Abstract
Introduction
The incidence rate of newly diagnosed HIV infection in Indonesia decreased from 21 per 100,000 in 2011 to 10 per 100,000 in 2021. Despite this progress, AIDS-related deaths among people living with HIV (PLWH) increased from 3.4% in 2010 to 4.8% in 2020. Determining risk factors for mortality may identify areas to intervene and reduce mortality.
Methods
A multicenter, prospective, observational cohort study of HIV infection, coinfections, and comorbidities (INA-PROACTIVE) was carried out at 19 hospitals across major islands in Indonesia. The study enrolled PLWH from 2018–2020 and followed them for 3 years. For this analysis, PLWH ≥ 18 years old with one year of follow-up data were included. Cox regression was used to identify variables at enrollment that correlated with one-year mortality.
Results
Among the 4,050 PLWH analysed in the study, 68.8% were male, 53.5% acquired HIV through heterosexual transmission, 92.4% were on antiretroviral treatment (ART) at enrollment, and 72.4% had an undetectable viral load. At one year, 115 (2.8%) had died. Detectable viremia at enrollment was significantly associated with mortality, with the risk increasing as the viral load (VL) category increased (adjusted hazard ratio [aHR] 4.47, 95% CI: 1.47–13.56 for VL 50 to < 1,000 copies/mL; aHR 7.88, 95% CI: 2.80–22.20 for VL 1,000 to 10,000 copies/mL; and aHR 18.33, 95% CI: 7.94–42.34 for VL > 10,000 copies/mL; compared to VL < 50 copies/mL). Other factors at enrollment significantly associated with mortality were a CD4 + count < 200 (aHR 8.02, 95% CI: 2.69–23.86; compared to ≥ 350), age 40–49 years (aHR 2.19, 95% CI 1.23–3.87; compared to 18–29 years) and being underweight (aHR 1.84, 95% CI: 1.18–2.85; compared to normal weight).
Conclusions
Among predominantly treatment-experienced PLWH, detectable viremia and continued immunosuppression were significantly associated with one-year mortality. This study highlights the importance of ART with complete viral suppression as well as immune recovery to prevent mortality.
Trial registration
Clinical Trial Number: NCT03663920, registration date: 4 January 2018.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10354-8.
Keywords: HIV, Indonesia, Mortality, Risk factors, Adult cohort, Viremia
Introduction
Since the first AIDS case was reported in 1987 [1], the HIV/AIDS epidemic in Indonesia has resulted in approximately 372,000 deaths and an estimated 543,100 people living with HIV (PLWH) [2, 3]. National policies and collaborative actions aimed at treating and preventing the spread of the virus have led to a 50% decrease in the annual incidence of reported HIV infections, from 21 per 100,000 in 2011 to 10 per 100,000 in 2021. Free antiretroviral therapy (ART) is provided by the government and consisted of 4 drug classes: a backbone of nucleoside reverse transcriptase inhibitors (NRTIs), including zidovudine (AZT), lamivudine (3TC), emtricitabine (FTC), tenofovir (TDF), and abacavir (ABC), combined with non-nucleoside reverse transcriptase inhibitors (NNRTIs), such as efavirenz (EFV) and nevirapine (NVP), as the first-line regimen; the protease inhibitor (PI) lopinavir/ritonavir (LPV/r) as the second-line regimen [4]; and the integrase inhibitor dolutegravir (DTG) as the third-line regimen. WHO then recommended DTG with 3TC and TDF as the first-line regimen in 2019 [5]; which was followed by an updated regulation in Indonesia in July 2020 [6]. While new users (over 10 years old and without co-infections) were recommended to start on DTG, the second-line and third-line regimens remained unchanged [6].
Despite free ART and ‘test and treat’ guidelines in Indonesia since 2018 [7], ART coverage and availability remains uneven across Indonesia due to the country's decentralized healthcare system [8], with varying retention rates ranging from 46% to 84% [9–11]. The low treatment retention has been attributed to stigma, discrimination, lack of family support, asymptomatic infection, and medication side effects [10–16]. While CD4 + cell count testing is covered by the universal national health insurance system since 2016, viral load (VL) testing is only provided when clinically indicated [17]. As of January 2023, only 27% of PLWH on ART were tested for VL, among whom 91% had viral suppression [3]. However, the 2022 estimate of viral suppression from UNAIDS, which considers all PLWH on ART in Indonesia, was only 14% [2].
The proportion of PLWH dying from AIDS increased from 3.4% (14,000/410,000) in 2010 to 4.8% (26,000/540,000) in 2022, and mortality risk factors included late diagnosis, delayed treatment initiation, unequal distribution of ART nationally, low ART coverage and retention, and co-occurrence with other chronic diseases and coinfections [18]. In this analysis of the Prospective Observational Cohort Study on HIV Infection and Risk-Related Coinfections/Comorbidities in Indonesia (INA-PROACTIVE), variables associated with one-year all-cause mortality in adults are reported.
Methods
Study design and sites
INA-PROACTIVE was a prospective, multicenter, observational cohort study of PLWH in Indonesia. The primary objective of the study was to describe socio-demographic and clinical characteristics at baseline, coinfections and comorbidities, treatment patterns and outcomes, and causes of mortality over a 3-year period. Using reported mortality data from 2016 [19], it was determined that a minimum of 4,000 participants was needed to observe 101 deaths at 9% accuracy.
The study was conducted at 19 hospitals in provincial capitals and major cities across 8 islands in Indonesia (Fig. 1). All hospitals provided routine HIV diagnostic and treatment services according to the national standard of care (SoC). Each site had a dedicated research unit staffed by qualified study personnel. INA-PROACTIVE was coordinated centrally by the INA-RESPOND Secretariat in Jakarta [20, 21], which oversaw all aspects of study planning and management. The study is registered on ClinicalTrials.gov under NCT03663920.
Fig. 1.
Map of INA-PROACTIVE study sites
Ethical approval
The study protocol was reviewed and approved by the central Ethics Committee of the National Institute of Health Research and Development (NIHRD) (LB.02.01/2/KE.012/2018) and the Ethics Committees of 2 study sites requiring additional reviews. All participants or their guardians signed informed consent to participate in the study, with minors signing assent as appropriate.
Enrollment
INA-PROACTIVE enrolled participants from January 2018 to June 2020. We randomized all patients attending their local outpatient clinic for initial or follow-up HIV care, giving each patient an equal chance of selection. We used a predefined, systematic randomization method outlined in the site’s standard operating procedure. This approach allowed for customization to local circumstances while minimizing selection bias and ensuring equal opportunities for patients to be invited to join the study. Study inclusion criteria consisted of a diagnosis of HIV infection made according to the SoC and a willingness to comply with study procedures. Study exclusion criteria consisted of plans to permanently relocate or having served time in prison.
Study procedures
Only adult participants (18 years and older) were included in the analysis. Participants were seen at baseline study enrollment and then every 6 months (± 3 months) for up to 3 years. At baseline, demographic data and medical history were collected, including the date of initial HIV diagnosis, clinical stage at HIV diagnosis, any treatment history, current and past illnesses, vital signs, and body mass index (BMI). BMI was classified as underweight (< 18.5), normal (18.5- < 25), or overweight/obesity (> = 25) [22]. Blood specimens were obtained to determine baseline CD4 + count, HIV VL, hepatitis B virus (HBV) and hepatitis C virus (HCV) infection status, and syphilis reactivity by rapid diagnostic test. Participants were clinically managed according to the Indonesian HIV/AIDS Treatment Guidelines [23] and/or the local SoC. This included providing ART, adjusting ART regimens based on clinical and laboratory observations, managing new and existing co-infections, and acting upon results from study testing. The guidelines specified several first-line ART regimens, including TDF + 3TC (or FTC) + EFV; TDF + 3TC (or FTC) + NVP; AZT + 3TC + EFV; and AZT + 3TC + NVP. For the second-line regimen in adults without coinfections, if the first-line treatment was based on AZT or d4T, it should be switched to TDF + 3TC (or FTC) + LPV/r. If the initial regimen was based on TDF, then it should be switched to AZT + 3TC + LPV/r. For the third-line regimen, the recommendation is to switch to Etravirine (ETR) + Raltegravir (RAL) + Darunavir/ritonavir (DRV/r).
During follow-up visits, new clinical data were collected and blood specimens were obtained for CD4 + count, HIV VL, and syphilis screening in those with a non-reactive baseline result. Following WHO guidance, virological failure was defined as VL > 1,000 copies/mL, and undetectable was defined as VL ≤ 50 copies/mL [24]. Opportunistic infections, coinfections, and comorbidities reported at any time during the study were verified based on the participant’s medical record and treated or managed according to SoC procedures.
