations with high relevance for understanding both organismal biology and gastric cancer microenvironment, shedding light into functionally salient programs in these diseases.
Fig. 8.
The distribution and gene expression characteristics of different cell types in single-cell RNA sequencing analysis. A and B t-SNE clustering and annotation plot C MARK gene expression heatmap D bar plot showing cell type proportions in the samples
Concluding versus cellular distribution of FTH1, ZFP36 and CIRBP at high expression sites
A t-SNE plot in Fig. 9A illustrates the distinct distribution of cells with high expression (in red) and low expression (in blue, relative to their own median measures for FTH1), suggesting clustering for various cell functions or states. Figure 9B shares a similar distribution pattern with the expression of ZFP36 clustered in different areas within osteoclasts which signals its probable crucial action for cell fitness or physiological adaptation. As can be seen from Fig. 9C, CIRBP has differential clustering features, most cells show little expression and only few have high or intermediate-level of expression These results suggest that the expression of these genes may regulate cell distribution and function in TME affecting tumorigenesis, providing some new datum for researches on their functions in gastrointestinal cancer biology.
Fig. 9.
Heat map of FTH1, ZFP36, and CIRBP gene expression
Discussion
Gastric cancer (GC) is the most prevalent, globally significant human health challenge associated with a substantial variate of mortality and incidence rates across regions but also among populations [28–31]. Ferroptosis is an iron-dependent andlipid-peroxidationregulated cell death pathway that has recently attracted significant attention in the field of cancer research. On one hand, ferroptosis may facilitate the development of gastric cancer in response to canonical metabolic stress and epithelial–mesenchymal transition. Ferroptosis can act as a tumor suppressive mechanism to induce oxidative stress and kill cancer cells, but it may also fuel tumorigenesis since the adaption of ferroptotic cell death by regulation on iron metabolism-related genes expression in tumors [10, 32, 33].
Increasing evidence suggests that ferroptosis plays a dual role in human cancers, acting as both a tumor-suppressing mechanism and a promoter of tumorigenesis. In gastric cancer, ferroptosis may exert anti-cancer effects by inducing oxidative stress and killing cancer cells, but it can also promote tumor development by regulating the expression of iron metabolism-related genes. The interaction between ferroptosis and immune cells, particularly T cells and macrophages, is crucial in anti-tumor immunity. Activated CD8 + T cells promote ferroptosis in tumor cells by releasing IFNγ [34, 35], which inhibits the expression of SLC3A2 and SLC7A11 in tumor cells. Additionally, the interplay between macrophages and ferroptosis plays a key role in anti-tumor immunity. For example, HMGB1, a DAMP released by ferroptotic cells, can stimulate inflammatory responses in macrophages [36, 37].
By these analyses, we extracted the possible molecular mechanisms of ferroptosis in gastric cancer cells including cell-metal ion response and oxidative stress. The WGCNA results highlighted the relationships between certain gene modules and clinical characteristics, especially ferroptosis-related features. These modules may regulate the process of ferroptosis in GC, and could be utilized as new biomarkers to elucidate molecular subtypes or therapeutic responses of GC. In this context, we developed a data-driven model from machine learning algorithms to determine important genes associated with the survival of gastric cancer patients. These models not only pinpoint gene expression patterns linked to ferroptosis, but also help predict how patients might respond to a treatment – paving the way for personalized medicine. The expression patterns of the key genes, such as CTSB, indicated that they play crucial roles in immune cell interactions within the GC microenvironment. Specifically, correlation analysis of CTSB with various immune cells subtypes indicates consistently that it plays an essential role in inhibiting the action of immune cells during ferroptosis. CTSB plays a multifaceted role in the immune microenvironment. It is involved in various physiological and pathological processes, such as cell proliferation, migration, autophagy, antigen presentation, and apoptosis, and is closely related to tumor development. CTSB affects tumor progression and treatment response by influencing the infiltration levels of immune cells in the tumor microenvironment, such as macrophages, dendritic cells, neutrophils, and T cells. Its role in immune regulation is complex; CTSB may impact tumor immune evasion and the effectiveness of immunotherapy by modulating immune cell infiltration and function. For example, CTSB is positively correlated with the infiltration level of regulatory T cells (Tregs), which may contribute to the formation of an immunosuppressive tumor microenvironment [38–40]. The involvement of CTSB in ferroptosis may offer pathways for inducing tumor cell death. By promoting ferroptosis, CTSB could potentially suppress tumor growth and enhance sensitivity to certain treatments, offering new therapeutic avenues. Understanding how CTSB modulates these processes provides insights into targeting it for therapeutic interventions. Inhibiting CTSB's role in immune suppression and enhancing its ferroptosis-inducing capabilities could improve treatment outcomes for gastric cancer patients.
