Abstract
The poor prognosis of pancreatic cancer is often attributed to difficulties of early detection due to a lack of appropriate risk factors. Previously, we demonstrated the presence of Enterococcus faecalis (E. faecalis) in pancreatic juice and tissues obtained from patients with cancers of the duodeno-pancreato-biliary region, suggesting the possible involvement of this bacterial species in chronic and malignant pancreatic diseases. However, it remains unclear if and how E. faecalis can infect pancreatic ductal cells. In this study, we used immortalized normal human pancreatic ductal epithelial cells (iPDECs) and pancreatic ductal cancer cell lines to demonstrate that E. faecalis adheres to and invades pancreatic ductal lineage epithelial cells. Inhibitors of micropinocytosis or clathrin- or caveolae-mediated endocytosis suppressed iPDEC invasion by E. faecalis. Mechanistically, bacterial expression of enterococcal fibronectin-binding protein A (EfbA) was correlated with adhesive potential of E. faecalis strains. Knockout of fibronectin 1, a binding partner of EfbA, in iPDECs resulted in suppressed E. faecalis adhesion and invasion, suggesting the importance of the EfbA-fibronectin axis in infection of pancreatic ductal epithelial lineage cells. Overall, these results suggest that E. faecalis can colonize pancreatic tissue by infecting iPDECs, at least in part, via the expression of the cell adhesion factor EfbA.
Subject terms: Microbiology, Molecular biology
Introduction
Enterococcus faecalis (E. faecalis) is a gram-positive facultative anaerobe and commensal bacterium that resides in the normal guts of healthy individuals. Outside of the gut, however, E. faecalis infection can be pathogenic. For example, E. faecalis is the third major cause of infectious endocarditis following bloodstream infection1,2 and is one of the pathogens frequently responsible for urinary tract infections3. While bloodstream and urothelial infections are often catheter-associated, translocation through the gut epithelial barrier is an important route of colonization outside the gut4. Bacterial colonization, a process in which bacteria in the fluid recognize and colonize tissue surfaces, is a critical precursor to tissue infection or translocation through the gut. Bacterial colonization follows one of two major models: (i) bacteria recognize host tissue damage and colonize exposed extracellular matrix or (ii) bacteria directly attach to the undamaged host surface5. Previous reports suggest that E. faecalis follows the latter model; it directly attaches to various types of cells including endothelial cells5, urinary tract cells6, and intestinal epithelial cells7 in in vitro experiments. Mechanistically, infection by E. faecalis consists of bacterial adhesion to epithelial cells7 followed by invasion into8 and across epithelial monolayers9. Therefore, investigating the ability of E. faecalis to attach to and invade a particular cell type can provide insights into the mechanisms of infection of the corresponding tissue type.
Pancreatic cancer is one of the most lethal cancer types. Its poor prognosis can be attributed in part to the challenges of early detection. In addition, it is difficult to identify high-risk populations for pancreatic cancer due to the lack of appropriate risk factors. In our efforts to identify such risk factors, we previously demonstrated the presence of Enterococcus spp., mostly E. faecalis, in pancreatic juice and tissues obtained from patients with cancers of the duodeno-pancreato-biliary region10,11. These reports suggest the involvement of E. faecalis in the formation of pancreatic microbiota12–14 in pancreatic malignancies or chronic pancreatitis associated with pancreatic cancer15. Pancreatic juice is thought to be an important factor in limiting the colonization of pathogenic bacteria in the gastrointestinal tract with its antibacterial activity, including high alkalinity16. However, E. faecalis is alkaline-resistant and can survive in experimental alkaline conditions up to pH 11.517. This alkaline resistance may contribute to the enhanced survival capacity of E. faecalis compared to other intestinal bacteria in the pancreatic duct. While reflux of duodenal fluid is the most possible route of retrograde infection of the pancreatic duct, the capability and mechanism of E. faecalis infection of pancreatic ductal cells remain unclear.
In the present study, we demonstrate that E. faecalis attaches to pancreatic ductal lineage cells and subsequently invades via endocytosis-mediated mechanisms. We also show that E. faecalis can translocate through Madin-Darby canine kidney (MDCK) epithelial monolayers via transcytosis. Moreover, our results reveal an association between E. faecalis fibronectin-binding protein (EfbA) and the bacteria’s capacity to adhere to pancreatic ductal cells. This attachment mechanism also involves fibronectin of the host cells. Together, these results provide mechanistic insights into the process of pancreatic colonization by E. faecalis.
Results
E.faecalis adheres to pancreatic ductal epithelial cells
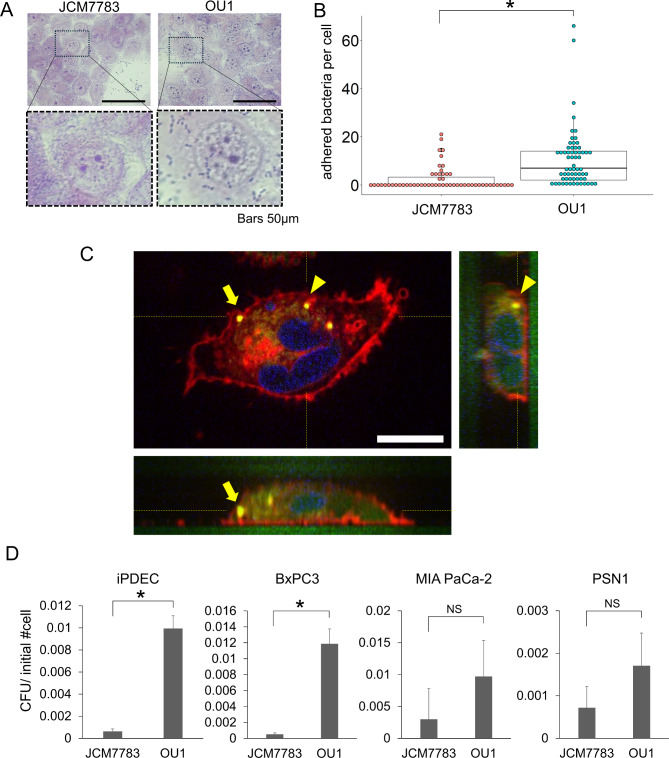
To examine the potential pathogenic role of E. faecalis in pancreas, we evaluated adhesion to immortalized normal human pancreatic ductal epithelial cells (iPDECs). Two E. faecalis strains were used to evaluate adhesion: JCM7783, which was obtained from RIKEN, and OU1, which we isolated from pancreatic ductal juice of a patient with pancreatic cancer. Both strains exhibited adhesion to iPDECs that persisted even after thorough washing steps (Fig. 1A,B), with the OU1 strain adhering to a greater extent.
Fig. 1.
E. faecalis adhere to and invade in pancreatic ductal lineage cells. (A) Giemsa stained images of iPDECs and E. faecalis (JCM7783 and OU1 strains) adherent to iPDECs. Dotted boxes in the upper panels are enlarged in the lower panels. Bars; 50 μm (B) Bacteria adherent to iPDECs were quantified by strain (JCM7783 and OU1). Data is presented as a box-whisker plot. N = 56 and 63 for JCM7783 and OU1, respectively. *P < 0.05, Wilcoxon rank sum test. (C) Optical sectioning images and cross-sectional images of iPDECs invaded by E. faecalis labeled with CFSE (green). Plasma membranes of iPDECs were labeled with Plasmem bright red reagent (red), and nuclei were labeled with Hoechst 33342 (blue). Yellow dotted lines indicate the sectioning planes; yellow arrows and arrowheads indicate E. faecalis labeled with CFSE. Scale bar; 10 μm (D) Invasion assay data for pancreatic ductal lineage cell lines iPDEC, BxPC3, MIA PaCa-2, and PSN1. JCM7783 and OU1 were compared and CFU was normalized to the number of cells initially plated. Data are presented as mean + SD, N = 3, each. *P < 0.05, t-test with Bonferroni correction; NS not significant.