Specimen handling and laboratory tests
Each study site was provided with SD Bioline HIV/Syphilis Duo rapid tests (Standard Diagnostics, Inc., Republic of Korea), Oncoprobe HIV 1&2 Antibody rapid tests (Formosa Corp., Taiwan), Determine HIV-1/2 Ag/Ab Combo tests (Alere, USA), and GeneXpert HIV-1 RNA Viral Load cartridges (Cepheid, USA). For participants with evidence of an HIV diagnosis made by SoC, results were validated using the SD Bioline test. For participants who were unable to provide evidence of their HIV diagnosis, both Oncoprobe and SD Bioline tests were performed. In case of discordant results between the intial SoC test and validating SD Bioline test, or between the Oncoprobe and SD Bioline tests, a Determine Combo test and/or Western blot was used to confirm HIV infection status. Participants who were syphilis-reactive at baseline or during follow-up were referred to healthcare providers for confirmation and management according to the local SoC. Collected blood specimens were processed and tested for HIV VL and CD4 + count on-site. Plasma specimen aliquots were centrally tested at the INA-RESPOND Reference Laboratory using ELISAs for hepatitis B surface antigen (LIAISON® XL Murex HBsAg, DiaSorin Italia S.p.A., Italy) and anti-hepatitis C antibody (LIAISON® XL Murex anti-HCV, DiaSorin Italia S.p.A., Italy). Lipid profile, liver function, and renal function were also tested centrally using an ABX Pentra 400 (Horiba Medical, USA). All tests were performed according to the manufacturer’s instructions.
Data management and review
Data were captured in case report forms (CRFs) and entered into an OpenClinica database (OpenClinica, LLC, Waltham, MA, USA) in duplicate. Data quality was periodically monitored by site investigators to ensure that data were complete, accurate, and entered in a timely manner. Data related to causes of death were reported by attending clinicians but not investigated further by study personnel. A group of experienced clinical staff reviewed all reports following the WHO guidelines [24] to determine if deaths were AIDS-related. For this analysis, participant virological, immunological, and all-cause mortality outcomes at one year were assessed.
Statistical analysis and risk factors
Risk factors included demographics (gender, education level, age group, region) and clinical characteristics at enrollment (suspected HIV transmission routes, VL, CD4 + count, BMI, type and duration of ART, HBV and HCV status, and clinical stage at HIV diagnosis). Reported routes of HIV transmission for adult participants were categorized as heterosexual, men who have sex with men (MSM), or injection drug use (IDU). Participants who never received any ART prior to enrollment were categorized as treatment-naïve, while participants with a history of ART use before or at enrollment were categorized as treatment-experienced by duration (< 6, 6–12, and > 12 months). ART regimen at enrollment was categorized as first-line (nevirapine-based, efavirenz-based, rilpivirine-based), second-line (lopinavir/ritonavir-based), third-line (dolutegravir-based), unknown, interrupted, or none. The "interrupted" category was used for experienced respondents who had stopped their ART and had not received any ART for at least 30 days prior to enrollment [25]. Dolutegravir-based regimens were usually initiated after the failure of first- and second-line regimens, especially between 2019–2022. The WHO clinical stage categorization for adult patients was used [24].
Study participant characteristics and follow-up data at one year post-enrollment were analyzed using R Statistical Software (v4.3.1; R Core Team 2023). For continuous variables, means and standard deviations were calculated, while count and percentages were used for binary data. Survival analysis was performed using Kaplan-Meier curves to assess the survival between each VL group at enrollment. A univariable Cox regression analysis was performed for each potential risk factor separately, followed by a multivariable Cox regression analysis to assess all potential risk factors for mortality. Due to multicollinearity between ART regimen and duration (all ART-naïve participants at enrolment have ART regimen “none”), only ART duration was included in the analysis. Participants with missing data were excluded from the multivariable analysis.
Results
Characteristics of the subjects
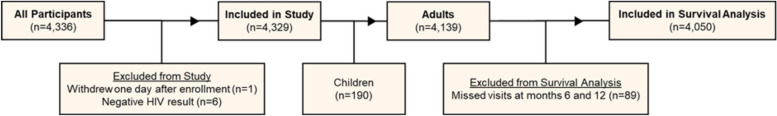
Of the 4,336 HIV infected outpatients enrolled, one individual withdrew immediately after enrollment and 6 were excluded upon confirmation of negative HIV status, leaving 4,329 participants. Among 4,139 adult participants, 89 were excluded for missing both their 6- and 12-month follow-up visits, leaving 4,050 adult participants in the one year survival analysis (Fig. 2).
Fig. 2.
STROBE diagram of patient selection for analysis
Over the study period, 4,050 PLWH were included (Table 1). The number of women was 1,262 (31.2%), and the median (Q1, Q3) age of all PLWH was 35 (29, 41). The majority of infections were reported to be sexually-acquired, with 53.5% by heterosexual sex and 34.6% by MSM. At original HIV diagnosis, 55.5% of participants had reached WHO clinical stage III-IV. At study enrollment, 92.4% were already on ART, primarily efavirenz-based regimens (66.9%), with 72.4% having an undetectable VL and 39.8% with CD4 + count ≥ 350 cells/μL. After one year of follow-up, the proportion virally suppressed increased to 88.8% (3,282/3,694). A higher suppression rate of 91.6% (2,328/2,542) was observed among those who entered the study on ART for more than 12 months.
Table 1.
Baseline characteristics of INA-PROACTIVE participants
| Variables | N (%) | |
|---|---|---|
| Sex | Male | 2788 (68.8) |
| Female | 1262 (31.2) | |
|
Education level (years of education) |
Elementary or junior high (0–9 years) | 869 (21.5) |
| Senior high (10–12 years) | 1972 (48.7) | |
| University diploma or higher (> 12 years) | 1208 (29.8) | |
| Unavailable | 1 (0.0) | |
|
Age group (years) |
18–29 | 1149 (28.4) |
| 30–39 | 1727 (42.6) | |
| 40–49 | 870 (21.5) | |
| ≥ 50 | 304 (7.5) | |
| Region | Java & Bali | 2246 (55.5) |
| Other | 1804 (44.5) | |
| Suspected transmissiona | Heterosexual | 2166 (53.5) |
| MSM | 1400 (34.6) | |
| IDU | 431 (10.6) | |
| Unavailable | 53 (1.3) | |
|
BMI (kg/m2) |
< 18.5 (Underweight) | 669 (16.5) |
| 18.5—< 25 (Normal weight) | 2502 (61.8) | |
| ≥ 25 (Overweight/obesity) | 879 (21.7) | |
|
ART duration (months) |
0 | 309 (7.6) |
| > 0—< 6 | 588 (14.5) | |
| 6–12 | 476 (11.8) | |
| > 12 | 2677 (66.1) | |
|
Viral load (copies/mL) |
< 50 | 2931 (72.4) |
| 50—< 1000 | 332 (8.2) | |
| 1000—10,000 | 187 (4.6) | |
| > 10,000 | 594 (14.7) | |
| Unavailable | 6 (0.1) | |
|
CD4 + count (cells/μL) |
< 200 | 896 (22.1) |
| 200–349 | 1025 (25.3) | |
| ≥ 350 | 1613 (39.8) | |
| Unavailable | 516 (12.7) | |
| ART regimen | First-line | |
| Nevirapine-based | 805 (19.9) | |
| Efavirenz-based | 2710 (66.9) | |
| Rilpivirine-based | 21 (0.5) | |
| Second-line | ||
| Lopinavir/Ritonavir-based | 169 (4.2) | |
| Third-line | ||
| Dolutegravir-based | 1 (0.0) | |
| Unknown | 16 (0.4) | |
| Interrupted | 19 (0.5) | |
| None | 309 (7.6) | |
| Hepatitis B surface antigena | Positive | 386 (9.5) |
| Hepatitis C antibodya | Positive | 365 (9.0) |
| Syphilis screeninga | Reactive | 615 (15.2) |
| Stage at diagnosisb | WHO Clinical stage I-II | 1628 (40.2) |
| WHO Clinical stage III-IV | 2246 (55.5) | |
| Unavailable | 176 (4.3) |
aNo missing value
bBased on history obtained at baseline
Factors associated with one-year mortality
During one year of observation, 115 participants died, 7 discontinued due to moving away, and 7 withdrew from the study. Table 2 shows the univariable and multivariable associations of baseline characteristics with all-cause mortality at one year. In the multivariable analysis, mortality was significantly associated with being aged 40-49 years (adjusted hazard ratio (aHR) 2.19, 95% confidence interval (CI): 1.23–3.87, compared to age 18–29 years), being underweight (aHR 1.84, 95% CI: 1.18–2.85, compared to normal weight), having a CD4 + count < 200 cells/μL at enrollment (aHR 8.02, 95% CI: 2.69–23.86, compared to ≥ 350 cells/μL), and having detectable VL at enrollment (increasing aHR with increasing VL category, compared to undetectable) (Table 2). In the univariable analysis, duration of prior ART treatment was associated with a decreased hazard of mortality compared to no treatment, and WHO clinical stage III-IV at diagnosis was associated with an increased hazard of mortality compared to stage I-II. However, neither of these associations remained significant in the multivariable analysis. A series of bivariable Cox models were conducted as a sensitivity analysis to understand why these variables were no longer significant in the multivariable analysis. Antiretroviral treatment duration no longer remained significant when adjusted for baseline viral load (Additional File 1), and WHO stage III-IV no longer remained significant when adjusted for baseline CD4 + count (Additional File 2).