On the basis of gastric cancer cells single-cell RNA sequencing data, we examined the distribution and gene expression properties in distinct cell types within stomach tumour microenvironment relevant for ferroptosis. The findings could help to shed light on how the iron ion-dependent oxidative stress response of cells in the tumor microenvironment is related with tumorigenesis and treatment responsiveness. The cell distribution profiles and combined functions of CIRBP, ZFP36, FTH1 dynamics in the absence or presence of BAP played essential role for gene signals on cells composition within tumor microenvironment during ferroptosis process. The alteration in expression of these genes might suggest the plasticity governed by tumor cells to use iron ions and oxidative stress as a signaling agent.
Limitations
The sample size of our study may limit the statistical power of the results, especially when exploring subgroup-specific effects. Additionally, since the samples primarily come from a specific region, they may not fully represent the genetic and clinical diversity of the global gastric cancer population. There may be selection bias, as we primarily analyzed publicly available datasets, which may not include all types of gastric cancer patients. Furthermore, differences in sample processing and preservation conditions across datasets could affect the molecular analysis results. Although our findings reveal the potential role of CTSB in the ferroptosis process in gastric cancer, further research is needed to determine whether these findings can be generalized to other types of cancer or populations. Validation in larger, more diverse sample sets is required to confirm the universality and applicability of these results.
Conclusion
Our comprehensive study has shed light on the pivotal role of ferroptosis in the context of gastric cancer (GC), offering novel insights that could reshape our understanding of this disease. Through systematic multidimensional bioinformatics analysis, we have unveiled the complex interplay between ferroptosis and the gastric cancer microenvironment, highlighting its potential as a biomarker for molecular classification, prognosis prediction, and the development of therapeutic strategies.
Author contributions
Shupeng Zhang and Zhaojin Li contributed to the conception and design of the study. Shupeng Zhang and Gang Hu performed the data analysis and interpretation. Zhaojin Li and Hekai Chen wrote the main manuscript text. Gang Hu prepared Figures and contributed to the critical revision of the manuscript. All authors reviewed and approved the final manuscript.
Funding
This study was supported by grants from Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-079D), Tianjin Health Research Project (TJWJ2022MS051) and Scientific Research Foundation of Tianjin Fifth Central Hospital (WZX201813).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gang Hu, Email: doctorhuhu@126.com.
Hekai Chen, Email: hakkie@126.com.
References
- 1.Hong Y, Li X, Liu Z, Fu C, Nie M, Chen C, Feng H, Gan S, Zeng Q. Predicting tumor invasion depth in gastric cancer: developing and validating multivariate models incorporating preoperative IVIM-DWI parameters and MRI morphological characteristics. Eur J Med Res. 2024;29(1):431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin X, Yang P, Wang M, Huang X, Wang B, Chen C, Xu A, Cai J, Khan M, Liu S, et al. Dissecting gastric cancer heterogeneity and exploring therapeutic strategies using bulk and single-cell transcriptomic analysis and experimental validation of tumor microenvironment and metabolic interplay. Front Pharmacol. 2024;15:1355269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng Q, Zhang P, Liu G, Lu L. Integrated single-cell and bulk RNA sequencing analyses identify an immunotherapy nonresponse-related fibroblast signature in gastric cancer. Anticancer Drugs. 2024. 10.1097/CAD.0000000000001651. [DOI] [PubMed] [Google Scholar]
- 4.Tang X, Gao L, Jiang X, Hou Z, Wang Y, Hou S, Qu H. Single-cell profiling reveals altered immune landscape and impaired NK cell function in gastric cancer liver metastasis. Oncogene. 2024. 10.1038/s41388-024-03114-0. [DOI] [PubMed] [Google Scholar]
