Abstract
Background
Blood transfusions are the most common procedure performed in American hospitals. The steps required for blood product delivery are often misunderstood by providers, leading to numerous phone calls to the blood bank requesting order status. Distracting calls can lengthen turnaround time, especially during blood product or staff shortages. We sought a tool to address these questions and reduce distraction. This study highlights a novel blood tracker tool implemented in the electronic health record (EHR) that allows providers to see their blood order's status.
Study Design and Methods
With a multidisciplinary team of healthcare professionals, we constructed a user‐friendly blood tracker highly visible in our EHR. It shows the status of a blood product in real time from “order printed” to “preparing” to “on its way.” We surveyed blood bank technologists to determine if call volume and distraction changed.
Results
We counted the number of views per month as a surrogate of usage. The blood tracker was viewed 109,626 times per month on average from January through December 2023. The fraction of technologists who received 21 or more calls per shift decreased by 47%.
Discussion
We successfully constructed and implemented a novel blood tracker into our EHR that relays the status of a blood product. It is a highly viewed piece of information in our EHR and decreases blood bank call volume as reported by blood bank technologists. Its success demonstrates that closed‐loop communication between the lab and providers regarding blood products is beneficial within our organization and potentially others.
Abbreviations
- electronic health record
EHR
- Vanderbilt University Medical Center
VUMC
- turnaround time
TAT
- red blood cells
RBC
- operating room
OR
- information technology
IT
- blood bank laboratory information system
BBLIS
1. INTRODUCTION
Blood product transfusions are the most common procedures performed in American hospitals. 1 Multiple healthcare providers located throughout the hospital complete several steps to get a blood product to a patient. These include the provider ordering the product, the completion of pre‐transfusion tests including the type and screen, crossmatching, and any additional steps such as irradiation, and product delivery. 2 Despite being a widely prevalent procedure, most clinicians are unaware of the complexities of blood transfusion. Many call the blood bank with questions, prolonging turnaround times. These inquiries are more likely to occur during times of added stress, such as blood shortages, staff shortages, or patients who require additional testing due to high alloantibody burden or a rare blood type. 2 , 3 , 4 , 5 , 6
At Vanderbilt University Medical Center (VUMC), the blood bank services the adult and children's hospital. In 2023, approximately 40,000 units of red blood cells, 16,000 units of plasma, 13,000 units of platelets, and 3000 units of cryoprecipitate were transfused to about 13,000 patients, or about 20% of our annual patient discharges. Our electronic health record (EHR) and blood bank laboratory information system (BBLIS) are Epic (Verona, Wisconsin) and Softbank (Clearwater, Florida), respectively. A provider first orders the product in the computerized physician order entry (CPOE) portion of our EHR. The transfusionist sees the order and requests the product from the EHR when ready. The request is electronically routed to the blood bank where it is printed and viewed by a technologist. The technologist selects the appropriate unit, completes additional steps like irradiation if needed, notes these steps in the BBLIS, and dispenses the unit to the patient's location. For inpatients or clinic patients, the product is transported via pneumatic tube system. For patients in the operating room (OR), the product is prepared in a cooler for the requesting team to pick up. In 2023, our expected turnaround time (TAT) for nonemergency orders from order receipt to product release was approximately 20 min for uncomplicated red blood cells (RBCs) (which are crossmatched on demand), 30 min for irradiated RBCs, 30 min for thawed plasma, and 20 min for uncomplicated platelets. This process is routinely disrupted by phone calls from providers querying the product's status. Common questions we receive are: “Did my order print?” “When will my blood be ready?” and “When does my patient's type and screen expire?” These frequent queries likely stem from a knowledge gap in transfusion medicine and blood bank workflow. Studies have shown that medical providers, including attendings, residents, and fellows, have poor transfusion medicine knowledge, lack of training in the field, and believe additional transfusion medicine education would be useful. 3 , 4 , 5 Among 474 internal medicine residents at 23 programs in nine countries who were administered a transfusion medicine assessment tool, the average correct response rate was 45.7%. 3 As such, clinicians often call the blood bank with questions about order status. Phoning in directly is required due to the lack of a method to answer these questions in the EHR. 7
These frequent calls interrupt blood bank staff and distract them from their workflow, potentially increasing blood product TAT. Fast blood product delivery is not only a quality indicator of efficient and satisfactory laboratory services but is critical during surgical emergencies. Surgical blood loss during scheduled procedures may often be unexpected and accounts for about 40% of all RBC transfusions administered in hospitals in the United States. 2 , 7 It is essential that the receipt of these products occurs in a timely fashion to ensure surgical success and patient stability. Distractions of blood bank staff jeopardize this. Thus, our goal was to create a tool that could be easily viewed by clinicians and answer the most common questions the blood bank staff were being asked, most of which were related to the order status. We developed a tracker module built into a preexisting summary report in our EHR, the “Transfusion Dashboard,” which later expanded into a different, more visible place within our EHR. 8 With this novel blood product tracker, clinicians can directly view the stage their order is at with timepoints and automated updates, diminishing the need to call the blood bank.