Table 2.
Baseline characteristics associated with all-cause mortality at one year of follow-up by Cox regression analysis
| Variables | Univariable Hazard Ratio | Multivariable Hazard Ratio | |
|---|---|---|---|
|
Education level (years of education) |
Elementary or junior high (0-9 years) | Reference | Reference |
| Senior high (10-12 years) | 0.79 (0.52-1.21, p=0.277) | 0.88 (0.53-1.45, p=0.613) | |
| University diploma or higher (>12 years) | 0.47 (0.27-0.81, p=0.006) | 0.60 (0.31-1.15, p=0.125) | |
|
Age group (years) |
18-29 | Reference | Reference |
| 30-39 | 0.72 (0.45-1.14, p=0.157) | 0.79 (0.46-1.35, p=0.381) | |
| 40-49 | 1.24 (0.77-2.00, p=0.371) | 2.19 (1.23-3.87, p=0.007) | |
| ≥50 | 0.96 (0.46-2.00, p=0.913) | 1.54 (0.65-3.67, p=0.327) | |
| Region | Java & Bali | Reference | Reference |
| Other | 1.26 (0.87-1.81, p=0.222) | 0.83 (0.54-1.27, p=0.397) | |
| Suspected transmission | Heterosexual | Reference | Reference |
| IDU | 1.08 (0.59-1.97, p=0.796) | 1.35 (0.49-3.73, p=0.566) | |
| MSM | 1.09 (0.73-1.61, p=0.685) | 1.07 (0.65-1.75, p=0.801) | |
|
BMI (kg/m2) |
18.5 - <25 (Normal weight) | Reference | Reference |
| <18.5 (Underweight) | 3.54 (2.42-5.17, p<0.001) | 1.84 (1.18-2.85, p=0.007) | |
| ≥25 (Overweight/obesity) | 0.40 (0.19-0.84, p=0.016) | 0.72 (0.33-1.56, p=0.403) | |
| ART duration (months) | 0 | Reference | Reference |
| >0 - <6 | 0.42 (0.26-0.67, p<0.001) | 1.41 (0.75-2.63, p=0.285) | |
| 6-12 | 0.08 (0.03-0.21, p<0.001) | 0.43 (0.13-1.45, p=0.173) | |
| >12 | 0.12 (0.08-0.19, p<0.001) | 0.94 (0.55-1.61, p=0.835) | |
|
Viral load (copies/mL) |
<50 | Reference | Reference |
| 50 - <1000 | 9.06 (3.93-20.89, p<0.001) | 4.47 (1.47-13.56, p=0.008) | |
| 1000 - 10,000 | 13.40 (5.55-32.33, p<0.001) | 7.88 (2.80-22.20, p<0.001) | |
| >10,000 | 41.64 (22.20-78.11, p<0.001) | 18.33 (7.94-42.34, p<0.001) | |
|
CD4+ count (cells/μL) |
≥350 | Reference | Reference |
| 200-349 | 2.22 (0.71-7.01, p=0.172) | 1.45 (0.41-5.05, p=0.562) | |
| <200 | 33.33 (13.53-82.08, p<0.001) | 8.02 (2.69-23.86, p<0.001) | |
| Hepatitis B surface antigen positive | No | Reference | Reference |
| Yes | 1.33 (0.76-2.33, p=0.317) | 1.09 (0.56-2.15, p=0.794) | |
| Hepatitis C antibody positive | No | Reference | Reference |
| Yes | 1.06 (0.57-1.97, p=0.856) | 1.71 (0.57-5.16, p=0.340) | |
| Stage at diagnosis | WHO clinical stage I-II | Reference | Reference |
| WHO clinical stage III-IV | 3.01 (1.89-4.80, p<0.001) | 1.18 (0.68-2.05, p=0.547) |
Number in dataframe = 4050, Number in model = 3313, Missing = 737, Number of events = 95, Concordance = 0.927 (SE = 0.010), R-squared = 0.090( Max possible = 0.370), Likelihood ratio test = 313.428 (df = 21, p = 0.000)
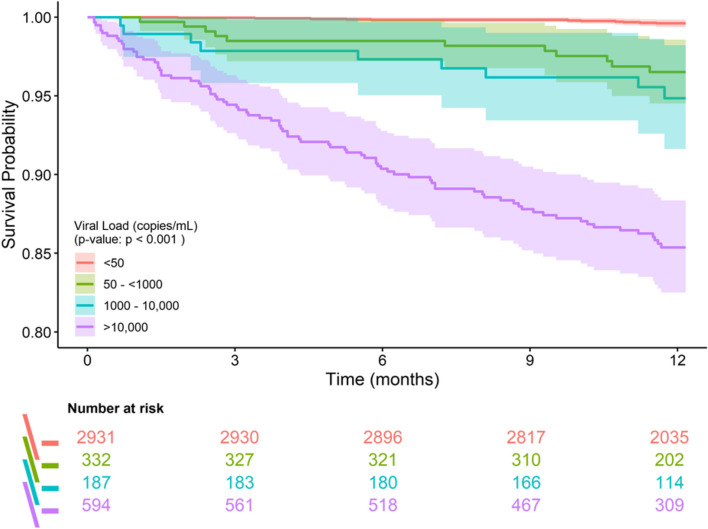
Among study participants, where 92.4% entered the study already on ART and 66.1% were on ART for > 1 year (Table 1), detectable viremia at study enrollment was a major risk factor for mortality (Table 2). Figure 3 shows a Kaplan–Meier curve of decreasing survival with increasing VL category. Among the 115 deaths that occurred in the first year, 103 (89%) occurred in those with detectable viremia at baseline, while 11 (1%) were in those with undetectable viremia (1 participant did not have VL results) (Table 3). Rates of death were 9.3% and 0.4% in those with and without detectable viremia, respectively. Deaths among ART-experienced participants were distributed relatively evenly across all months. In contrast, among 36 deaths observed in ART-naïve participants, 30 (83.3%) occurred during the first 6 months of observation despite ART being started within 1 month in 24 (80%) participants and within 3 months in 27 (90%). The remaining 9 participants never received ART because of ongoing treatment for other infections (7/9, 77.8%), death occurring within 5 days of enrollment (1/9, 11.1%), or the participant never returning to take medication (1/9, 11.1%). The majority of deaths in both groups were AIDS-related: 33 (91.7%) among ART-naïve and 66 (83.5%) among ART-experienced. The most commonly reported underlying comorbidities contributing to death were tuberculosis (34.3%), multiple infections (25.3%), pneumonia (16.2%), and toxoplasmosis (10.1%). Of the 11 participants with undetectable VL who died, 9 had CD4 + counts < 200 cells/μL. The median CD4 + counts among ART-naïve and -experienced participants who died were 38 cells/μL and 59 cells/μL, respectively.
Fig. 3.
Kaplan–Meier plot comparing all-cause mortality over one year among study participants grouped by HIV viral load category
Table 3.