Next, we examined entry of E. faecalis into iPDECs following adhesion. iPDECs were treated with fluorescently labeled E. faecalis (OU1 strain) and imaged using fluorescence microscopy. Image analysis confirmed that E. faecalis entered the cytoplasm of iPDECs (Fig. 1C). E. faecalis invasion of iPDECs was quantified using an invasion assay (see Materials and Methods) in which E. faecalis was extracted from epithelial cell lysates following infection and then cultured and quantified. The results demonstrated that both strains of E. faecalis entered iPDECs, with the highly adhesive OU1 strain invading to a greater extent than the JCM7783 strain (Fig. 1D). Cellular invasion by E. faecalis was also confirmed in three additional pancreatic ductal epithelial lineage cell lines: BxPC3, MIA PaCa-2, and PSN1 (Fig. 1D). Notably, the OU1 strain was significantly more invasive than the JCM7783 strain in BxPC3, a comparable trend was observed in the other two cell lines. These results suggest that E. faecalis adheres to and enters pancreatic ductal epithelial cells, which can be regulated by both bacterial and cellular factors.
E. faecalis invades pancreatic ductal cells via endocytosis-mediated uptake
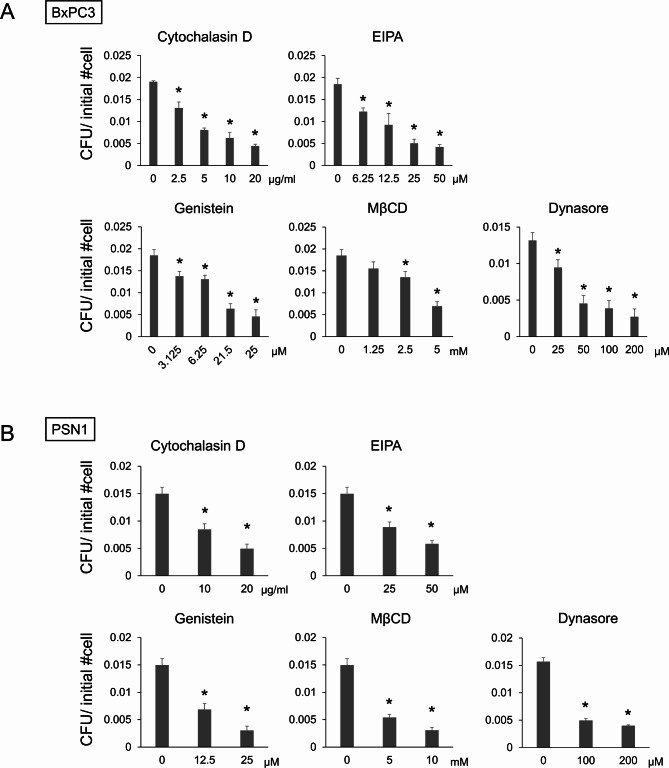
Endocytosis is a well-studied route of bacterial internalization not only in phagocytic cells but also in epithelial cells18,19. However, precise mechanisms of pancreatic ductal cell invasion by E. faecalis have not been fully described. Thus, we sought to investigate which internalization mechanism is operational for the entry of E. faecalis into pancreatic ductal lineage cells. BxPC3 cells and PSN1 cells were pretreated with micropinocytosis inhibitors (cytochalacin D20,21 and 5-[N-ethyl-N-isopropyl] amiloride (EIPA)21,22), caveolae-mediated endocytosis inhibitors (genistein23,24 and methyl-beta-cyclodextrin (MβCD)23), and a dual clathrin- and caveolae-mediated endocytosis inhibitor (Dynasore20) prior to conduction of the invasion assay. These inhibitors significantly suppressed the number of E. faecalis colonies retrieved from infected cell lysates in BxPC3 (Fig. 2A) and PSN1 cells (Fig. 2B). Note that none of the inhibitors were toxic to either epithelial cells or E. faecalis at concentrations used in the assays (Fig. S1A,B). These results suggest both that micropinocytosis and caveolae-mediated endocytosis are involved in E. faecalis invasion into pancreatic ductal lineage cells.
Fig. 2.
E. faecalis invades pancreatic ductal lineage cells via endocytosis. (A,B) Invasion assay data in the presence of endocytosis inhibitors at indicated concentrations in pancreatic ductal lineage cell lines BxPC3 (A) and PSN1 (B). For BxPC3, a single data set for the untreated group was used as a control for multiple assays (cytochalacin D, EIPA, genistein, and MβCD). CFU was normalized by the number of cells initially plated. Data are presented as mean + SD, N = 3, each. *P < 0.01, Dunnett’s test.
E. faecalis penetrates through epithelial monolayers by transcytosis
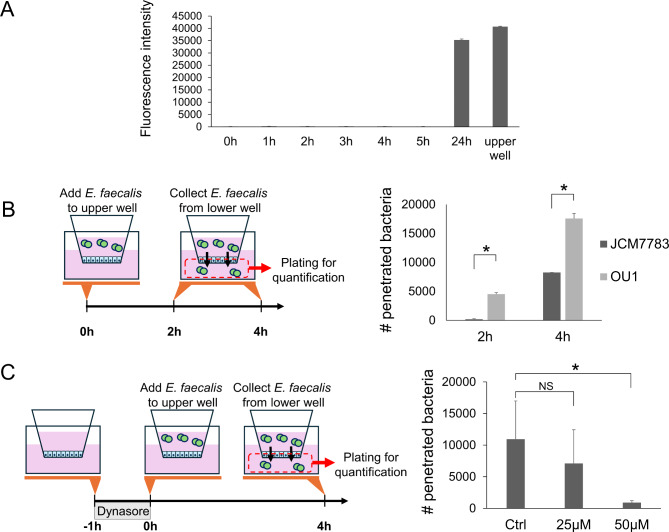
Penetration of the epithelial monolayer is an important mechanism by which bacteria migrate into, colonize, and eventually damage tissues. The epithelial monolayer can be penetrated via both paracellular and transcellular routes25. Given that invasion by E. faecalis involves endocytic processes in which bacteria are expected to be trapped in vesicles, we next evaluated whether E. faecalis utilizes this same mechanism for penetrating epithelial monolayers. First, we examined whether JCM7783 and OU1 strains penetrate through epithelial monolayers. Because iPDECs do not form differentiated monolayers partitioned by adhesion bonds and tight junctions, MDCK cells cultured using a transwell culture system were instead used as an epithelial monolayer model. MDCK cells were infected by adding E. faecalis to the upper chamber of the transwell, and migration of bacteria to the lower chamber was quantified. Penetration was monitored at 2 and 4 h after infection because the epithelial monolayer remained intact within this time frame (Fig. 3A). The results demonstrated that both strains penetrated the monolayer, and significantly higher penetration was observed for the OU1 strain (Fig. 3B). This result is consistent with a correlation between higher adhesion potential and more invasion. Next, to examine whether the penetration through the epithelial monolayer occurs via transcytosis, intracellular trafficking of vesicles was inhibited by Dynasore, a GTPase inhibitor that inhibits multiple steps of vesicle trafficking26. MDCK monolayers were pretreated with the indicated concentration of Dynasore for 1 h before addition of E. faecalis (Fig. 3C, left panel). As shown in Fig. 3C, Dynasore significantly suppressed the amount of OU1 strain bacteria that penetrated through MDCK monolayer. These results suggest that the mechanism of epithelial layer penetration by E. faecalis involves transcytosis.
