Abstract
Mice with heterozygous deletion of the PTEN tumor suppressor gene develop a range of epithelial neoplasia as well as lymphoid hyperplasia. Previous studies suggest that PTEN suppresses tumor formation by acting as a phosphoinositide phosphatase to limit signaling by phosphoinositide 3-kinase (PI3K). Here, we examined the effect of deleting various regulatory subunits of PI3K (p85α and p85β) on epithelial neoplasia and lymphoid hyperplasia in PTEN+/- mice. Interestingly, we found the loss of one p85α allele with or without the loss of p85β led to increased incidence of intestinal polyps. Signaling downstream of PI3K was enhanced in the PTEN+/-p85α+/-p85β-/- polyps, as judged by an increased fraction of both cells with cytoplasmic staining of the transcription factor FKHR and cells with positive staining for the proliferation marker Ki-67. In contrast, the incidence of prostate intraepithelial neoplasia was not significantly altered in PTEN+/- mice heterozygous for p85α or null for p85β, whereas the fraction of proliferating cells in prostate intraepithelial neoplasia was reduced in mice lacking p85β. Finally, there was no significant change in T lymphocyte hyperplasia in the PTEN+/- mice with various p85 deletions, although anti-CD3-stimulated AKT activation was somewhat reduced in the p85α+/- background. These results indicate that decreasing the levels of different p85 regulatory subunits can result in enhanced PI3K signaling in some tissues and decreased PI3K signaling in others, supporting the model that, although p85 proteins are essential for class IA PI3K signaling, they can function as inhibitors of PI3K signaling in some tissues and thus suppress tumor formation.
Keywords: intestinal polyps, prostate intraepithelial neoplasia, AKT
Phosphoinositide 3-kinases (PI3Ks) are a family of lipid kinases that regulate a wide range of cellular processes, among which are cell survival, growth, and proliferation, processes whose misregulation contributes to cancer (1, 2). Class I PI3Ks phosphorylates the 3′-hydroxyl group on the inositol ring of the lipid phosphatidylinositol-4,5-bisphosphate to generate the membrane-bound second messenger phosphatidylinositiol-3,4,5-trisphosphate (PIP3), which in turn activates a number of downstream proteins through interaction with their pleckstrin-homology domains.
A major downstream target of PIP3 is the protein serine-threonine kinase Akt (also known as PKB). PIP3 recruits Akt to the plasma membrane through binding to its pleckstrin-homology domain. At the plasma membrane, the protein kinase PDK1 phosphorylates Thr-308 in the catalytic loop of Akt and hence activates it (1). Full activation of Akt requires additional phosphorylation at Ser-473 in its C terminus (3). Akt regulates the activity of a number of cellular proteins and mediates many of the downstream effects of PI3K. The FOXO (forkhead) family of transcription factors act as negative regulators of cell survival and cell cycle progression through their transcriptional activation of proapoptotic proteins such as FAS-ligand and cell-cycle repressors such as p27kip1 (4). The phosphorylation of FOXO proteins by Akt leads to their cytoplasmic sequestration through binding to 14-3-3 proteins. Therefore, Akt promotes cell survival and cell cycle entry in part by inhibiting the activities of FOXO proteins (1). Akt also phosphorylates tuberin, the protein product of the tuberous sclerosis 2 gene and turns off its function as a GTPase activating protein for the small GTPase Rheb. Rheb activates the raptor/mTOR signaling pathway that ultimately regulates protein translation and cell growth, at least in part, through the regulation of p70 S6 kinase and its downstream targets ribosomal S6 protein and 4E-BP1 (5).
Several PIP3 phosphatases act to terminate PI3K signaling in the cell; of particular importance is the 3′-phosphatase PTEN, which dephosphorylates the 3′-position of PIP3 to regenerate phosphatidylinositol-4,5-bisphosphate (6). Loss-of-function mutations, gene deletion, or gene silencing of the PIP3 phosphatase PTEN are frequent events in human cancers. Germ-line mutations in PTEN cause the familial hamartoma syndromes Cowden's disease and Bannayan-Riley-Ruvalcaba syndrome. Somatic mutations, gene deletion, or inactivation of PTEN occur at high frequencies in glioblastomas (-30%), endometrial cancers (>50%), and prostate cancers (20-60%), and at lower frequencies in breast, lung, thyroid, liver, and lymphoid cancers and melanomas (2, 6). Studies using knockout mice have firmly established the important role of PTEN as a tumor suppressor. Mice with heterozygous deletion of the PTEN gene develop lymphoid hyperplasia and epithelial neoplasia of the thyroid, prostate, endometrium, breast, liver, and intestine (7-10). Furthermore, mice with tissue-specific deletion of PTEN in T cells and in the prostate develop T cell lymphomas and metastatic prostate carcinomas, respectively (11-13).