Characteristics of study participants who died during the first year of follow-up
| Variables | ART-experienced N=3741 |
ART-naïve N=309 |
Total N=4050 |
|
|---|---|---|---|---|
| Total deaths | 79 (2.1%) | 36 (11.7%) | 115 (2.8%) | |
| Reported cause of death | AIDS-related | 66 (83.5%) | 33 (91.7%) | 99 (86.1%) |
| Tuberculosis | 19 (28.8%) | 15 (45.5%) | 34 (34.3%) | |
| Multiplea | 17 (25.8%) | 8 (24.2%) | 25 (25.3%) | |
| Pneumonia | 15 (22.7%) | 1 (3%) | 16 (16.2%) | |
| Toxoplasmosis | 8 (12.1%) | 2 (6.1%) | 10 (10.1%) | |
| Other | 5 (7.6%) | 3 (9.1%) | 8 (8.1%) | |
| Unknownb | 2 (3%) | 4 (12.1%) | 6 (6.1%) | |
| Non-AIDS-related | 12 (15.2%) | 3 (8.3%) | 15 (13%) | |
| No data | 1 (1.3%) | 0 (0%) | 1 (0.9%) | |
| Stage at diagnosis | WHO clinical stage I-II | 16 (20.3%) | 6 (16.7%) | 22 (19.1%) |
| WHO clinical stage III-IV | 60 (75.9%) | 30 (83.3%) | 90 (78.3%) | |
| Unavailable | 3 (3.8%) | 0 (0%) | 3 (2.6%) | |
|
Timing of death (months since enrollment) |
0 - 3 | 21 (26.6%) | 23 (63.9%) | 44 (38.3%) |
| >3 - 6 | 22 (27.8%) | 7 (19.4%) | 29 (25.2%) | |
| >6 - 12 | 36 (45.6%) | 6 (16.7%) | 42 (36.5%) | |
|
Time from enrollment to ART initiation (months) |
Died before ART initiation | N/A | 9 (25%) | 9 (25%) |
| <1 month | N/A | 24 (66.7%) | 24 (66.7%) | |
| 1 - <3 months | N/A | 3 (8.3%) | 3 (8.3%) | |
|
ART duration from enrollment to death (months) |
0 | 2 (2.5%) | 9 (25%) | 11 (9.6%) |
| >0 - <1 | 3 (3.8%) | 8 (22.2%) | 11 (9.6%) | |
| 1 -<3 | 16 (20.3%) | 10 (27.8%) | 26 (22.6%) | |
| 3 -<6 | 22 (27.8%) | 4 (11.1%) | 26 (22.6%) | |
| ≥6 | 36 (45.6%) | 5 (13.9%) | 41 (35.7%) | |
| CD4+ count at enrollment (cells/μL) (median, IQR) | 59 (23–143) | 38 (21–101) | 52 (21–126) | |
| VL (copies/mL) and CD4 (cells/µL) at enrollment |
<50 CD4+ count (median, IQR) |
11 (13.9%) 215 (144-468) |
0 (0%) 0 |
11 (9.6%) 215 (144-468) |
|
50 - <1000 CD4+ count (median, IQR) |
11 (13.9%) 125 (95.5-149) |
0 (0%) 0 |
11 (9.6%) 125 (95.5-149) |
|
|
1000 - 10,000 CD4+ count (median, IQR) |
6 (7.6%) 77 (35.8-85.3) |
3 (8.3%) 106 (72-157.5) |
9 (7.8%) 83 (38-106) |
|
|
>10,000 CD4+ count (median, IQR) |
50 (63.3%) 40.5 (11.8-80.5) |
33 (91.7%) 36 (18.5-86) |
83 (72.2%) 37 (14-86) |
|
| No viral load results | 1 (1.3%) | 0 (0%) | 1 (0.9%) |
aMultiple: a combination of more than one etiologies, including pneumonia, pulmonary TB, extra-pulmonary TB, pulmonary abscess, toxoplasmosis cerebri, cryptococcus, CMV, chronic diarrhea, sepsis, HIV wasting syndrome, HIV-associated nephropathy (HIVAN), lymphoma, and progressive multifocal leukoencephalopathy. The combinations: pulmonary TB + pneumonia (5), pneumonia + extra-pulmonary TB (3), chronic diarrhea + HIV wasting syndrome (2), pulmonary TB + pneumonia + sepsis (1), pulmonary TB + pneumonia + CMV (1), pulmonary TB + pneumonia + toxoplasmosis (1), pulmonary TB + pulmonary abscess + meningoencephalitis TB (1), pulmonary TB + toxoplasmosis (1), pulmonary TB + toxoplasmosis + CMV (1), pulmonary TB + chronic diarrhea (1), pneumonia + acute diarrhea (1), pneumonia + HIV wasting syndrome (1), pneumonia + lymphoma (1), pneumonia + progressive multifocal leukoencephalopathy (1), pneumonia + encephalopathy + HIVAN (1), toxoplasmosis + non-Hodgkin lymphoma (1), toxoplasmosis + chronic diarrhea (1), toxoplasmosis + cryptococcus + meningitis TB (1)
bUnknown: respondents died at home, with CD4+ counts prior to death ranging from 7-68 cells/μL
Discussion
Among the 4,050 adult PLWH included in this analysis, the majority were male, < 40 years old, had up to a high school education, and were infected heterosexually. These observations are all consistent with previous reports in Indonesia [26–29]. Nearly 56% originally presented to the healthcare system with advanced HIV (WHO stage III-IV), and although the majority of participants had already been on ART for > 1 year at the time of study enrollment and had an undetectable VL, over 47% still had a CD4 + count < 350 cells/μL. The percentages of undetectable VL observed at enrollment and after one year of follow-up were both below the 95% UNAIDS target of viral suppression [30]. These observations highlight a clear need to expand early HIV identification, treatment, and monitoring programs. Currently, free HIV testing and counselling are available at primary health centers and mobile facilities, but these services are not well-integrated with clinical services or treatment, resulting in barriers to early initiation of ART. Efforts are needed to increase testing in persons at risk for HIV so that infections can be detected earlier and ART initiated prior to the development of advanced HIV. VL monitoring, which is not part of the national SoC, should be made available to all PLWH to aid in maintaining viral suppression and reaching the 95% UNAIDS target.
We found that in our cohort of mostly treatment experienced participants, detectable viremia, low CD4 + count, and underweight BMI were all significant baseline risk factors for one-year mortality. A high VL indicates active HIV replication, leading to systemic inflammation, immune activation, and faster progression to AIDS and death. A CD4 + count < 200 cells/μL at enrollment was a significant risk factor for mortality, and in the late-stage of HIV, full reconstitution of CD4 + and CD8 + T-cells may not occur even if there is complete virological suppression [31, 32]. In resource-limited settings where many infectious diseases are prevalent, opportunistic infections are a leading cause of death, particularly in those with CD4 + counts < 200 cells/μL. This was seen in our cohort, where the reported causes of death in participants included tuberculosis, pneumonia, toxoplasmosis, and multiple other causes. Underweight BMI was another significant risk factor for mortality, even after controlling for CD4 + count and VL. This suggests that BMI underlies other physiological mechanisms besides the risk reflected by a modest correlation between BMI and CD4 + count [33]. Previous research has indicated that in PLWH initiating ART, lower baseline BMI is associated with poorer viral suppression during follow-up, while higher baseline BMI is linked to improved immune reconstitution [34, 35].
During the initial year of follow-up, 115 of 4,050 (2.8%) participants died. This overall mortality rate is lower than the 4.8% rate estimated nationally for 2022 [3] and may be due to several factors. First, most study sites were referral hospitals with more clinical specialties, advanced testing capacity, ongoing research, educational activities, better access to ART regimens, and in most cases, dedicated clinics for HIV patients; all of which may have contributed to better outcomes for participants. Second, there is likely a survival bias in our cohort, as most (66.1%) were already ART-experienced for more than 1 year. Many deaths occur during the initial year on treatment, particularly in those who present with advanced HIV. Finally, our study may have provided better HIV care than the national average due to closer follow-up and provision of VL testing.
The mortality rate among ART-naïve participants was significantly higher than that of ART-experienced participants (11.7% vs. 2.1%, respectively). However, prior ART treatment duration was no longer a risk factors for mortality in the multivariable analysis because, as shown in the sensitivity analysis, adjusting for VL at enrollment reduced its significance. This is consistent with the knowledge that achieving undetectable viremia, not the length of treatment, is what reduces mortality [36]. The study population being predominantly treatment-experienced is important because there is likely a survival bias since a substantial proportion of deaths in the general population may have occurred within the first year of treatment, as was seen in the ART-naïve study participants. Although some ART-naïve participants never received ART, the overall mortality rate among ART-naïve participants and the early occurrence of death after ART initiation in this cohort are consistent with similar studies in Mozambique, Tanzania, and Malawi [37]; Latin America and the Caribbean [38]; Guangxi, China [39]; KwaZulu-Natal, South Africa [40]; and Northwest Ethiopia [41].
Previous research has shown that changing ART regimens based on VL monitoring can lead to improved viral suppression and better clinical outcomes for PLWH [42]. However, in resource-limited settings such as Indonesia, this can be challenging due to limited laboratory capacity and high costs. As VL monitoring is rarely conducted in Indonesia, clinicians rely on worsening clinical status to initiate a change in ART regimens. This results in delayed adherence counseling, the delayed provision of effective new regimens, likely increases in VL, and likely decreases in CD4 + count. In addition to the mortality risk posed by detectable viremia, the next greatest risk factor observed in this study was continuing immune suppression (CD4 + count < 200 cells/μL) independent of viremia. This is easily understood for ART-naïve participants, but for ART-experienced participants, this means that the lack of appropriate immune reconstitution poses a continuing risk of mortality even when viremia is suppressed. In addition, individuals who begin treatment with very low CD4 + counts may not attain normal CD4 + levels, often plateauing at suboptimal levels [43]. This persistent immunodeficiency significantly raises the risk of morbidity and mortality, increasing susceptibility to opportunistic infections and non-AIDS related diseases.
Therefore, early diagnosis and early treatment should be the priority to reduce mortality. Engaging community members and key populations such as MSM and IDU has shown great potential in expanding HIV testing coverage and ensuring timely initiation of ART [44–46]. Additionally, the Indonesian TB guidelines emphasize the importance of integrating TB and HIV services to promote timely ART initiation. ART should begin within two weeks of starting TB treatment for patients with severe immunosuppression (CD4 count < 50 cells/mm3) and within the first eight weeks for all other co-infected patients, regardless of CD4 count, to reduce mortality [47].