- 5.Shen Y, Chi H, Xu K, Li Y, Yin X, Chen S, Yang Q, He M, Zhu G, Li X. A novel classification model for lower-grade glioma patients based on pyroptosis-related genes. Brain Sci. 2022. 10.3390/brainsci12060700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu B, Chen Z, Li Z, Zhao X, Zhang W, Zhang A, Wen L, Wang X, Zhou S, Qian D. Hsp90alpha promotes chemoresistance in pancreatic cancer by regulating Keap1-Nrf2 axis and inhibiting ferroptosis. Acta Biochim Biophys Sin. 2024. 10.3724/abbs.2024138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu D, Ren L, Liu J. METTL14 promotes chondrocyte ferroptosis in osteoarthritis via m6A modification of GPX4. Int J Rheum Dis. 2024;27(8): e15297. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Guo G, Chen Y, Lu L, Li H, Zhou Z, Guo J, Gan X, Hu Y, Li Q, et al. HCP5 derived novel microprotein triggers progression of gastric cancer through regulating Ferroptosis. Adv Sci (Weinh). 2024;11(46): e2407012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Gan X, Cheng X, Jia Y, Wang G, Tang X, Du H, Li X, Liu X, Xing X, et al. ABCC2 induces metabolic vulnerability and cellular ferroptosis via enhanced glutathione efflux in gastric cancer. Clin Transl Med. 2024;14(8): e1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun K, Gao L, Li S, Zheng J, Zhu Z, Zhi K, Ren W. Circ-CDK8 regulates SLC7A11-mediated ferroptosis by inhibiting miR-615-5p to promote progression in oral squamous cell carcinomas. Front Pharmacol. 2024;15:1432520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan Y, Wang Q, Guo Y, Zhang N, Xu Y, Bai X, Liu J, Bi X. Dexmedetomidine mitigates lidocaine-induced spinal cord injury by repressing ferritinophagy-mediated ferroptosis by increasing CISD2 expression in rat models. J Bioenerg Biomembr. 2024. 10.1007/s10863-024-10034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Mo D, Zhang N, Yu H. Ferroptosis in diabetic cardiomyopathy: advances in cardiac fibroblast-cardiomyocyte interactions. Heliyon. 2024;10(15): e35219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez-Gamarra M, Salgado-Roo A, Dominguez E, GoiricelayaSeco EM, Veiga-Rua S, Pedrera-Garbayo LF, Carracedo A, Allegue C. CARTAR: a comprehensive web tool for identifying potential targets in chimeric antigen receptor therapies using TCGA and GTEx data. Brief Bioinform. 2024. 10.1093/bib/bbae326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patricio A, Costa RS, Henriques R. Pattern-centric transformation of omics data grounded on discriminative gene associations aids predictive tasks in TCGA while ensuring interpretability. Biotechnol Bioeng. 2024;121(9):2881–92. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Zhou H, Ma R, Guo W, Yang X, Li X, Liu Z, Zhong Y, Jing Z. Structure of POU2AF1 recombinant protein and it affects the progression and treatment of liver cancer based on WGCNA and molecular docking analysis. Int J Biol Macromol. 2024;278(Pt 1): 134629. [DOI] [PubMed] [Google Scholar]
- 16.Wu K, Chen H, Li F, Meng X, Chen L, Li N. Identification of potential biomarkers for atrial fibrillation and stable coronary artery disease based on WGCNA and machine algorithms. BMC Cardiovasc Disord. 2024;24(1):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadalam PK, Ramadoss R, Arumuganainar D. Weighted gene co-expression network analysis (WGCNA) of wnt signaling related to periodontal ligament formation: a bioinformatics-based analysis. Cureus. 2024;16(7): e63639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abulfaraj AA, Shami AY, Alotaibi NM, Alomran MM, Aloufi AS, Al-Andal A, AlHamdan NR, Alshehrei FM, Sefrji FO, Alsaadi KH, et al. Exploration of genes encoding KEGG pathway enzymes in rhizospheric microbiome of the wild plant Abutilon fruticosum. AMB Express. 2024;14(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castaneda EU, Baker EJ. KNeXT: a networkX-based topologically relevant KEGG parser. Front Genet. 2024;15:1292394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dvorak P, Hlavac V, Hanicinec V, Rao BH, Soucek P. Genes divided according to the relative position of the longest intron show increased representation in different KEGG pathways. BMC Genomics. 2024;25(1):649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Intagliata NM, Rahimi RS, Higuera-de-la-Tijera F, Simonetto DA, Farias AQ, Mazo DF, Boike JR, Stine JG, Serper M, Pereira G, et al. Procedural-related bleeding in hospitalized patients with liver disease (PROC-BLeeD): an international, prospective. Multicenter Observ Study Gastroenterol. 2023;165(3):717–32. [DOI] [PubMed] [Google Scholar]
- 22.Shu W, Li M, Xiao H, Amaerjiang N, Khattab NM, Zunong J, Guan M, Vermund SH, Hu Y. Validation of “life’s essential 8” metrics with cardiovascular structural status in children: the PROC Study in China. J Am Heart Assoc. 2023;12(12): e029077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu F, Zhang K, Xu Q, Ye L, Zeng M, Jin Y, Wang M, Yang L. Analysis of PROC mutations and clinical features in 22 unrelated families with inherited protein C deficiency. Ann Hematol. 2024;103(2):645–52. [DOI] [PubMed] [Google Scholar]