2. STUDY DESIGN AND METHODS
We assembled a multidisciplinary team of physicians, nurses, health information technology (IT) staff, blood bank leadership, quality improvement department leadership, and Transfusion Safety Officers, who collaborated to design the blood tracker. While brainstorming, we studied commercial package trackers for e‐commerce or food delivery. These tools describe when products are ordered, what stage of processing they are in, and when the customer should expect delivery. With user‐centered health technologies and accessibility in mind, our health IT specialists constructed the tracker integrating information in real time from the BBLIS into the highly visible “Transfusion Dashboard” within our EHR. 9 , 10 Because of its immediate, widespread use, the blood tracker was quickly copied into another location, the “Storyboard” portion of our EHR, which is always visible in a patient chart (Figure 1A).
FIGURE 1.
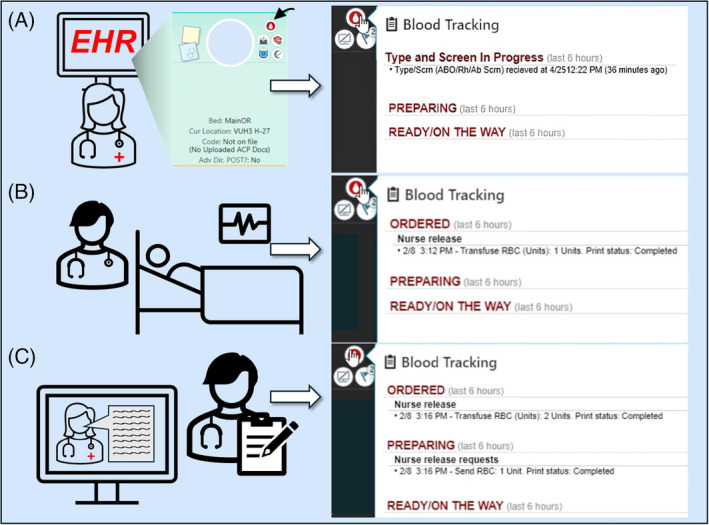
(A). The provider orders a Type and Screen, triggering the “blood drop” icon to appear on the Storyboard (indicated by arrow). Hovering over the blood drop will display the first step of the tracker: “Type and Screen in Progress” and the order's date and time. (B). When the provider orders blood products (in this example, one unit of pRBCs), they will display under “Ordered” with the order date, time, and shows a completed print status. The nurse then prepares the patient for transfusion. (C). The nurse releases the blood product, which prints the request for blood products in the blood bank. This is shown on the tracker under “Preparing,” with the date and time of the release request and print status. [Color figure can be viewed at wileyonlinelibrary.com]
The tracker shows the blood product's status through ordering to dispensation and gives a delivery time estimate. To start, a physician places an order in CPOE for a blood product, which prompts a “blood drop” icon to appear on the patient's chart in a highly visible location, the “Storyboard” (Figure 1A). Hovering over this icon allows the physician to continuously monitor the status of the blood product order since the blood tracker is automatically updated in real time by the BBLIS. After the physician places the order, it is visible on the transfusionist's task list in Epic, who can release the blood product request when ready. At this point, hovering over the icon will show “Ordered, Nurse release” (Figure 1B). Once the order is released, the blood bank receives a printed request to send the blood product. While the technologist prepares the product, the BBLIS will communicate with the EHR, prompting the tracker to display “Preparing” (Figure 1C). Once the blood product is ready for delivery, the technologist will issue the product in the BBLIS, prompting the blood tracker to display “Ready/On The Way” (Figure 2A). The products are sent either via pneumatic tube (which is complex and can take up to 30 min for delivery between our hospitals) or cooler as indicated on the order. Once the blood is delivered to the patient and scanned, the “blood drop” icon disappears from the patient's chart. The “Transfusion Dashboard” will then display the start time and finish of one or multiple blood products and the expiry of the type and screen. This dashboard also shows all past blood products administered to the patient in their lifetime at VUMC (Figure 2B).