Moreover, the treatment of latent TB infection (LTBI) plays a crucial role in reducing TB incidence among people living with HIV. Regulations, including the Indonesian Minister of Health Regulation No. 67 of 2016, target both children under five who are in contact with active TB cases and people living with HIV/AIDS (PLWHA). However, by 2019, only 9.4% and 12% respectively of these two groups received TB Preventive Therapy in Indonesia [48]. The 2020 National TB Control Program expanded this focus to include household contacts over five years old and other at-risk groups, such as inmates, immunocompromised patients, healthcare workers, and other high risk groups [49]. While widespread implementation of the expanded 2020 guidelines is still ongoing, the emphasis on these key populations highlights the need for continued monitoring and evaluation to assess the program's full impact. Integrating and further promoting LTBI treatment within routine HIV care is vital for preventing TB reactivation and addressing the significant TB burden in Indonesia.
This study has several limitations. First, all known factors influencing mortality outcomes, such as socioeconomic status [50, 51], mental health and psychological well-being, social support, health literacy [52], and medication adherence [53], were not captured. Second, the population in this study may not be representative of the general population of PLWH in Indonesia. Study sites only included large or referral hospitals, which may draw more participants with advanced disease but may also provide better care. This study also did not enroll incarcerated persons, which is an important population of PLWH in Indonesia [54, 55].
Conclusions
In conclusion, the INA-PROACTIVE study provides the largest nation-wide observational data on PLWH in Indonesia to-date. Results from the first year of observation highlight the importance of routine VL and CD4 + cell count monitoring. Current ART treatment guidelines suggest that HIV VL should become undetectable within 6 months of starting ART [24]. If this does not happen, additional patient counseling on treatment adherence, as well as evaluation for primary drug resistance, should be undertaken to achieve an undetectable VL. Controlling viremia allows reconstitution of the immune system. If the CD4 + cell count does not slowly increase to > 200 cells/μL and beyond, the participant is still at increased risk of death compared to those with a CD4 + cell count ≥ 350 cells/μL. Large cohort studies have observed reduced mortality even when starting ART below 500 cells/μL [56], and current treatment guidelines recommend treatment for all PLWH regardless of CD4 + cell count [24]. Most patients, however, are still identified late in the course of their disease. To achieve higher rates of early HIV diagnosis and treatment, several crucial steps must be taken, including educating individuals on the benefits of early HIV testing, establishing more HIV diagnostic clinics with trained staff who can provide proper education, and encouraging prompt initiation of ART. Consideration should be given to including routine VL testing, in addition to CD4 + count, in the national treatment program, as this should aid decision making to reduce mortality and improve treatment outcomes.
Supplementary Information
Additional file 1: Combination of each additional variable to find out which one changed ART duration from significant to not significant. Additional file 2: Combination of each additional variable to find out which one changed clinical stage from significant to not significant.
Acknowledgements
We gratefully acknowledge the significant contributions of our late colleague, Dr. Nia Kurniati, to this research. Sadly, Dr. Kurniati passed away prior to the publication of this work. We also extend our heartfelt thanks to H. Clifford Lane, Sophia Siddiqui, Bachti Alisjahbana, Pratiwi Pudjilestari Sudarmono, Vivi Lisdawati, Mansyur Arif, M. Hussein Gasem, Abu Tholib Aman, and Erlina Burhan for their leadership and support in making this study possible. Our deepest gratitude goes to all the participants of this study, whose cooperation and dedication were essential to our research. We also express our sincere appreciation to the staff at the various study sites across Indonesia. Their diligent efforts in coordinating study logistics, managing data collection, and engaging participants have been crucial to the success of our research.
INA-PROACTIVE Study Group Members
Indonesia
Dr. Hasan Sadikin General Hospital/Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia: Rudi Wisaksana (PI), Agnes Rengga Indrati, Anggraini Alam, Syndi Nurmawati, Nurul Hidayah Chairunnisa, Octavia Kartika Dwi, Hofiya Djauhari, Sigit Sunarko. Faculty of Medicine, Universitas Udayana, Denpasar, Indonesia: Tuti Parwati Merati (PI). Ngoerah Hospital/Faculty of Medicine, Universitas Udayana, Denpasar, Indonesia: I Ketut Agus Somia (PI), I Made Susila Utama, Ni Made Dewi Dian Sukmawati, Anak Agung Yuli Gayatri, Ketut Dewi Kumara Wati, Anak Agung Wiradewi Lestari, Ni Nengah Dwi Fatmawati, Ni Luh Putu Ariastuti, Ni Putu Satriawati, Ni Ketut Lestari, Ni Wayan Desi Jumanti, Ni Nyoman Setiani, I Gede Purinawa, Desak Putra Artini, Ni Nyoman Seri Sutarni. Cipto Mangunkusumo Hospital/Faculty of Medicine, Universitas Indonesia, Central Jakarta, Indonesia: Evy Yunihastuti (PI), Nia Kurniati†, July Kumalawati, Fadlika Harinda, Tiara Kumala Putri, Madyaningati, Wusthi Ali Chasanah. Prof. Dr. Sulianti Saroso Infectious Disease Hospital, North Jakarta, Indonesia: Desrinawati Muhammad Amin (PI), Teguh Sarry Hartono, Adria Rusli, Nisrina Fatin Mardiyah, Ayu Reskianingsih, Evi Hindawati, Fitriani. Faculty of Medicine, Universitas Hasanuddin/Dr. Wahidin Sudirohusodo Hospital, Makassar, Indonesia: Sudirman Katu (PI), Ninny Meutia Pelupessy, Munawir. Dr. Wahidin Sudirohusodo Hospital, Makassar, Indonesia: Asvin Nurulita, Dhiny Reskita Ayu, Rizky Auliah Bakri, Muhammad Fiqhi Hardiansyah, Dewi Sriyanti, Kartini, Nasruh Nurdin, Andi Tessyoja. Faculty of Medicine, Universitas Diponegoro/Dr. Kariadi Hospital, Semarang, Indonesia: Nur Farhanah (PI), Muchlis Achsan Udji Sofro, Nahwa Arkhaesi, Muji Rahayu. Dr. Kariadi Hospital, Semarang, Indonesia: Rohedy Adlina, Erni, Trie Wahyuningtias, Bayu Sampurmantoro Kusumo, Ngatno. Faculty of Medicine, Universitas Airlangga/Dr. Soetomo General Academic Hospital, Surabaya, Indonesia: Usman Hadi (PI), Dwiyanti Puspitasari,Munawaroh Fitriah, Muhammad Vitanata Arfijanto, Bramantono, Musofa Rusli, Myrna Evanda Adeline, Rifaa'ah Rosyiidah, Misutarno, Yuanita Bahar, Diah Wahyuni. Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia: Hera Nirwati. Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada/Dr. Sardjito General Hospital, Yogyakarta, Indonesia: Ida Safitri Laksanawati (PI), Yanri Wijayanti Subronto, Umi Solekhah Intansari, Vitia Ajeng Nur Linda, Diajeng Gayatri Kusumaningtyas, Siti Binzanah, Linda Oktabriana. Persahabatan Central General Hospital, East Jakarta, Indonesia: Eppy Eppy (PI), Heidy Agustin, Fauzi Mahfuzh, Dewi Yennita Sari, Titin Dani Martiwi, Putri Permata Sari, Ariesta Putra Indoin, Susetianingsih, Tri Mulyani. Adam Malik Hospital, Medan, Indonesia: Tambar Kembaren (PI), Rita Evalina, Nelly Elfrida Samosir, Cicimei Putri Siregar, Iramaya Oktariana Harahap, Junita Siahaan, Ennyke Karolina Ginting, Melpinna Fretty Marpaung. District Hospital, Tangerang, Indonesia: I Gede Rai Kosa (PI), Chakrawati Hayuningsih, Arif Budiman, Cintya Naya Danastri, Ivana Yulian Hendarsin, Husnul Chotimah, Evi Herawati, Suyono. Dr. Moch. Ansari Saleh Hospital, Banjarmasin, Indonesia:Wiwit Agung Sri Nur Cahyawati (PI), Dwiana Savitri, Rahmawati, Priyanti Kisworini, Regina Septiana, Rizka Dwi Puteri, Lutfia Rahimah, Silvia Rahmi Astuti, Irma Meilyana Fajar Utami, Setiawati. St. Carolus Hospital, Central Jakarta, Indonesia:Emon Winardi Danudirgo (PI), Elizabeth Yohmi, Deborah Theresia, Janice Tandraeliene, Cicilia Ina Prihsetyati, Lili. Budi Kemuliaan Hospital, Batam, Indonesia:Danang Legowo Norosingomurti (PI), Willy Anthony Iqnatius Wullur, Nisa Trini Asnil, Francisca Lianiwati Tanzil, Glegery Putri Hardoni, Winny Wangun Lestari Wullur, Mutamimah, Diana Sulastri, Friska Sitorus. Abdoel Wahab Sjahranie General Hospital/Faculty of Medicine, Universitas Mulawarman, Samarinda: Carta A. Gunawan (PI). Abdoel Wahab Sjahranie General Hospital, Samarinda, Indonesia: Sunarto Ang, Sri Wahyunie, Hendra, Andini Agustyana, Edwin Prasetya, Monika Lestari Palondongan, Heniastuti, Nursiah Mukano, Ady Achmaddany. Dr. Zainoel Abidin Hospital/Faculty of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia: Kurnia Fitri Jamil (PI), Mulya Safri, Vivi Keumala Mutiawati, Muhammad Abrar Azhar, Ika Novita, Muslim, Nurul Husna, Ella Firbria, Arfajah. Dr. Soedarso General Hospital, Pontianak, Indonesia: Ivan Lumban Toruan (PI), Muchamad Budi Nugroho, Handoko Halim, Wiwi Endang Susanti, Redha Vebrina, Ike Rahmawati, Herlina, Sudaryanto, Emy Suyanti. Abepura Regional General Hospital, Jayapura, Papua, Indonesia: I Made Gede Darmaja (PI), Immaculata Purwaningsih, Justina Sembiring, Nur Yanti, Nani Emma, Widya Amalia Swastika, Sunarti, Martha Magdalena Ayer. Dr. T. C. Hillers Public Hospital, Maumere, Indonesia: Asep Purnama (PI), Mario Bernardinus Realino Nara, Dwi Kurniawan Nugroho, Putri Nur Indah Sari, Ulul Azmiyah Riawan, Fransiska Mathilde, Kurniawati Handayani Koli, Stefani Marwah Tolok, Maria Yupina Yuanti. Health Policy Agency, Ministry of Health, Central Jakarta, Indonesia: Muhammad Karyana (PI), Dona Arlinda. Indonesia Research Partnership on Infectious Diseases (INA-RESPOND), Central Jakarta, Indonesia: Herman Kosasih, Dewi Lokida, Adhella Menur Naysilla, Aly Diana, Chandra Ilham El Anwary Junior, Deni Pepy Rentina Butarbutar, Eka Windari Rusman, I Wayan Adi Pranata, Lois Eirene Bang, Meity Siahaan, Melinda Setiyaningrum, Nada Ariqa, Nugroho Harry Susanto, Nur Latifah Hanum, Nurhayati Lukman, Restu Amalia Mukti, Rizki Amalia Sari, Tiya Saraswati, Virtania Meirina Agusta, Wahyu Nawang Wulan.