Fig. 3.
E. faecalis penetrates epithelial monolayer via transcytosis. (A) Membrane integrity evaluated by dextran-FITC leakage. E. faecalis strain OU1 and dextran-FITC were added to the upper well of a transwell culture system containing a cultured MDCK monolayer. Fluorescence of the media in the lower well was measured at indicated time points. Data is presented as mean + SD. (B) Schema (left) and results (right) of the transwell penetration assay. CFUs in the lower well media were normalized to the number of E. faecalis applied in the upper well. Data are presented as mean + SD, N = 3, each. *P < 0.05, t-test with Bonferroni correction. (C) Schema (left) and results (right) of the transwell penetration assay with Dynasore treatment. CFUs in the lower well media were normalized to the number of E. faecalis applied in the upper well. Data are presented as mean + SD, N = 4, each. *P < 0.05, t-test with Bonferroni correction; NS not significant.
Invasion capacity of E. faecalis correlates with EfbA expression
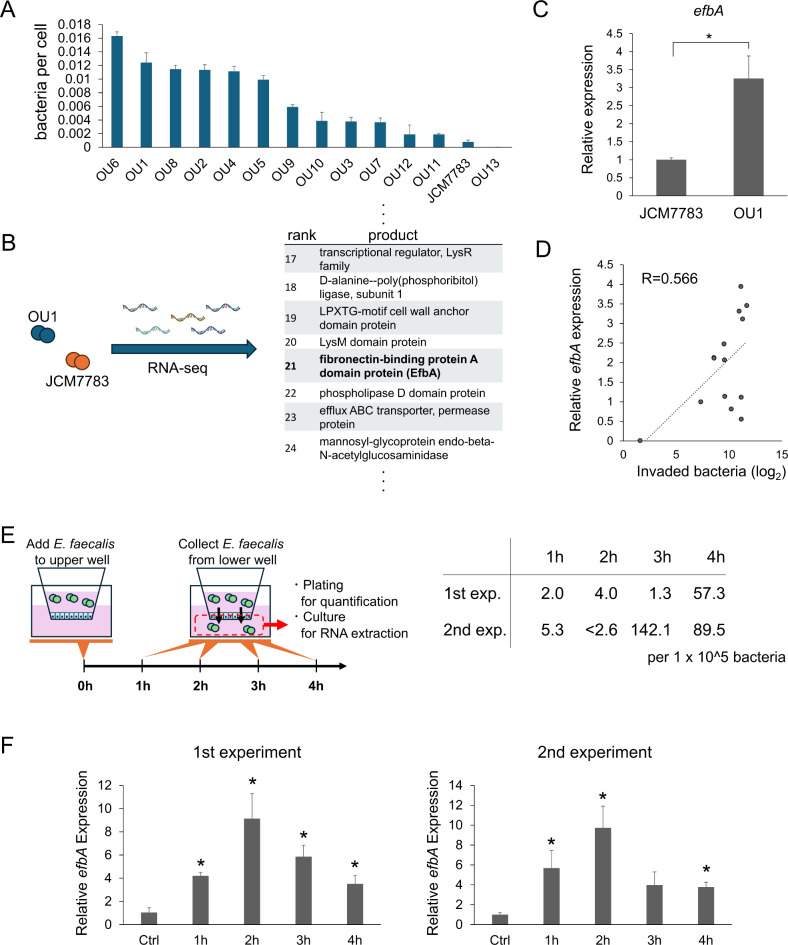
As described above, E. faecalis can adhere to and invade pancreatic ductal cells and penetrate epithelial monolayers. We next explored the mechanisms by which these two processes are regulated. We began by comparing the invasion capacity of 14 different E. faecalis strains, including JCM7783 and OU1, and we found that the degree of invasion as determined by the invasion assay varied among the strains (Fig. 4A). Because the phenotype, or pathogenesis, of E. faecalis can be regulated by conjugative plasmids, major known virulence factors included in conjugative plasmids were evaluated in each strain (Fig. S2). However, no correlation could be identified between any of the virulence factors and invasion capacity. Next, we employed RNA sequencing to compare gene expression in JCM7783 and OU1, strains with low and high invasion capacity, respectively. Among the 43 genes that were highly expressed in OU1 at more than 5-fold in fragments per kilobase of transcript per million mapped reads (FPKM), we identified two genes related to attachment of gram-positive bacteria: LPXTG-motif cell wall anchor domain protein and efbA27 (Fig. 4B). We focused on EfbA because EfbA is a PavA-like fibronectin adhesin reported to be involved in urinary tract infections28 and infectious endocarditis29. Higher efbA expression in OU1 compared to JCM7783 was confirmed by quantitative polymerase chain reaction (qPCR) (Fig. 4C). In addition, efbA expression was evaluated in 14 E. faecalis strains (Fig. S3) and found to correlate weakly but significantly with invasion capacity (Fig. 4D, R = 0.57, p = 0.035).
Fig. 4.
Invasion capacity of E. faecalis correlates with EfbA expression. (A) Quantification of iPDEC invasion by 14 E. faecalis strains. Data was sorted by invasion capacity and is presented as mean + SD, N = 3, each. (B) Summary of the RNA-seq analysis comparing JCM7783 and OU1 strains. From the RNA-seq results, genes with normalized FPKM > 10 in both strains were identified (Supplementary Table S2). Adhesion-related gene efbA was selected among highly-expressed genes for further study. (C) qPCR quantification of efbA expression in E. faecalis strains JCM7783 and OU1. Data is presented as mean + SD, N = 3, each. *P < 0.05, t-test (D) Correlation of efbA expression and number of invaded bacteria in 14 E. faecalis strains. Correlation coefficient is denoted as R. (E) Schema (left) and the results (right) of the transwell penetration time course assay. CFUs of the lower-well media were normalized to E. faecalis strain JCM7783 applied in the upper well. Experiment was performed in duplicate and both datasets are presented. (F) qPCR quantification of efbA expression in E. faecalis (JCM7783 strain) collected in the experiments in (E). Data is presented as mean + SD, N = 3, each. *P < 0.05, t-test with Bonferroni correction.
We further examined the involvement of EfbA in bacterial penetration through epithelial monolayers. A time-course experiment was used to evaluate penetration of an MDCK monolayer cultured in a transwell system by JCM7783, a strain with low invasion/penetration capacity and low efbA expression (Fig. 4E). An increase in membrane penetration was detected 3 to 4 h following application of E. faecalis to the upper well (Fig. 4E). Accordingly, efbA expression was increased in E. faecalis that penetrated the monolayer compared to control bacteria (Fig. 4F). These results are consistent with the involvement of EfbA in bacterial infection of epithelial cells.