To date, only class IA PI3Ks, which are activated by growth factor receptors, have been implicated in cancers. These enzymes are heterodimers consisting of a p110 catalytic subunit and a p85 regulatory subunit, with the p85 regulatory subunit being essential both for the stability of the p110 catalytic subunit and for its recruitment to activated growth factor receptors (14, 15). In human and mouse, there are three p110 isoforms (p110α, p110β, and p110δ) and five p85 isoforms (p85α, p55α, p50α, p85β, and p55γ). The p85α, p55α, and p50α isoforms arise from alternative transcription initiation sites on a single gene pik3r1, whereas p85β and p55γ are encoded by separate genes pik3r2 and pik3r3, respectively. All five p85 isoforms contain two SH2 domains that recognize phospho-tyrosine residues on activated growth factors receptors (or their adaptors), the two longer isoforms p85α and p85β also contain a SH3 and a BH domain in the N terminus. No clear preference has been demonstrated for regulatory-catalytic subunit pairing and it is generally assumed that each p85 isoform can bind to all p110 isoforms and vice versa (15).
The class IA PI3K signaling pathway is among the most frequently mutated signaling pathway in human cancers and presents an attractive target for cancer therapeutics (2, 16). Mutations in this pathway that lead to enhanced PI3K signaling are often selected for in tumor cells because they confer survival and growth advantage. For example, gene amplification of p110α and Akt, as well as oncogenic mutations in p85α that leads to enhanced PI3K activation have been identified in ovarian, colon, and breast cancers (2). Recently, point mutations in p110α that confer enhanced kinase activity have been found in a wide range of tumors, with ≈20-30% of brain, colon, breast, and liver tumors and a smaller percentage of lung, ovarian, and gastric tumors harboring such mutations (17-20). When expressed in chicken embryonic fibroblasts, these p110α mutants induce oncogenic transformation that is associated with the constitutive activation of Akt and p70 S6-kinase (21).
Mice with targeted deletion of various class IA PI3K isoforms have been generated to elucidate theirs roles in vivo. Homozygous deletion of either p110α or p110β results in early embryonic death, whereas mice heterozygous for p110α and p110β show blunted PI3K signaling in insulin-sensitive tissues (22, 23). Mice lacking all three p85α isoforms (p85α/p55α/p50α) exhibit perinatal lethality that is associated with hypoglycemia and liver necrosis, whereas heterozygotes are viable (24, 25). Mice deficient in p85β are viable but are slightly smaller (26). Germ-line deletion of both p85α and p85β results in embryonic lethality with a phenotype similar to the loss of p110α (27). Surprisingly, viable mice that result from any combination of deletions for p85α, p55α, p50α, or p85β exhibit increased insulin sensitivity (24, 26, 28-30). The enhanced insulin signaling despite a reduction in p85 levels in these animals is thought to be due to a change in the molecular balance between p85 monomers and p85/p110 dimers (31). The p85/p110 heterodimer has a half-life for dissociation of many hours and thus cannot dynamically exchange with monomeric p85 in vivo (23). In some cell types, p85 is in excess of p110, and free monomeric p85 acts as a dominant negative by competing with p85/p110 dimers to bind to tyrosine-phosphorylated IRS proteins, adaptors through which the insulin and the IGF-1 receptors activate PI3K. A partial loss of p85 leads to a net reduction in the ratio of p85 monomers to p85/p110 dimers, thereby alleviating the negative regulation of PI3K by monomeric p85, and results in improved PI3K signaling (23, 31).