The United States of America (USA)
National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, United States of America: Ray Y. Chen, Aaron T. Neal, C. Jason Liang, Renee Ridzon, Chuen-Yen Lau. Leidos Biomedical Research, Inc., Maryland, USA: Louis Grue, Jacqueline Perodin, Katie Watkins.
Abbreviations
- 3TC
: Lamivudine
- ABC
: Abacavir
- AIDS
: Acquired Immunodeficiency Syndrome
- ART
: Antiretroviral Therapy
- aHR
: Adjusted Hazard Ratio
- AZT
: Zidovudine
- BMI
: Body Mass Index
- CI
: Confidence Interval
- CRF
: Case Report Form
- DRV/r
: Darunavir/ritonavir
- DTG
: Dolutegravir
- EFV
: Efavirenz
- ETR
: Etravirine
- FTC
: Emtricitabine
- HBV
: Hepatitis B Virus
- HCV
: Hepatitis C Virus
- HIV
: Human Immunodeficiency Virus
- IDU
: Injection Drug Use
- INA-PROACTIVE
: Prospective Observational Cohort Study on HIV Infection and Risk- Related Coinfections/Comorbidities in Indonesia
- LPV/r
: Lopinavir/ritonavir
- MSM
: Men Who Have Sex With Men
- NRTI
: Nucleoside Reverse Transcriptase Inhibitor
- NNRTI
: Non-Nucleoside Reverse Transcriptase Inhibitor
- NVP
: Nevirapine
- PI
: Protease Inhibitor
- PLWH
: People Living With HIV
- RAL
: Raltegravir
- SoC
: Standard of Care
- TDF
: Tenofovir
- VL
: Viral Load
- WHO
: World Health Organization
Authors’ contributions
T.P.M., E.Y., R.W., N.K., D.A., M.K., N.H.S., H.K., C.J.L., A.T.N., were responsible for conceptualization and study design. T.M.P., E.Y., R.W., N.K., D.M.A., E.E., W.A.S.N.C., E.W.D., I.M.G.D., N.F., C.A.G., U.H., T.S.H., K.F.J., S.K., T.K., I.G.R.K., D.L.N., A.P., I.S., I.K.A.S., I.L.T. recruited subjects and collected and validated clinical data. L.E.B. coordinated the preparation of sites to implement the study, and ensuring sites to implement the study according to good clinical practice. D.L. managed specimen collection and storage, performed biomarker qualification at the laboratory. M.S. managed data collection and validation of the data. N.H.S., C.J.L., R.Y.C. performed the statistical analysis. T.P.M., E.Y., R.W., N.K., D.A., M.K., N.H.S., H.K., R.R., C.J.L., A.T.N., R.Y.C. interpreted the data. T.P.M., D.A., M.K., H.K., A.D., R.R., A.T.N., R.Y.C. drafted the paper. T.P.M., E.Y., R.W., N.K., D.A., M.K., N.H.S., D.L., H.K., A.D., R.R., C.J.L., A.T.N., R.Y.C. performed critical data review and manuscript revision. All authors read and approved the final version of the manuscript.
Funding
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under contract Nos. HHSN261201500003I and 75N91019D00024. The content of this manuscript does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was reviewed and approved by the central Ethics Committee of the National Institute of Health Research and Development (NIHRD) (LB.02.01/2/KE.012/2018) and the Ethics Committees of 2 study sites requiring additional reviews. All participants or their guardians signed informed consent to participate in the study, with minors signing assent as appropriate.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nia Kurniati is deceased.
Contributor Information
Dona Arlinda, Email: arlindona@ina-respond.net.
INA-PROACTIVE Study Group:
Evy Yunihastuti, Rudi Wisaksana, Nia Kurniati, Agnes Rengga Indrati, Anggraini Alam, Syndi Nurmawati, Nurul Hidayah Chairunnisa, Octavia Kartika Dwi, Hofiya Djauhari, Sigit Sunarko, Tuti Parwati Merati, IKetut Agus Somia, IMade Susila Utama, Ni Made Dewi Dian Sukmawati, Anak Agung Yuli Gayatri, Ketut Dewi Kumara Wati, Anak Agung Wiradewi Lestari, Ni Nengah Dwi Fatmawati, Ni Luh Putu Ariastuti, Ni Putu Satriawati, Ni Ketut Lestari, Ni Wayan Desi Jumanti, Ni Nyoman Setiani, IGede Purinawa, Desak Putra Artini, Ni Nyoman Seri Sutarni, July Kumalawati, Fadlika Harinda, Tiara Kumala Putri, Madyaningati, Wusthi Ali Chasanah, Desrinawati Muhammad Amin, Teguh Sarry Hartono, Adria Rusli, Nisrina Fatin Mardiyah, Ayu Reskianingsih, Evi Hindawati, Fitriani, Sudirman Katu, Ninny Meutia Pelupessy, Munawir, Asvin Nurulita, Dhiny Reskita Ayu, Rizky Auliah Bakri, Muhammad Fiqhi Hardiansyah, Dewi Sriyanti, Kartini, Nasruh Nurdin, Andi Tessyoja, Nur Farhanah, Muchlis Achsan Udji Sofro, Nahwa Arkhaesi, Muji Rahayu, Rohedy Adlina, Erni, Trie Wahyuningtias, Bayu Sampurmantoro Kusumo, Ngatno, Usman Hadi, Dwiyanti Puspitasari, Munawaroh Fitriah, Muhammad Vitanata Arfijanto, Bramantono, Musofa Rusli, Myrna Evanda Adeline, Rifaa’ah Rosyiidah, Misutarno, Yuanita Bahar, Diah Wahyuni, Hera Nirwati, Ida Safitri Laksanawati, Yanri Wijayanti Subronto, Umi Solekhah Intansari, Vitia Ajeng Nur Linda, Diajeng Gayatri Kusumaningtyas, Siti Binzanah, Linda Oktabriana, Eppy Eppy, Heidy Agustin, Fauzi Mahfuzh, Dewi Yennita Sari, Titin Dani Martiwi, Putri Permata Sari, Ariesta Putra Indoin, Susetianingsih, Tri Mulyani, Tambar Kembaren, Rita Evalina, Nelly Elfrida Samosir, Cicimei Putri Siregar, Iramaya Oktariana Harahap, Junita Siahaan, Ennyke Karolina Ginting, Melpinna Fretty Marpaung, IGede Rai Kosa, Chakrawati Hayuningsih, Arif Budiman, Cintya Naya Danastri, Ivana Yulian Hendarsin, Husnul Chotimah, Evi Herawati, Suyono, Wiwit Agung Sri Nur Cahyawati, Dwiana Savitri, Rahmawati, Priyanti Kisworini, Regina Septiana, Rizka Dwi Puteri, Lutfia Rahimah, Silvia Rahmi Astuti, Irma Meilyana Fajar Utami, Setiawati, Emon Winardi Danudirgo, Elizabeth Yohmi, Deborah Theresia, Janice Tandraeliene, Cicilia Ina Prihsetyati, Lili, Danang Legowo Norosingomurti, Willy Anthony Iqnatius Wullur, Nisa Trini Asnil, Francisca Lianiwati Tanzil, Glegery Putri Hardoni, Winny Wangun Lestari Wullur, Mutamimah, Diana Sulastri, Friska Sitorus, Carta A. Gunawan, Sunarto Ang, Sri Wahyunie, Hendra, Andini Agustyana, Edwin Prasetya, Monika Lestari Palondongan, Heniastuti, Nursiah Mukano, Ady Achmaddany, Kurnia Fitri Jamil, Mulya Safri, Vivi Keumala Mutiawati, Muhammad Abrar Azhar, Ika Novita, Muslim, Nurul Husna, Ella Firbria, Arfajah, Ivan Lumban Toruan, Muchamad Budi Nugroho, Handoko Halim, Wiwi Endang Susanti, Redha Vebrina, Ike Rahmawati, Herlina, Sudaryanto, Emy Suyanti, IMade Gede Darmaja, Immaculata Purwaningsih, Justina Sembiring, Nur Yanti, Nani Emma, Widya Amalia Swastika, Sunarti, Martha Magdalena Ayer, Asep Purnama, Mario Bernardinus Realino Nara, Dwi Kurniawan Nugroho, Putri Nur Indah Sari, Ulul Azmiyah Riawan, Fransiska Mathilde, Kurniawati Handayani Koli, Stefani Marwah Tolok, Maria Yupina Yuanti, Muhammad Karyana, Dona Arlinda, Herman Kosasih, Dewi Lokida, Adhella Menur Naysilla, Aly Diana, Chandra Ilham El Anwary Junior, Deni Pepy Rentina Butarbutar, Eka Windari Rusman, IWayan Adi Pranata, Lois Eirene Bang, Meity Siahaan, Melinda Setiyaningrum, Nada Ariqa, Nugroho Harry Susanto, Nur Latifah Hanum, Nurhayati Lukman, Restu Amalia Mukti, Rizki Amalia Sari, Tiya Saraswati, Virtania Meirina Agusta, Wahyu Nawang Wulan, Ray Y. Chen, Aaron T. Neal, C. Jason Liang, Renee Ridzon, Chuen-Yen Lau, Louis Grue, Jacqueline Perodin, and Katie Watkins