E. faecalis binding is regulated by fibronectin from pancreatic ductal epithelial cells
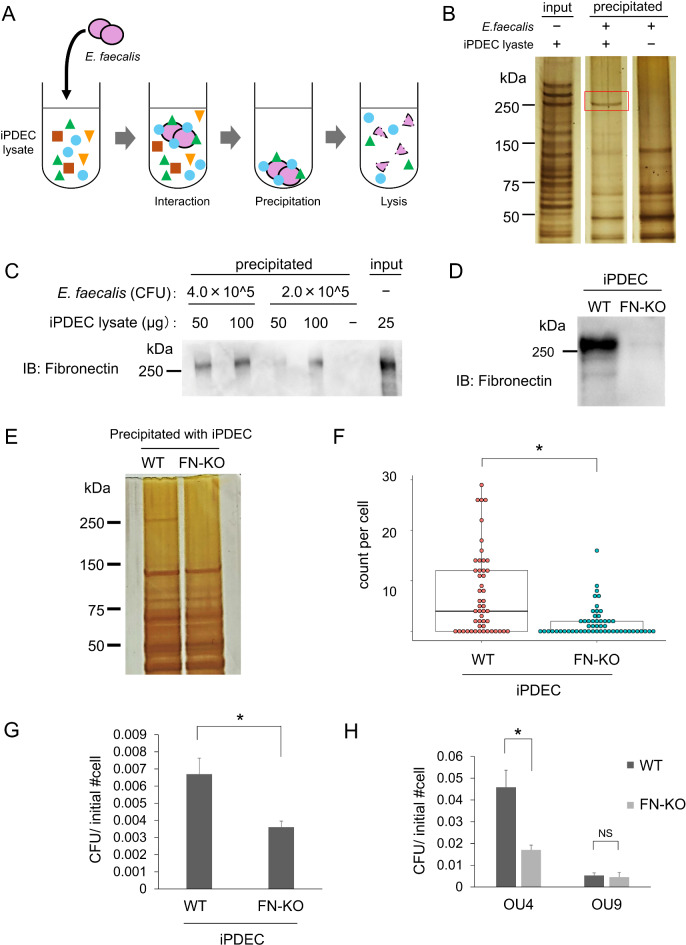
Next, we investigated which proteins on pancreatic epithelial cells bind E. faecalis. iPDEC-derived proteins that bind to E. faecalis were recovered by co-precipitation; iPDEC lysate was mixed with E. faecalis and cell components captured by E. faecalis were collected by centrifugation (Fig. 5A). The recovered proteins were compared to those present in the lysate of E. faecalis bacteria alone by silver staining, and a ~ 260-kDa protein band that resulted from the co-precipitation experiment was observed (Fig. 5B). To identify the protein, the captured lysate was immunoblotted for fibronectin, a known target of EfbA binding with a size of 210–250 kDa that can be detected as a slightly higher band in SDS-PAGE30. Western blot confirmed detection of fibronectin as a ~ 260-kDa band, indicating that fibronectin was captured by E. faecalis (Fig. 5C).
Fig. 5.
E. faecalis binding is regulated by fibronectin from pancreatic ductal epithelial cells. (A) Schema of binding target precipitation with E. faecalis. Live E. faecalis (OU1 strain) was mixed with iPDEC lysate, centrifuged, washed, and lysed together with iPDEC binding proteins (see Materials and Methods for details). (B) Silver staining of SDS-PAGE gel to evaluate iPDEC proteins that precipitated with E. faecalis. Red-boxed band indicates a ~ 260 kDa iPDEC protein that precipitated with OU1. The images were cropped and reordered from an original gel staining image presented in (Fig. S4A). (C) Immunoblotting analysis of proteins precipitated with E. faecalis. E. faecalis and iPDEC lysates were mixed at varying ratios as indicated. Proteins were detected using the indicated anti-fibronectin antibody. The image was cropped from an original blotting image presented in (Fig. S4B). (D) Immunoblotting analysis of wild type (WT) and fibronectin knockouts (FN-KO) of iPDEC. Protein was detected with an anti-fibronectin antibody. The image was cropped from an original blotting image presented in (Fig. S4C). (E) Silver staining of SDS-PAGE gel to evaluate proteins derived from iPDEC WT or FN1-KO cells that precipitated with E. faecalis (OU1 strain). The image was cropped from an original gel staining image presented in (Fig. S4D). (F) Quantification of bacteria (OU1 strain) adherent to WT vs. FN1-KO iPDECs. Data is presented as a box-whisker plot. N = 49 and 54 for WT and FN1-KO, respectively. *P < 0.05, Wilcoxon rank sum test. (G) Quantification of WT and FN1-KO iPDEC bacterial invasion (OU1 strain). CFU was normalized to the number of initially plated iPDECs. N = 3, each. *P < 0.05, t-test. (H) Comparison of WT and FN1-KO iPDEC invasion by OU4 and OU9 strains. CFU was normalized by the number of initially plated iPDECs. N = 3, each. *P < 0.05, t-test with Bonferroni correction.
Lastly, the role of fibronectin in iPDEC infection by E. faecalis was evaluated using fibronectin knockouts (FN1-KO). CRISPR-mediated knockout of FN1, which encodes the human fibronectin monomer, was confirmed by western blot (Fig. 5D). Notably, the ~ 260-kDa band observed in wild-type (WT) iPDEC lysates co-precipitated with E. faecalis was not detected in FN1-KO iPDEC lysates (Fig. 5E). Adhesion of E. faecalis (OU1 strain) to iPDECs was significantly reduced in FN1-KO compared to WT cells (Fig. 5F). Additionally, invasion by the OU1 strain following adhesion was inhibited in FN1-KO iPDEC (Fig. 5G). Fibronectin involvement in invasion was further confirmed using two additional E. faecalis strains, OU4 and OU9 (efbA-high and -low strains, respectively) (Fig. S3). iPDEC invasion by E. faecalis was significantly suppressed by FN1-KO in the efbA-high strain OU4, but not in the efbA-low strain OU9 (Fig. 5H). Taken together, E. faecalis infection of pancreatic ductal cells is regulated by bacteria-host interaction via adhesion molecules including bacterial EfbA and host fibronectin.
Discussion
In this study, we demonstrated that E. faecalis attaches to and invades epithelial cells, particularly pancreatic ductal epithelial lineage cells. Attachment was mediated by E. faecalis EfbA and iPDEC fibronectin. Various types of endocytosis were involved in the entry of E. faecalis into the epithelial cells. Additionally, E. faecalis was capable of penetrating epithelial monolayers via transcytosis in transwell experiments using MDCK cells.