FIGURE 2.

(A). After completing preparation of the blood products and issuing to the patient in the BBLIS, the blood tracker will display “Ready/On the Way” with the date and time of issue for each product ordered. The blood bank technologist then sends the blood product via pneumatic tube system, or a cooler picked up by the requesting team. In this way, providers and nurses can mointor the status of the blood product until the patient receives it. (B). Following product administration, the “Transfusion Dashboard” displays all blood products given. BBLIS: blood bank laboratory information system. [Color figure can be viewed at wileyonlinelibrary.com]
Following go‐live in January 2023, we counted the number of views per month as a surrogate of usage. We achieved this by implementing custom code. Each month, this code calculates the total views by summing up the instances where the blood tracker print group is displayed for an individual user. Each instance counts as one view. Custom code was essential because the blood tracker print group appears in multiple reports and only displays under specific conditions, such as when there are blood orders. We also collected electronic survey data from blood bank technologists to gauge their perceptions of the tracker and workflow changes following the go live. This voluntary survey was electronically delivered and asked what volume of calls (0–10, 11–20, or >21) was received during the shift and how distracting the volume was (very mild, mild, moderate, and severe). The survey was administered in the month just prior to tracker go‐live and readministered after 6 months (Figure 3).
FIGURE 3.

A voluntary survey was electronically delivered to blood bank technologists and asked what volume of calls (0–10, 11–20, or > 21) was received during the shift and how distracting the volume was (very mild, mild, moderate, and severe). The survey was administered in the month just prior to tracker go‐live and readministered after 6 months. [Color figure can be viewed at wileyonlinelibrary.com]
3. RESULTS
The blood tracker was viewed 109,626 times per month on average from January 2023 through December 2023. This is comparable to common reports such as Patient Summary (118,471 views in December 2023) and Meds History (88,635 views in December 2023). The technologist survey demonstrated that prior to the tracker's implementation, 65% of respondents received over 10 calls per shift, and there was a positive relationship between call volume and distraction. Six months after the go‐live, the fraction of respondents who received over 21 calls per shift decreased from 39% to 20% (Figure 3).
4. DISCUSSION AND CONCLUSION
We have successfully constructed and implemented a novel blood tracker into our EHR that relays the status of a blood product from ordering to dispensation. It displays all relevant information to ordering providers, including if their order printed, when the blood product is being prepared, and when the product is en route. It averaged almost 110,000 views per month in 2023. Importantly, our survey data suggests the tracker limits the distraction from phone calls of our blood bank technologists, which has been linked to significant transfusion delays and subsequently associated with patient safety concerns especially in an emergency setting. 7 Using survey data, call volume is also estimated to have decreased following tracker implementation. However, this survey is limited by low response rate, perhaps due to its voluntary nature. Readministration 6 months after tracker implementation, rather than sooner, might have introduced recall bias. Moreover, the only “severely distracting” responses (N = 4 of 15) in the post‐implementation survey were recorded during a platelet shortage, which might have skewed the data.