References
- 1.Indonesia, World Health Organization, editors. Review of the health sector response to HIV and AIDS in Indonesia, 2007. New Delhi: World Health Organization, Regional Office for South-East Asia; 2007. Available from: https://iris.who.int/handle/10665/205869.
- 2.UNAIDS. Country Factsheets Indonesia 2022. 2022. Available from: https://www.unaids.org/en/regionscountries/countries/indonesia. Cited 2022 Dec 3.
- 3.Ministry of Health Republic Indonesia. Hepatitis, HIV, and STI in Indonesia, Status of the Epidemic and National Response (in Bahasa Indonesia). 2022.
- 4.Ministry of Health Republic Indonesia. Regulation of the Minister of Health of the Republic of Indonesia Number 87 of 2014 on Guidelines for Antiretroviral Treatment (in Bahasa Indonesia). 2014. Available from: https://siha.kemkes.go.id/portal/files_upload/Buku_Permenkes_ARV_Cetak.pdf. Cited 2024 Oct 23.
- 5.World Health Organization. Update of recommendations on first-and second-line antiretroviral regimens. 2019. Available from: https://www.who.int/publications/i/item/WHO-CDS-HIV-19.15. Cited 2024 Oct 10.
- 6.Recommendations of the Expert Panel on HIV/AIDS and STI Control from the Online Meeting, Thursday, July 2, 2020 (in Bahasa Indonesia). 2020. Available from: https://hivaids-pimsindonesia.or.id/download/file/REKOMENDASI_PANEL_AHLI_HIV_DAN_IMS.pdf. Cited 2024 Oct 23.
- 7.Ministry of Health Republic Indonesia. Circular letter No. HK.02.02/I/1564/2018 concerning management of people with HIV AIDS to eliminate HIV AIDS by 2030 (in Bahasa Indonesia). 2018. Available from: https://siha.kemkes.go.id/portal/files_upload/surat_edaran_test_and_treat.pdf. Cited 2024 Oct 10.
- 8.Gedela K, Wirawan DN, Wignall FS, Luis H, Merati TP, Sukmaningrum E, et al. Getting Indonesia’s HIV epidemic to zero? One size does not fit all. Int J STD AIDS. 2021;32(3):290–9. [DOI] [PubMed] [Google Scholar]
- 9.Nurfalah F, Yona S, Waluyo A. The relationship between HIV stigma and adherence to antiretroviral (ARV) drug therapy among women with HIV in Lampung. Indonesia Enferm Clínica. 2019;29:234–7. [Google Scholar]
- 10.Suryana K, Suharsono H, Antara IGPJ. Factors associated with adherence to anti-retroviral therapy among people living with HIV/AIDS at Wangaya Hospital in Denpasar, Bali, Indonesia: a cross-sectional study. HIV AIDS - Res Palliat Care. 2019;11:307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Januraga PP, Reekie J, Mulyani T, Lestari BW, Iskandar S, Wisaksana R, et al. The cascade of HIV care among key populations in Indonesia: a prospective cohort study. Lancet HIV. 2018;5(10):e560–8. [DOI] [PubMed] [Google Scholar]
- 12.Lumbantoruan C, Kermode M, Giyai A, Ang A, Kelaher M. Understanding women’s uptake and adherence in Option B+ for prevention of mother-to-child HIV transmission in Papua, Indonesia: a qualitative study. PLOS One. 2018;13(6):e0198329. Sacks E, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell E, Lazuardi E, Anintya I, Rowe E, Whitford K, Wirawan DN, et al. A qualitative exploration of family, work, community, and health service influences on HIV treatment uptake and adherence among female sex workers in three cities in Indonesia. AIDS Educ Prev. 2020;32(3):243–59. [DOI] [PubMed] [Google Scholar]
- 14.Fauk NK, Merry MS, Ambarwati A, Sigilipoe MA, Ernawati, Mwanri L. A qualitative inquiry of adherence to antiretroviral therapy and its associated factors: a study with transgender women living with HIV in Indonesia. Indian J Public Health. 2020;64(2):116–23. [DOI] [PubMed] [Google Scholar]
- 15.Sianturi EI, Perwitasari DA, Islam MdA, Taxis K. The association between ethnicity, stigma, beliefs about medicines and adherence in people living with HIV in a rural area in Indonesia. BMC Public Health. 2019;19(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wardhani SF, Yona S. Spousal intimacy, type of antiretroviral drug and antiretroviral therapy adherence among HIV patients in Bandung, Indonesia. J Public Health Res. 2021;10(1_suppl):jphr.2021.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ministry of Health Republic Indonesia. Guidebook on the implementation of national health insurance (JKN/BPJS) financing for HIV/AIDS and STI services at healthcare facilities (in Bahasa Indonesia). 2015. Available from: https://siha.kemkes.go.id/portal/files_upload/1_h__Draft_JKN_HIV_211215_Final.pdf.
- 18.Jeong SJ, Italiano C, Chaiwarith R, Ng OT, Vanar S, Jiamsakul A, et al. Late presentation into care of HIV disease and its associated factors in Asia: results of TAHOD. AIDS Res Hum Retroviruses. 2016;32(3):255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wijayanti F, Tarmizi SN, Tobing V, Nisa T, Akhtar M, Trihandini I, et al. From the millennium development goals to sustainable development goals.: The response to the HIV epidemic in Indonesia: challenges and opportunities. J Virus Erad. 2016;2(Suppl 4):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karyana M, Kosasih H, Samaan G, Tjitra E, Aman AT, Alisjahbana B, et al. INA-RESPOND: a multi-centre clinical research network in Indonesia. Health Res Policy Syst. 2015;13(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karyana M, Kosasih H, Neal AT, Lau CY. Maintaining international research collaborations in the setting of a pandemic: approach in Indonesia. J Glob Health. 2021;30(11):03087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Body Mass Index (BMI). 2024. Available from: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/body-mass-index?introPage=intro_3.html. Cited 2024 Nov 16.
- 23.Directorate General of Disease Prevention and Control, Ministry of Health. Pocketbook for the HIV/AIDS and PIMS Control Program in Primary Healthcare Facilities (in Bahasa Indonesia). 2017. Available from: https://siha.kemkes.go.id/portal/files_upload/BUKU_3_PENGENDALIAN_HIV_COLOR_A5_15x21_cm.pdf.