Infection by pathogenic bacteria involves an initial attachment to the host cells. To investigate factors that contribute to the ability to adhere and subsequently invade, we initially began our experiments by comparing JCM7783 (equivalent to ATCC 29212), a commonly used laboratory strain of E. faecalis with low invasion ability, and OU1, which showed high invasion ability and possessed the most virulence factors (Fig. S2). However, no correlation was observed between the virulence factors examined across multiple strains and invasion ability. Therefore, we next focused on EfbA, a fibronectin-binding protein, which was identified from a comparison of gene expression between the two strains. Fibronectin-binding proteins are well-studied molecules known to be involved in this step, especially in Streptococcus sp27. In Streptococcus pyogenes, fibronectin-binding proteins not only regulate binding to fibronectin of the host cells but also facilitate rearrangement of cytoskeletal actin in host cells following bacterial invasion31. EfbA of E. faecalis is a homolog of fibronectin-binding proteins of Streptococcus sp. such as Fbp54 of S. pyogenes32, FbpA of S. gordonii33, SmFnB of S. mutans34, and SfbA of group B streptococci35, collectively referred to as PavA-like proteins27. In contrast to the well-studied streptococcal fibronectin-binding proteins, EfbA has been investigated in only a limited number of studies. For example, EfbA has been reported to contribute to the pathogenesis of infective endocarditis caused by E. faecalis via binding to both fibronectin and collagen types I and V29. Additionally, EfbA involvement in the pathogenesis of urinary tract infections via fibronectin binding has been reported28. This present study is the first report demonstrating the involvement of E. faecalis EfbA in infection of pancreatic ductal epithelial lineage cells. Previously, we reported the detection of E. faecalis in pancreatic tissues10 and the pancreatic ductal juice11 of patients with cancers of the duodeno-pancreato-biliary region, suggesting its possible involvement in the pathogenesis or progression of cancer in this region. Because pancreatic cancer is a complex disease involving many cellular processes unrelated to bacterial infection, the overall extent of the contribution of E. Faecalis to disease progression remains unclear. Nevertheless, accumulating reports provide evidence of microorganism involvement in pancreatic cancer14,36–38. Regarding Enterococcus, several reports have described its possible involvement in colorectal cancer progression39–41. This present study suggests involvement of E. faecalis in pancreatic diseases, specifically via the fibronectin-binding protein EfbA.
Endocytosis is a cellular process by which cells internalize extracellular material through invagination of the plasma membrane, forming vesicles. Endocytosis plays crucial roles in nutrient uptake, receptor internalization, and maintenance of cellular homeostasis. Bacteria can hijack this process to enter host cells, even in non-phagocytes such as epithelial cells19. Clathrin-dependent endocytosis, the predominant subtype of endocytosis, is utilized by diverse bacterial species including gram-positive bacteria such as Staphylococcus and Streptococcus18. Additionally, clathrin-independent endocytosis is involved in bacterial entry to host cells; macropinocytosis is used by group B streptococci to invade brain endothelial cells42, and caveolae-mediated endocytosis is used by S. aureus to invade fibroblasts19. In E. faecalis, previous studies have reported that macropinocytosis is involved in entry into HeLa cells43 and keratinocytes20. Interestingly, E. faecalis does not use clathrin-mediated nor caveolae-mediated endocytosis when entering keratinocytes20. On the other hand, our results demonstrated that E. faecalis utilizes both caveolae-mediated endocytosis and macropinocytosis. Collectively, these data suggest that the mechanism of bacterial entry depends on the combination of the bacterial species and host cell type. The results of this study also shed light on how E. faecalis penetrates epithelial monolayers. Both paracellular and transcellular routes can be proposed as possible means of translocation through the epithelium25. Paracellular transport involves materials leaking through the intercellular spaces by damaging tight junctions. Transcellular transport, on the other hand, consists of material transport by transcytosis, in which materials in vesicles traffic across a polarized cell via endocytosis from one side and exocytosis from the other. We demonstrated that penetration of E. faecalis through MDCK monolayers was inhibited by Dynasore, which inhibits multiple steps of transcytosis by suppressing clathrin-mediated and caveolae-mediated endocytosis as well as intracellular trafficking of vesicles26,44,45. These results suggest that E. faecalis penetrates through epithelial monolayers via transcytosis.
Taken together, we propose a model in which E. faecalis attaches to the pancreatic duct via EfbA-fibronectin interactions and is internalized into host cells via endocytosis. A portion of the endocytosed E. faecalis can then migrate to pancreatic tissues via transcytosis. Future studies, including in vivo experiments, will clarify how this model is involved in the pathogenesis of pancreatic cancers. Additionally, these studies may contribute to the identification of patient populations that are at high risk for pancreatic cancer and the development of prevention and therapeutic strategies.
Materials and methods
Cell culture
To generate iPDECs, normal human pancreatic ductal epithelial cells (HPDECs) purchased from Cell Systems Corporation (ACBRI 515; Kirkland, WA, USA) were immortalized. A cDNA fragment encoding murine ecotropic retrovirus receptor (EcoVR) was obtained from the retroviral plasmid pCX4hyg-EcoVR46 and subcloned into pCX4 vector. cDNA fragments for human TP53R175H were generated by PCR and subcloned into pCX4.1hisD (GenBank; AB086389) retroviral vector. pCX4bsr_CDK4 and pCX4neo_hTERT retroviral vectors have been described previously47. Retroviral-mediated gene transfer was carried out as described previously47 using retrovirus-packaging constructs (pGP, pE-Ampho, pE-Eco; Takara Bio, Shiga, Japan). EcoVR was first introduced into HPDECs using amphotropic virus to induce susceptibility to subsequent infection with ecotropic viral vectors. The EcoVR-expressing HPDECs were then infected with retroviruses expressing CDK4, hTERT, and a dominant negative form of p53 (TP53R175H). The infected cell populations were selected in culture media containing blasticidin (2 µg/ml), G418 (500 µg/ml), and L-histidinol (4 mM) for 2 weeks. iPDECs were established following polyclonal cell expansion. MiaPaCA2, PSN-1, and MDCK cells were obtained from JCRB Cell Bank (NIBIOHN, Osaka, Japan). iPDECs were grown and maintained in iPDEC culture medium [DMEM/F12 GlutaMAX (Gibco Thermo Fisher Scientific, Waltham, MA) containing 2 mg/ml BSA (fatty acid free) (Fujifilm Wako, Osaka, Japan), 5 mM nicotinamide (Sigma-Aldrich, St. Louis, MO), 1× insulin-transferrin-selenium (Thermo Fisher Scientific), 50 ng/ml recombinant human EGF (PeproTech, Cranbury, NJ), and 12.5 ng/ml recombinant human FGF10 (PeproTech)] using collagen type I–coated 60-mm dishes (IWAKI, Tokyo, Japan). MIA PaCa-2 cells were grown and maintained in DMEM (Nacalai Tesque, Kyoto、Japan) containing 10% fetal bovine serum (FBS, MERCK, Darmstadt, Germany), 100 U/ml penicillin and 100 µg/ml streptomycin (Nacalai Tesque). PSN-1 cells were cultured in RPMI1640 (Nacalai Tesque) supplemented with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin. MDCK cells were grown and maintained in DMEM (Nacalai Tesque) containing 10% FBS, 1× MEM Non-essential Amino Acids Solution (Wako), 100 U/ml penicillin, and 100 µg/ml streptomycin. All cells were cultured at 37 °C in a 5% CO2 humidified atmosphere. Cell viability was evaluated by ATP assay using CellTiter-Glo (Promega, Madison, WI) according to the manufacturer’s instructions, and luminescence was measured with an INFINITE M PLEX plate reader (Tecan, Männedorf, Switzerland).
Enterococcus faecalis
The ethical committee at Osaka University Hospital approved the study protocol (protocol IDs 14107 and 15212), and written informed consent was obtained from each participant. This study was performed in accordance with the Declaration of Helsinki. E. faecalis strain JCM7783 (derived from ATCC29212 and deposited by R. Sakazaki) was obtained from RIKEN BRC (Ibaraki, Japan), and other strains were isolated from pancreatic juice of patients with cancers of the duodeno-pancreato-biliary region. E. faecalis cells were cultured using Colombia media [3.5% Difco Columbia broth (BD, Franklin Lakes, NJ) in water] or Brain Heart Infusion (BHI) agar [3.7% BBL Brain Heart Infusion (BD) and 1.35% Bacto Agar (BD) in water]. Unless otherwise stated, glycerol stocks of bacterial strains were prepared using the following method: cell culture was performed in Columbia media for 3.5 h, then centrifuged at 4,000 × g for 5 min at room temperature, followed by pellet resuspension in phosphate-buffered saline (PBS) containing 25% glycerol. The suspension was aliquoted and stored at − 80 °C until each experiment. To determine CFU, a portion of the aliquot was withheld and cultured on BHI agar overnight. Bacterial growth was evaluated by absorbance of the culture media at 660 nm measured by a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA).