- 24.Su K, Shen Q, Tong J, Gu T, Xu K, Li H, Chi H, Liu Y, Li X, Wen L, et al. Construction and validation of a nomogram for HBV-related hepatocellular carcinoma: a large, multicenter study. Ann Hepatol. 2023;28(4): 101109. [DOI] [PubMed] [Google Scholar]
- 25.Kim GD, Lim C, Park J. A practical handbook on single-cell RNA sequencing data quality control and downstream analysis. Mol Cells. 2024;47(9): 100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin K, Cai J, Pan S, Yu X, Qiu Y, Ying Z, Feng H, Zhang L, Liu Y, Shen H, et al. Screening of PDSS1 as a potential biomarker for hepatocellular carcinoma based on a copper-related prognostic signature through bulk and single-cell rna-sequencing analysis. J Cancer. 2024;15(15):5028–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong J, Chen G, Lin B, Zhong L, Jiang X, Lu H. Integrative analysis of single-Cell RNA sequencing and experimental validation in the study of abdominal aortic aneurysm progression. Gene. 2024;929: 148820. [DOI] [PubMed] [Google Scholar]
- 28.Chang Y, Jin H, Cui Y, Yang F, Chen K, Kuang W, Huo C, Xu Z, Li Y, Lin A, et al. PUS7-dependent pseudouridylation of ALKBH3 mRNA inhibits gastric cancer progression. Clin Transl Med. 2024;14(8): e1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Z, Liang C, Xu Q, Yuan Z, Chen M, Li R, Zhou S, Li P, Wei B, Zhao X. The safety and efficacy of neoadjuvant PD-1 inhibitor plus chemotherapy for patients with locally advanced gastric cancer: a systematic review and meta-analysis. Int J Surg. 2024. 10.1097/JS9.0000000000002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng L, Lu J, Kong D, Zhan Y. Single-cell sequencing analysis revealed that WDR72 was a novel cancer stem cells related gene in gastric cancer. Heliyon. 2024;10(15): e35549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhuo H, Hou J, Hong Z, Yu S, Peng H, Zhang L, Xie W, Hong X. TAGLN2 induces resistance signature ISGs by activating AKT-YBX1 signal with dual pathways and mediates the IFN-related DNA damage resistance in gastric cancer. Cell Death Dis. 2024;15(8):608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S, Wang J, Huang G, Xiao X, Xu S, Weng P, Wang Y, Tian H, Huang H, Chen Y. TCP1 expression alters the ferroptosis sensitivity of diffuse large B-cell lymphoma subtypes by stabilising ACSL4 and influences patient prognosis. Cell Death Dis. 2024;15(8):611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang XD, Sun J, Zheng XM, Zhang J, Tan LL, Fan LL, Luo YX, Hu YF, Xu SD, Zhou H, et al. Plin4 exacerbates cadmium-decreased testosterone level via inducing ferroptosis in testicular Leydig cells. Redox Biol. 2024;76: 103312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye Z, Ding J, Huang J, Hu Z, Jin F, Wu K. Ginsenoside Rg3 activates the immune function of CD8+ T cells via circFOXP1-miR-4477a-PD-L1 axis to induce ferroptosis in gallbladder cancer. Arch Pharm Res. 2024;47(10–11):793–811. [DOI] [PubMed] [Google Scholar]
- 35.Zhu B, Yang C, Hua S, Li K, Shang P, Chen X, Hua ZC. Lithium enhances ferroptosis sensitivity in melanoma cells and promotes CD8(+) T cell infiltration and differentiation. Free Radic Biol Med. 2024;227:233–45. [DOI] [PubMed] [Google Scholar]
- 36.Schwantes A, Wickert A, Becker S, Baer PC, Weigert A, Brune B, Fuhrmann DC. Tumor associated macrophages transfer ceruloplasmin mRNA to fibrosarcoma cells and protect them from ferroptosis. Redox Biol. 2024;71: 103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao H, Du X, Tao Z, Jing N, Bao S, Gao WQ, Dong B, Fang YX. Taurine inhibits ferroptosis mediated by the crosstalk between tumor cells and tumor-associated macrophages in prostate cancer. Adv Sci (Weinh). 2024;11(3): e2303894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Y, Li S, Huang S, Tu J, Chen X, Xiao L, Liu B, Yuan X. Comprehensive and integrative analysis of two novel SARS-CoV-2 entry associated proteases CTSB and CTSL in healthy individuals and cancer patients. Front Bioeng Biotechnol. 2022;10: 780751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Liang Y, Lian C, Peng F, Xiao Y, He Y, Ma C, Wang Y, Zhang P, Deng Y, et al. CST6 protein and peptides inhibit breast cancer bone metastasis by suppressing CTSB activity and osteoclastogenesis. Theranostics. 2021;11(20):9821–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin C, Yang H, Zhao W, Wang W. CTSB(+) macrophage repress memory immune hub in the liver metastasis site of colorectal cancer patient revealed by multi-omics analysis. Biochem Biophys Res Commun. 2022;626:8–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.