- 24.Consolidated guidelines on HIV prevention. testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva, Switzerland: World Health Organization; 2021. [PubMed] [Google Scholar]
- 25.Steingrover R, Pogány K, Fernandez Garcia E, Jurriaans S, Brinkman K, Schuitemaker H, et al. HIV-1 viral rebound dynamics after a single treatment interruption depends on time of initiation of highly active antiretroviral therapy. AIDS Lond Engl. 2008;22(13):1583–8. [DOI] [PubMed] [Google Scholar]
- 26.Utami S, Sawitri AAS, Wulandari LPL, Artawan Eka Putra IWG, Astuti PAS, Wirawan DN, et al. Mortality among people living with HIV on antiretroviral treatment in Bali, Indonesia: incidence and predictors. Int J STD AIDS. 2017;28(12):1199–207. [DOI] [PubMed] [Google Scholar]
- 27.Pribadi GS, Cahyono ABF. Characteristics and opportunistic infections of AIDS patients in East Java Province in 2018. J Berk Epidemiol. 2021;9(1):96. [Google Scholar]
- 28.Kadri A, Yandra E. Demographic, clinical, and laboratory characteristics of HIV patients with cerebral toxoplasmosis at Haji Adam Malik General Hospital Medan. J Kedokt Brawijaya. 2022;4:116–9. [Google Scholar]
- 29.Ministry of Health Republic Indonesia. HIV Epidemiology Review Indonesia 2016 (in Bahasa Indonesia). 2017. Available from: https://siha.kemkes.go.id/portal/files_upload/HIV_EPIDEMIOLOGY_REVIEW_INDONESIA_2016.pdf. Cited 2023 Feb 3.
- 30.UNAIDS. Understanding fast-tract: Accelerationg action to end the AIDS epidemic by 2030. 2015. Available from: https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Understanding_FastTrack_en.pdf.
- 31.Estes JD, Haase AT, Schacker TW. The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Semin Immunol. 2008;20(3):181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schacker TW, Nguyen PL, Beilman GJ, Wolinsky S, Larson M, Reilly C, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110(8):1133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Sande MAB, van der Loeff MFS, Aveika AA, Sabally S, Togun T, Sarge-Njie R, et al. Body mass index at time of HIV diagnosis: a strong and independent predictor of survival. JAIDS J Acquir Immune Defic Syndr. 2004;37(2):1288–94. [DOI] [PubMed] [Google Scholar]
- 34.Zhu J, Huang H, Wang M, Zhang Y, Mo J, Tian W, et al. High baseline body mass index predicts recovery of CD4+ T lymphocytes for HIV/AIDS patients receiving long-term antiviral therapy. PLOS One. 2022;17(12):e0279731. Onwuamah CK, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Ding H, Geng W, Liu J, Jiang Y, Xu J, et al. Predictive effects of body mass index on immune reconstitution among HIV-infected HAART users in China. BMC Infect Dis. 2019;19(1):373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bijker R, Kiertiburanakul S, Kumarasamy N, Pujari S, Sun LP, Ng OT, et al. Survival after long-term ART exposure: findings from an Asian patient population retained in care beyond 5 years on ART. Antivir Ther. 2020;25(3):131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marazzi MC, Liotta G, Germano P, Guidotti G, Altan AD, Ceffa S, et al. Excessive early mortality in the first year of treatment in HIV type 1-infected patients initiating antiretroviral therapy in resource-limited settings. AIDS Res Hum Retroviruses. 2008;24(4):555–60. [DOI] [PubMed] [Google Scholar]
- 38.Tuboi SH, Schechter M, McGowan CC, Cesar C, Krolewiecki A, Cahn P, et al. Mortality during the first year of potent antiretroviral therapy in HIV-1-infected patients in 7 sites throughout Latin America and the Caribbean. JAIDS J Acquir Immune Defic Syndr. 2009;51(5):615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu J, Yousuf MA, Yang W, Zhu Q, Shen Z, Lan G, et al. Mortality and attrition rates within the first year of antiretroviral therapy initiation among people living with HIV in Guangxi, China: an observational cohort study. BioMed Res Int. 2021;2021:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naidoo K, Hassan-Moosa R, Yende-Zuma N, Govender D, Padayatchi N, Dawood H, et al. High mortality rates in men initiated on anti-retroviral treatment in KwaZulu-Natal, South Africa. PLOS One. 2017;12(9):e0184124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aemro A, Wassie M, Chekol B. Incidence and predictors of mortality within the first year of antiretroviral therapy initiation at Debre-Markos Referral Hospital, Northwest Ethiopia: a retrospective follow up study. PLOS One. 2021;16(5):e0251648. Madeddu G, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ART-LINC of IeDEA Study Group, Keiser O, Tweya H, Boulle A, Braitstein P, Schecter M, et al. Switching to second-line antiretroviral therapy in resource-limited settings: comparison of programmes with and without viral load monitoring. AIDS Lond Engl. 2009;23(14):1867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darraj M, Shafer LA, Chan S, Kasper K, Keynan Y. Rapid CD4 decline prior to antiretroviral therapy predicts subsequent failure to reconstitute despite HIV viral suppression. J Infect Public Health. 2018;11(2):265–9. [DOI] [PubMed] [Google Scholar]
- 44.LINKAGES. LINKAGES Indonesia: Summary of Achievements August 2015–September 2021. 2021. Available from: https://www.fhi360.org/wp-content/uploads/2024/02/linkages-indonesia-summary.pdf. Cited 2024 Oct 23.
- 45.Lazuardi E, Newman CE, Anintya I, Rowe E, Wirawan DN, Wisaksana R, et al. Increasing HIV treatment access, uptake and use among men who have sex with men in urban Indonesia: evidence from a qualitative study in three cities. Health Policy Plan. 2020;35(1):16–25. [DOI] [PubMed]
- 46.Tarigan YN, Woodman RJ, Miller ER, Wisaksana R, Wignall FS, Ward PR. Changes in the HIV continuum of care following expanded access to HIV testing and treatment in Indonesia: a retrospective population-based cohort study. PLOS One. 2020;15(9):e0239041. Francis JM, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ministry of Health Republic Indonesia. Decree of the Minister of Health of the Republic of Indonesia Number HK.01.07/Menkes/755/2019 on the National Guidelines for Medical Services in the Management of Tuberculosis (in Bahasa Indonesia). 2019. Available from: https://yankes.kemkes.go.id/unduhan/fileunduhan_1610422577_801904.pdf. Cited 2024 Oct 10.
- 48.World Health Organization. Global tuberculosis report. 2020. Available from: https://www.who.int/publications/i/item/9789240013131. Cited 2024 Oct 10.
- 49.Ministry of Health Republic Indonesia. Technical Guidelines for the Management of Latent Tuberculosis Infection, Ministry of Health, 2020 (in Bahasa Indonesia). 2020. Available from: https://yki4tbc.org/wp-content/uploads/2021/12/Petunjuk-Teknis-Penanganan-Infeksi-Laten-Tuberkulosis-ILTB.pdf.
- 50.Burkey MD, Weiser SD, Fehmie D, Alamo-Talisuna S, Sunday P, Nannyunja J, et al. Socioeconomic determinants of mortality in HIV: evidence from a clinical cohort in Uganda. JAIDS J Acquir Immune Defic Syndr. 2014;66(1):41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lua I, Silva AF, Guimarães NS, Magno L, Pescarini J, Anderle RVR, et al. The effects of social determinants of health on acquired immune deficiency syndrome in a low-income population of Brazil: a retrospective cohort study of 28.3 million individuals. Lancet Reg Health - Am. 2023;24:100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention. Decreasing Deaths Among People with HIV. 2020. Available from: https://www.cdc.gov/hiv/statistics/deaths/index.html.
- 53.Rai S, Mahapatra B, Sircar S, Raj PY, Venkatesh S, Shaukat M, et al. Adherence to antiretroviral therapy and its effect on survival of HIV-infected individuals in Jharkhand, India. PLoS One. 2013;8(6):e66860. Shukla D, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelwan EJ, Isa A, Alisjahbana B, Triani N, Djamaris I, Djaja I, et al. Routine or targeted HIV screening of Indonesian prisoners. Int J Prison Health. 2016;12(1):17–26. [DOI] [PubMed] [Google Scholar]
- 55.Nelwan EJ, Diana A, van Crevel R, Alam NN, Alisjahbana B, Pohan HT, et al. Indonesian prisons and HIV: part of the problem, part of the solution? Acta Medica Indones. 2009;41(Suppl 1):52–6. [PubMed] [Google Scholar]
- 56.Zhao Y, Wu Z, McGoogan JM, Shi CX, Li A, Dou Z, et al. Immediate antiretroviral therapy decreases mortality among patients with high CD4 counts in China: a nationwide, retrospective cohort study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2018;66(5):727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Combination of each additional variable to find out which one changed ART duration from significant to not significant. Additional file 2: Combination of each additional variable to find out which one changed clinical stage from significant to not significant.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.