Adhesion assay
iPDECs were grown to 90% confluence on 35-mm glass base dishes coated with type I collagen (IWAKI) and then incubated with iPDEC medium containing E. faecalis at MOI 100. After 2 h, the medium containing E. faecalis was removed, and the dishes were washed seven times with warm PBS containing calcium to remove nonadherent bacteria. The cells were fixed with methanol for 10 min at room temperature, washed, and stained with Giemsa staining reagent (Muto pure chemicals, Tokyo, Japan) for 30 min. The attachment of E. faecalis to iPDECs was observed and recorded with a BZ-9000 microscope (KEYENCE, Osaka, Japan).
Semi-quantitative real time RT-PCR
For RNA extraction, bacterial pellets were suspended in Sepasol RNA Super G (Nacalai Tesque) containing zirconia balls (YTZ-0.4, Nikkato, Osaka, Japan) and crushed with a TWIN MIXER TM-282 (AS ONE, Osaka, Japan). Total RNA was then extracted according to the manufacturer’s protocol. cDNA was synthesized with the GoScript reverse transcription system (Promega) using a T100™ Thermal cycler (Bio-Rad, Hercules, CA). A 10-fold dilution of the synthesized cDNA was used for each semi-quantitative real-time reverse transcription PCR (RT-PCR) by Thunderbird SYBR qPCR Mix with ROX reference dye (Toyobo, Osaka, Japan) using a MxPro Real-Time QPCR system (Mx3000P, Agilent Technologies, Santa Clara, CA). In the gene expression analysis, 16S rRNA was used as the reference gene, and the relative quantification method (ΔΔCT) was used for comparison. The primer pairs used were as follows: efbA F: 5’-ACAAGGCGGCGAACTAATTC-3’, efbA R: 5’-TCGCGACGGTAATTTTCAGC-3’, E. faecalis 16 S-Ef16S F: 5’-TTCGAAGCAACGCGAAGAAC-3’, E. faecalis 16 S-Ef16S R: 5’-TGCACCACTGTCACTTTGTC-3’.
Invasion assay
iPDECs were grown in collagen-coated 24-well plates (IWAKI). BxPC3 and PSN-1 cells were grown to 80–90% confluence in tissue culture 24-well plates (IWAKI), and then medium containing 3.0 × 106 CFU of E. faecalis was added. After 3 h, the cells were treated for an additional 2 h with 1,000 µg/ml ampicillin and 1,000 µg/ml streptomycin to kill all bacteria outside the epithelial cell surface. Invasive bacteria were recovered by cell lysis using a final concentration of 0.1% Triton X-100 (MP Biomedicals, Santa Ana, CA), and the number of cells recovered was determined by culturing cell lysate on BHI agar plates.
To evaluate the effect of the inhibitors on E. faecalis invasion, cytochalasin D (Wako), EIPA (Selleck), genistein (Wako), MβCD (Sigma-Aldrich), and Dynasore (Selleck) were added to the medium at the indicated concentrations for 1 h before bacterial infection.
Transwell penetration assay
MDCK cells were grown on the upper well of transwell culture plates (6-well, pore size 3 μm, Greiner Bio-One, Kremsmünster, Austria) to form epithelial monolayers48 for 2 to 3 weeks until trans-epithelial electrical resistance (TEER) increased and stabilized. TEER was measured using a Millicell®ฏ ERS-2 Epitherial Volt/Ohm Meter (Merck Millipore, Burlington, MA). For the penetration assay, 2 ml of MDCK medium containing 4.0 × 106 CFU/ml of E. faecalis was added to the upper well. E. faecalis migration through the MDCK cells was quantified by collecting the medium in the lower chamber at the indicated times and plating on BHI agar to assess the number of CFUs formed. To inhibit penetration, MDCK monolayers were pretreated with Dynasore for 1 h before addition of the indicated concentrations of E. faecalis.
Fluorescent microscopic analysis
To visualize entry of E. faecalis into epithelial cells, the E. faecalis OU1 strain was fluorescently labeled with 5(6)-CFDA N-succinimidyl ester (CFSE, Abcam, Cambridge, UK) prior to infection for 3 h at MOI 100. Cells were then washed with Hank’s Balanced Salt Solution (with calcium, Nacalai Tesque), and the bacteria outside iPDECs were eliminated by treating with 1,000 µg/ml ampicillin and 1,000 µg/ml streptomycin for 2 h. Cells were then fixed with 4% paraformaldehyde for 15 min, washed, and iPDEClabeled with Hoechst 33342 (Nacalai Tesque) and Plasmem bright red (Dojindo, Kumamoto, Japan). Optical sectioning images were obtained using the sectioning module of a BZ-9000 fluorescent microscope (KEYENCE), and z-stacked images were reconstructed.
Western blot
Cells were lysed in TNE buffer (10 mM Tris-HCl pH 7.8, 1 mM EDTA, 1% Nonidet P-40, and 0.15 M NaCl), and proteins were electrophoresed on polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Merck Millipore). After blocking with Tris-buffered saline-Tween 20 (TBST) containing 5% skim milk, proteins were labeled using the following primary and secondary antibodies: Anti-Fibronectin (Abcam, Cambridge, UK) and HRP-conjugated anti-rabbit IgG (Promega). Signals were developed using Chemi-Lumi One Super (Nacarai Tesque) and detected with a FUSION chemiluminescence imaging system (Vilber-Lourmat, Collegien, France).
Typing of the highly conjugative plasmids
E. faecalis has been identified as a causative bacteria of urinary tract infections and endocarditis and is known to harbor virulence factors encoded by conjugative plasmids. We selected the following virulence factors that are commonly detected in E. faecalis associated with urinary tract infections: asaI, CylA, aphA3, aacA/aphD, aadC, gelE, and esp. Product sizes and primer sequences are summarized in (Supplementary Table S1). The PCR amplification protocol was as follows: denaturation at 95 °C for 3 min; 35 cycles at 95 °C for 30 s, 50 °C for 30 s, and 72 °C for 1 min; and final extension at 72 °C for 7 min.
RNA sequencing
E. faecalis strains JCM7783 and OU1 were cultured in Columbia media and collected during the growth phase. Bacteria were centrifuged, and RNA was extracted from the pellets as described above. Data analysis using a next-generation sequencer was performed by the Department of Genome Informatics, Bioinformatics Center, Research Institute for Microbial Diseases, Osaka University. In brief, ribosomal RNA was removed from the extracted total RNA, and the remaining RNA was fragmented using the Ribo-Zero Plus rRNA Depletion Kit (Illumina, San Diego, CA). Library preparation was performed using the TruSeq stranded mRNA sample prep kit (Illumina) according to the manufacturer’s instructions. Sequencing was performed on an Illumina NovaSeq 6000 sequencer (Illumina) in 101-base single-read mode. After removing adapter sequences with Trimmomatic version 0.38, the reads were mapped to the E. faecalis genome sequences (GCF_001999625.1 and GCF_000147595.1) using Hisat version 2.1.0. Raw counts were calculated using featureCounts version 2.0.0. Cuffdiff version 2.2.1 was used to calculate the FPKMs. Bioinformatics analysis was performed using Integrated Differential Expression and Pathway Analysis (iDEP) v. 0.81.