The present study aims to address the question of whether the p85 regulatory subunits of PI3K play a negative role in the regulation of PI3K-dependent tumor formation, analogous to the negative role they play in insulin signaling. Alternatively, it is possible that, in some tissues where the p85 regulatory subunits are not in excess over the p110 catalytic subunits, a decrease in p85 levels would result in a major decrease in total PI3K and thereby suppress tumor formation. Here, we show that PTEN+/- mice that were also heterozygous for the pik3r1 gene that encodes p85α, p55α, and p50α (hereafter referred to as p85α+/- for clarity) showed increased incidence of intestinal polyps but no significant change in prostate intraepithelial neoplasia (PIN) compared to PTEN+/- littermates. PTEN+/- mice with homozygous deletion of the pik3r2 gene, which encodes p85β (hereafter referred to as p85β-/-), did not have a significant change in the incidence of intestinal polyps or PIN compared to PTEN+/- littermates, but exhibited a reduction in the fraction of proliferating cells in PIN. Although the incidence and severity of T cell hyperplasia was not significantly affect by heterozygous loss of p85α or homozygous loss of p85β or both, anti-CD3-dependent AKT activation was reduced in T cells that were heterozygous for p85α. These results indicate that p85 proteins are acting to suppress PI3K-dependent neoplasia in some (intestinal epithelium) but not all tissues, they also suggest that p85α and p85β may play competing roles in the regulation of PI3K signaling in PIN.
Materials and Methods
Generation of Mice. Male mice of the genotype PTEN+/-p85β+/- were mated to female mice of the genotype p85α+/-p85β+/- to generate age-matched littermates of the following eight genotypes: wild type, p85α+/-, p85β-/-, p85α+/-p85β-/-, PTEN+/-, PTEN+/-p85α+/-, PTEN+/-p85β-/-, and PTEN+/-p85α+/-p85β-/-. All mice were maintained on the 129Sv/C57BL6 mixed background. Genotyping for each locus was carried out as described (25, 26, 32). Animal care and experimentation were approved by the Institutional Animal Care and Use Committees of Beth Israel Deaconess Medical Center and Harvard Medical School.
Histology, Immunohistochemistry, and Analytical Procedures. Tissues from 5- to 10-month-old mice were fixed in 4% paraformaldehyde and embedded in paraffin. Macroscopic polyps in the gastrointestinal track of each mouse were counted and dissected out individually before fixation. Hematoxylin and eosin sections of all major organs were surveyed systematically for neoplasia/hyperplasia. The grading of the epithelial neoplasia of the prostate and the endometrium was based on the estimated area of neoplastic epithelium as a percentage of the total epithelium area on the section. Immunohistochemistry was carried out by using fixed tissue sections with heat-induced antigen retrieval. The antibodies were used at the following empirically determined dilutions: phospho-Akt (1:400), FKHR (1:100), Ki-67 (1:2000), and phospho-S6 (1:300). For the quantitation of FKHR localization and the percentage of Ki-67-positive cells in neoplastic lesions, ≈200 cells were counted per section and at least three mice per genotype were analyzed. The onset of s.c. lymphoid hyperplasia in mice was monitored by palpation of the major s.c. lymph nodes (superficial cervical, axillary, brachial, and inguinal lymph nodes) twice per week. Statistical analysis was carried out by using prism 4 (GraphPad, San Diego).
Molecular Biology and Antibodies. For in vitro T cell stimulation, T cells were isolated from lymph nodes of 6- to 8-week-old mice by using Spin-sep murine T cell enrichment kit (Stem Cell Technologies). T cells were stimulated with a mixture of 10 μg/ml biotin-conjugated anti-CD3ε antibody and 20 μg/ml streptavidin (eBioscience) for 5 min at 37°C, and total cell lysates were subjected to Western blotting analysis. Flow cytometry analysis of T and B cells was performed as described (9) with minor modifications using FACScan, and the data were analyzed by using cellquest software (BD Bioscience). The anti-p85 pan antibody was described (25); PTEN antibody was a gift from W. R. Sellers (Dana-Farber Cancer Institute); CD4 and CD44 antibodies were from BD Bioscience; and Ki-67 antibody was from Vector Laboratories; phospho-Akt (S473), Akt, phospho-p44/42 Erk (T202/Y204), p44/42 Erk, phospho-S6 (S240/244), and FKHR antibodies were from Cell Signaling Technologies.