This tracker's success is due to multidisciplinary input into its design and Vanderbilt Health IT's deep knowledge of the BBLIS and EHR. One limitation is its inability to track products after leaving the blood bank. Currently, it only lists “Ready/On The Way” for the entire journey from the blood bank to the patient, and we give a 30‐min disclaimer for arrival. Additionally, the tracker is not available during downtime events such as power outages or cyberattacks. Because the ordering and product preparation is distinct, this tool does not track massive transfusion protocols (MTPs) or distribution from offsite refrigerators such as those containing O units in the emergency department. It will track product orders for highly alloimmunized patients, even though these can sometimes take days to fill. Understandably, these scenarios represent special circumstances in which communication beyond what the tracker can provide is sometimes necessary. Our institution has separate EHR‐based communication tools for these situations beyond the scope of this report. Lastly, uncommon issues that would delay blood products, such as newly identifying a rare alloantibody, cannot be communicated by the tracker and may require direct communication with the clinical team.
We hope to improve the tracker by continuing to evaluate clinicians' usage and requesting their feedback. We will also continue monitoring calls, specifically to determine the most asked questions, and addressing these queries directly in the tracker if possible. The blood tracker has enhanced transparency for all stakeholders of the blood product transfusion process—from providers, nurses, and other care partners—and hopefully will eliminate the need to wonder, “Are my blood products coming?”
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose.
ACKNOWLEDGMENTS
We thank our multidisciplinary team of physicians, nurses, IT staff, blood bank leadership, quality improvement department leadership, Transfusion Safety Officers, and blood bank technologists.
Chai T, Hartzell C, Winstead R, Krokosky K, Atchison K, Mueller A, et al. “Are my blood products coming?”: Implementation of a novel blood product tracker in the electronic health record system. Transfusion. 2025;65(1):17–21. 10.1111/trf.18089
REFERENCES
- 1. Goel R, Zhu X, Patel E, Crowe EP, Ness PM, Katz LM, et al. Blood transfusion trends in the United States: national inpatient sample, 2015 to 2018. Blood Adv. 2021;5:4179–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee A‐J, Kim S‐G. Analysis of turnaround time for intraoperative red blood cell issues: a single‐center study. Lab Med. 2017;48:277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haspel R, Lin Y, Mallick R, Tinmouth A, Cid J, Eichler H, et al. Internal medicine resident knowledge of transfusion medicine: results from the BEST‐TEST international education needs assessment. Transfusion (Paris). 2015;55:1355–1361. [DOI] [PubMed] [Google Scholar]
- 4. Lin Y, Tinmouth A, Mallick R, Haspel RL, for the BEST‐TEST2 Investigators . BEST‐TEST2: assessment of hematology trainee knowledge of transfusion medicine. Transfusion (Paris). 2016;56:304–310. [DOI] [PubMed] [Google Scholar]
- 5. Halford B, Pinheiro A, Haspel RL. Hospital medicine Providers' transfusion knowledge: a survey study. Transfus Med Rev. 2021;35:140–145. [DOI] [PubMed] [Google Scholar]
- 6. Lotterman S, Sharma S. Blood transfusion. StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. [PubMed] [Google Scholar]
- 7. McClain C, Hughes J, Andrews JC, Blackburn J, Sephel S, France D, et al. Blood ordering from the operating room: turnaround time as a quality indicator. Transfusion (Paris). 2013;53:41–48. [DOI] [PubMed] [Google Scholar]
- 8. Andrews J, Delaney M. Leveraging technology to improve transfusion practices in children. AABB News. 2017;19:6–10. [Google Scholar]
- 9. De Vito Dabbs A, Myers B, McCurry KR, Dunbar‐Jacob J, Hawkins RP, Begey A, et al. User‐centered design and interactive health technologies for patients. Comput Inform Nurs CIN. 2009;27:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yung W, Andrews JC. Building a transfusion dashboard in an electronic medical record. Transfusion (Paris). 2016;56:246A. [Google Scholar]