Target protein precipitation with E. faecalis
iPDECs were suspended in TNE buffer and disrupted and lysed by sonication. iPDEC lysate (25 µg) iPDECwas mixed with 1.0 × 109E. faecalis (OU1 strain) in 200 µl PBS, and the mixture was incubated in a 1.5 ml centrifuge tube at 4 °C for 1 h using a rotator. After the incubation, E. faecalis was spun down by centrifugation at 4000 × g, washed twice with PBS, and resuspended in 15 µl of sterile deionized water. Next, bound proteins were eluted from the bacterial cells by adding SDS-Tris buffer (0.2 M Tris, 8% SDS) and boiling at 95 °C for 5 min, followed by centrifugation at 20,000 × g at 4 °C for 20 min. The supernatant was collected as a mixture of E. faecalis proteins and iPDEC-derived proteins bound to E. faecalis. Silver staining was performed using the Silver Stain MS Kit (Wako) according to the manufacturer’s protocol.
FN1 knockout by CRISPR/CAS9 system
Genome editing of FN1 was performed with the CRISPR/Cas9 system. The gRNA targeting sequences for FN1 were cloned into the BsmBI site of lentiCRISPR v2 puromycin (Addgene 52961, gift from Feng Zhang), which was obtained from Addgene (Watertown, MA). Pairs of oligonucleotides used for cloning are as follows: hFN sgRNA F: 5’-caccgCTCTTCGAGGCTCCCGTGGA-3’ and hFN sgRNA R: 5’-aaacTCCACGGGAGCCTCGAAGAGC-3’. To produce lentivirus, the lentiCRISPR plasmids containing packaging plasmids (pMD2.G and psPAX2) were transfected into HEK293T cells using lipofectamine 2000 (Thermo Fisher Scientific), and the medium was collected after 2 days. iPDECs were infected with the virus solution in the presence of 10 µg/ml polybrene (Nacalai tesque). Infected cells were selected with 4 µg/ml puromycin (InvivoGen, SanDiego, CA) to remove WT cells, and FN1-KO was confirmed by western blot.
Statistical analysis
Significance was tested using an unpaired Student’s t-test or Wilcoxon rank sum test for single comparisons. T-tests with Bonferroni corrections were applied for multiple comparisons, and multiple comparisons to untreated controls were made using the Dunnett test. For correlation, Pearson’s product-moment correlation coefficient was designated as the R-value. Statistical significance was set at P < 0.05.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Masaya Yamaguchi and Shigetada Kawabata from Osaka University Graduate School of Dentistry for scientific suggestions. We acknowledge the NGS core facility at the Research Institute for Microbial Diseases of Osaka University for assistance with sequencing and data analysis. This study was supported by Japanese KAKENHI from the Ministry of Education, Culture, Sports, Science and Technology of Japan (numbers A20H05675 and A22H02967).
Author contributions
M. Shimosaka: Investigation and Methodology. J. Kondo: Conceptualization, Validation, Formal analysis, Writing—Original Draft and Review & Editing, and Visualization. M. Sonoda, R. Kawaguchi, E. Noda, K. Nishikori, A. Ogata, and K. Sasai: Investigation. S. Takamatsu: Investigation and Writing—Review & Editing. H. Akita and H. Eguchi: Resources. Y. Kamada and S. Okamoto: Writing—Review & Editing. E. Miyoshi: Conceptualization, Writing—Review & Editing, Supervision, Project administration, and Funding acquisition. All authors reviewed the manuscript.
Data availability
The datasets generated and analyzed during the current study are available in the DDBJ repository, BioProject Accession number PRJDB18786. The other data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Munefumi Shimosaka and Jumpei Kondo contributed equally to this work.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-86531-9.
References
- 1.Muñoz, P. et al. Current epidemiology and outcome of infective endocarditis. Medicine94, e1816. 10.1097/md.0000000000001816 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cahill, T. J. & Prendergast, B. D. Infective endocarditis. Lancet387, 882–893. 10.1016/s0140-6736(15)00067-7 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Flores-Mireles, A. L., Walker, J. N., Caparon, M. & Hultgren, S. J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol.13, 269–284. 10.1038/nrmicro3432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiore, E., Van Tyne, D. & Gilmore, M. S. Pathogenicity of Enterococci. Microbiol. Spectr.710.1128/microbiolspec.gpp3-0053-2018 (2019). [DOI] [PMC free article] [PubMed]
- 5.Barnes, A. M. T. et al. Enterococcus faecalis colonizes and forms persistent biofilm microcolonies on undamaged endothelial surfaces in a rabbit endovascular infection model. FEMS Microbes. 210.1093/femsmc/xtab014 (2021). [DOI] [PMC free article] [PubMed]
- 6.Diederich, A. K. et al. Role of glycolipids in the pathogenesis of Enterococcus faecalis urinary tract infection. PLoS One9, e96295. 10.1371/journal.pone.0096295 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro, M. S. et al. Probiotic activity of Enterococcus faecalis CECT7121: effects on mucosal immunity and intestinal epithelial cells. J. Appl. Microbiol.121, 1117–1129. 10.1111/jam.13226 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Olmsted, S. B., Dunny, G. M., Erlandsen, S. L. & Wells, C. L. A plasmid-encoded surface protein on Enterococcus Faecalis augments its internalization by cultured intestinal epithelial cells. J. Infect. Dis.170, 1549–1556. 10.1093/infdis/170.6.1549 (1994). [DOI] [PubMed] [Google Scholar]
- 9.Zeng, J., Teng, F., Weinstock, G. M. & Murray, B. E. Translocation of < i > Enterococcus faecalis strains across a monolayer of polarized human enterocyte-like T84 cells. J. Clin. Microbiol.42, 1149–1154. 10.1128/jcm.42.3.1149-1154.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maekawa, T. et al. Possible involvement of Enterococcus infection in the pathogenesis of chronic pancreatitis and cancer. Biochem. Biophys. Res. Commun.506, 962–969. 10.1016/j.bbrc.2018.10.169 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Itoyama, S. et al. Enterococcus spp. have higher fitness for survival, in a pH-dependent manner, in pancreatic juice among duodenal bacterial flora. JGH Open.6, 85–90. 10.1002/jgh3.12703 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geller, L. T. et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science357, 1156–1160. 10.1126/science.aah5043 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas, R. M. et al. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis39, 1068–1078. 10.1093/carcin/bgy073 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pushalkar, S. et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov.8, 403–416. 10.1158/2159-8290.cd-17-1134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomita, Y. et al. Pancreatic fatty degeneration and fibrosis as predisposing factors for the development of pancreatic ductal adenocarcinoma. Pancreas43, 1032–1041. 10.1097/mpa.0000000000000159 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Rubinstein, E. et al. Antibacterial activity of the pancreatic fluid. Gastroenterology88, 927–932. 10.1016/s0016-5085(85)80009-3 (1985). [DOI] [PubMed] [Google Scholar]