Results
Loss of One p85α Allele Increases the Incidence of Intestinal Polyps. To investigate whether the loss of one p85α and/or both p85β alleles alters epithelial neoplasia and lymphoid hyperplasia in PTEN+/- mice, we crossed PTEN+/- mice with p85α+/- and p85β-/- mice to generate the four viable genotypes of mice that carry various combination of PTEN and p85 alleles (PTEN+/-, PTEN+/-p85β-/-, PTEN+/-p85α+/-, and PTEN+/-p85α+/-p85β-/-). Epithelial neoplasia, such as those of the prostate, endometrium, intestine, or mammary glands, are frequently found in PTEN+/- mice (7, 10, 32). Molecularly, these lesions were characterized by hyperactivation of Akt (7, 8) and elevated S6 phosphorylation (see below). In the particular genetic background of the mice (129Sv/C57BL6) used in this study, we found epithelial neoplasia of the intestine, prostate, and endometrium to be the most frequent lesions in PTEN+/- animals (data not shown). Morphologically, these lesions were mostly focal, and cells in the lesion still retained epithelial characteristics. Highly dedifferentiated and/or invasive carcinomas were rarely seen. The epithelia of various tissues from control p85β-/-, p85α+/-, or p85α+/-p85β-/- animals appeared normal, and no neoplastic lesions were seen (data not shown).
Approximately 30% of PTEN+/- mice develop neoplastic polyps of the intestinal epithelium between 5 and 10 months of age. The incidence of intestinal polyps in PTEN+/- mice was not affected by the homozygous deletion of p85β. In contrast, the loss of one p85α allele, either alone or in combination with p85β-/-, led to an ≈2-fold increase in incidence rate. Approximately 60% of PTEN+/-p85α+/- and PTEN+/-p85α+/- p85β-/- mice developed intestinal polyps, compared to an incidence rate of ≈30% in PTEN+/- and PTEN+/-p85β-/- littermates (Table 1). Furthermore, there was a tendency of PTEN+/-p85α+/- and PTEN+/-p85α+/-p85β-/- mice to develop multiple (more than three) macroscopic polyps, whereas PTEN+/- and PTEN+/-p85β-/- littermates of the same cohort mostly developed only one or two macroscopic polyps per animal (Fig. 1A). Despite the increased incidence rate, intestinal polyps from PTEN+/-p85α+/- and PTEN+/-p85α+/-p85β-/- mice appeared well defined: the neoplastic cells still retained epithelial morphology, and no invasive carcinoma was observed in these lesions.
Table 1. Incidence of intestinal polyps in PTEN+/- mice with various p85 alleles.
| Genotype | 5 months | 10 months | Total | Incidence rate, % |
|---|---|---|---|---|
| PTEN+/– | 2 (14) | 6 (13) | 8 (27) | 30 |
| PTEN+/–p85β-/- | 1 (11) | 7 (14) | 8 (25) | 32 |
| PTEN+/–p85α+/– | 5 (13) | 8 (11) | 13 (24) | 54* |
| PTEN+/–p85α+/–p85β-/- | 4 (7) | 9 (13) | 13 (20) | 65† |
The numbers of mice harboring intestinal polyps versus the total number of mice analyzed (in parentheses) per genotype are shown. *, P < 0.08 compared to PTEN+/– mice, χ2 test. †, P < 0.02 compared to PTEN+/– mice, χ2 test.
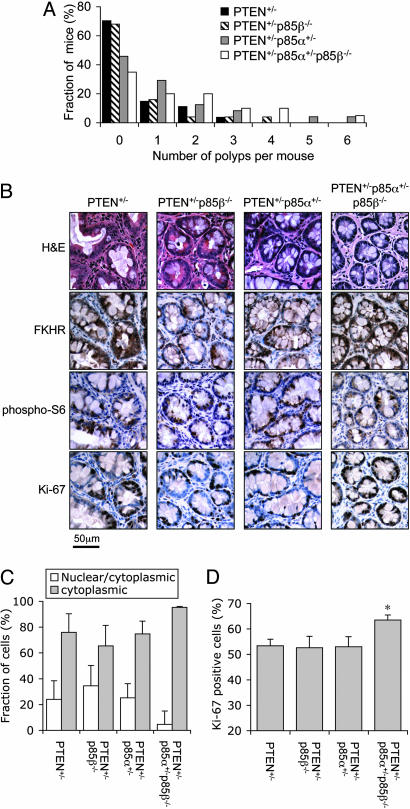
Fig. 1.