- 17.Weckwerth, P. H. et al. In vitro alkaline pH resistance of Enterococcus faecalis. Braz Dent. J.24, 474–476. 10.1590/0103-6440201301731 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Veiga, E. & Cossart, P. The role of clathrin-dependent endocytosis in bacterial internalization. Trends Cell Biol.16, 499–504. 10.1016/j.tcb.2006.08.005 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann, C. et al. Caveolin limits membrane microdomain mobility and integrin-mediated uptake of fibronectin-binding pathogens. J. Cell Sci.123, 4280–4291. 10.1242/jcs.064006 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Da Silva, R. A. G. et al. Enterococcus faecalis alters endo-lysosomal trafficking to replicate and persist within mammalian cells. PLoS Pathog.18, e1010434. 10.1371/journal.ppat.1010434 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, H. P. et al. Identification of novel macropinocytosis inhibitors using a rational screen of food and drug administration-approved drugs. Br. J. Pharmacol.175, 3640–3655. 10.1111/bph.14429 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koivusalo, M. et al. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol.188, 547–563. 10.1083/jcb.200908086 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamei, N. et al. Optimization of the method for analyzing endocytosis of fluorescently tagged molecules: impact of incubation in the cell culture medium and cell surface wash with glycine-hydrochloric acid buffer. J. Control. Release310, 127–140. 10.1016/j.jconrel.2019.08.020 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Itagaki, M., Nasu, Y., Sugiyama, C., Nakase, I. & Kamei, N. A universal method to analyze cellular internalization mechanisms via endocytosis without non-specific cross‐effects. FASEB J.3710.1096/fj.202201780r (2023). [DOI] [PubMed]
- 25.Backert, S., Boehm, M., Wessler, S. & Tegtmeyer, N. Transmigration route of campylobacter jejuni across polarized intestinal epithelial cells: paracellular, transcellular or both? Cell. Commun. Signal.11, 72. 10.1186/1478-811x-11-72 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preta, G., Cronin, J. G. & Sheldon, I. M. Dynasore - not just a dynamin inhibitor. Cell. Commun. Signal.1310.1186/s12964-015-0102-1 (2015). [DOI] [PMC free article] [PubMed]
- 27.Yamaguchi, M., Terao, Y. & Kawabata, S. Pleiotropic virulence factor. Streptococcus pyogenes fibronectin-binding proteins. Cell. Microbiol.15, 503–511. 10.1111/cmi.12083 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Torelli, R. et al. The PavA-like fibronectin-binding protein of Enterococcus faecalis, EfbA, is important for virulence in a mouse model of ascending urinary tract infection. J. Infect. Dis.206, 952–960. 10.1093/infdis/jis440 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Singh, K. V., Rosa, L., Somarajan, S. L., Roh, S. R., Murray, B. E. & J. H. & The fibronectin-binding protein EfbA contributes to pathogenesis and protects against infective endocarditis caused by Enterococcus faecalis. Infect. Immun.83, 4487–4494. 10.1128/iai.00884-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kornblihtt, A. R., Umezawa, K., Vibe-Pedersen, K. & Baralle, F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J.4, 1755–1759. 10.1002/j.1460-2075.1985.tb03847.x (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molinari, G., Talay, S. R., Valentin-Weigand, P., Rohde, M. & Chhatwal, G. S. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group a Streptococci by epithelial cells. Infect. Immun.65, 1357–1363. 10.1128/iai.65.4.1357-1363.1997 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawabata, S. et al. Systemic and mucosal immunizations with fibronectin-binding protein FBP54 induce protective immune responses against < i > Streptococcus pyogenes challenge in mice. Infect. Immun.69, 924–930. 10.1128/iai.69.2.924-930.2001 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christie, J., McNab, R. & F Jenkinson, H. Expression of fibronectin-binding protein FbpA modulates adhesion in Streptococcus gordonii. Microbiology148, 1615–1625. 10.1099/00221287-148-6-1615 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Miller-Torbert, T. A., Sharma, S. & Holt, R. G. Inactivation of a gene for a fibronectin-binding protein of the oral bacterium Streptococcus mutans partially impairs its adherence to fibronectin. Microb. Pathog.45, 53–59. 10.1016/j.micpath.2008.02.001 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mu, R. et al. Identification of a group B streptococcal fibronectin binding protein, SfbA, that contributes to invasion of brain endothelium and development of meningitis. Infect. Immun.82, 2276–2286. 10.1128/iai.01559-13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAllister, F., Khan, M. A. W., Helmink, B. & Wargo, J. A. The tumor microbiome in pancreatic cancer: Bacteria and beyond. Cancer Cell.36, 577–579 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Sethi, V., Vitiello, G. A., Saxena, D., Miller, G. & Dudeja, V. The role of the microbiome in immunologic development and its implication for pancreatic cancer immunotherapy. Gastroenterology 156, 2097–2115.e (2092). 10.1053/j.gastro.2018.12.045 (2019). [DOI] [PubMed]
- 38.Guo, W. et al. Tumor microbiome contributes to an aggressive phenotype in the basal-like subtype of pancreatic cancer. Commun. Biol.410.1038/s42003-021-02557-5 (2021). [DOI] [PMC free article] [PubMed]
- 39.Williamson, A. J. et al. Enterococcus faecalis promotes a migratory and invasive phenotype in colon cancer cells. Neoplasia27, 100787. 10.1016/j.neo.2022.100787 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Almeida, C. et al. Differential responses of colorectal cancer cell lines to Enterococcus faecalis’ strains isolated from healthy donors and colorectal cancer patients. J. Clin. Med.8, 388. 10.3390/jcm8030388 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Almeida, C. V., Taddei, A. & Amedei, A. The controversial role of Enterococcus faecalis in colorectal cancer. Therapet. Adv. Gastroenterol.11, 175628481878360. 10.1177/1756284818783606 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Espinal, E. R. et al. Group B Streptococcus-induced macropinocytosis contributes to bacterial invasion of brain endothelial cells. Pathogens11, 474. 10.3390/pathogens11040474 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertuccini, L., Ammendolia, M., Superti, F. & Baldassarri, L. Invasion of HeLa cells by Enterococcus faecalis clinical isolates. Med. Microbiol. Immunol.191, 25–31. 10.1007/s00430-002-0115-4 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Henley, J. R., Krueger, E. W. A., Oswald, B. J. & McNiven, M. A. Dynamin-mediated internalization of caveolae. J. Cell Biol.141, 85–99. 10.1083/jcb.141.1.85 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirchhausen, T., Macia, E. & Pelish, H. E. in Methods in Enzymology Vol. 438, 77–93 (Elsevier, 2008). [DOI] [PMC free article] [PubMed]
- 46.Akagi, T., Sasai, K. & Hanafusa, H. Refractory nature of normal human diploid fibroblasts with respect to oncogene-mediated transformation. Proc. Natl. Acad. Sci.100, 13567–13572. 10.1073/pnas.1834876100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasai, K. et al. Oncogene-mediated human lung epithelial cell transformation produces adenocarcinoma phenotypes in vivo. Cancer Res.71, 2541–2549. 10.1158/0008-5472.can-10-2221 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Irvine, J. D. et al. MDCK (madin-darby canine kidney) cells: a tool for membrane permeability screening. J. Pharm. Sci.88, 28–33. 10.1021/js9803205 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available in the DDBJ repository, BioProject Accession number PRJDB18786. The other data that support the findings of this study are available from the corresponding author upon reasonable request.