Loss of one p85α allele increased intestinal polyps incidence in PTEN+/- mice. (A) The distribution of the number of macroscopic intestinal polyps per animal in 5- to 10-month-old mice. The total numbers of mice analyzed for each genotype were the same as those in Table 1. (B) Immunohistochemistry of prostate PIN from 5- to 10-month-old animals revealed cytoplasmic localization of FKHR and elevated S6 phosphorylation. For each genotype, representative staining of adjacent sections from the same lesion are shown. (C) The relative distribution of FKHR staining in cells from intestinal epithelial neoplasia. Few cells with exclusive nuclear staining of FKHR were found in all lesions analyzed. Results shown are mean ± SEM (n = 4 mice per genotype). (D) The percentage of Ki-67-positive cells in intestinal epithelial neoplasia. Results shown are mean ± SEM (n = 4 mice per genotype; *, P < 0.05 compared to PTEN+/- mice, two-tailed t test).
We next examined the activation status of the PI3K signaling pathway in these lesions by immunohistochemistry. We used the serine-473 phosphorylation of Akt (i.e., activated Akt), the nuclear versus cytoplasmic distribution of the FOXO transcription factor FKHR, and the phosphorylation of the ribosomal S6 protein as the readout for the activation of this pathway. Unlike the prostate and endometrial neoplastic lesions (see below), phospho-Akt staining in the intestinal neoplastic epithelium was difficult to evaluate because of high background staining in this tissue from all genotypes (data not shown). However, we observed significant cytoplasmic distribution of FKHR and elevated S6 phosphorylation in these cells (Fig. 1B), suggesting that Akt was indeed activated. Therefore, we used the cellular localization (nuclear vs. cytoplasmic) of FKHR to quantify the extent of Akt activation in the neoplastic epithelium. We binned cells into three categories, defined as those with mostly nuclear FKHR staining, those with both nuclear and significant cytoplasmic FKHR staining, and those with mostly cytoplasmic FKHR staining, to indicate progressive elevation in cellular Akt activity in this order. In all four genotypes, most cells in the intestinal neoplastic epithelium show some extent of cytoplasmic FKHR staining. In addition, the intestinal neoplastic epithelium from PTEN+/-p85α+/-p85β-/- mice show increased cytoplasmic and decreased nuclear FKHR staining compared to that of PTEN+/- mice (Fig. 1C), indicating enhanced Akt activity in these cells. Although this difference failed to reach statistical significance, it was associated with a statistically significant increase in the percentage of Ki-67-positive cells in these lesions (Fig. 1D). Taken together, these findings suggest that the loss of one p85α allele actually enhanced PI3K signaling and the development of intestinal polyps in PTEN+/- mice.
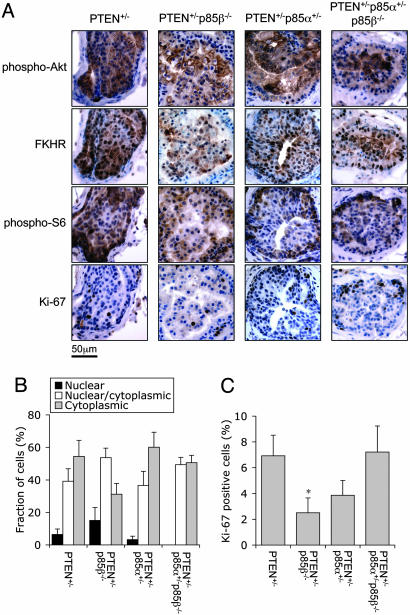
Loss of p85β Attenuates Akt Signaling and Cell Proliferation in Prostate Intraepithelial Neoplasia of PTEN+/- Mice. We next analyzed the effect of losing one or more p85 alleles on the incidence of PIN in PTEN+/- males. A histological survey of the prostate from PTEN+/-, PTEN+/-p85β-/-, PTEN+/-p85α+/-, and PTEN+/-p85α+/-p85β-/- males at the age of 5-10 months revealed PIN in a significant fraction of the animals, with lesions being found in several or all of the four lobes (ventral, lateral, dorsal, and anterior) of the prostate. However, the incidence frequencies were similar among the four genotypes (Table 2). The PIN in all four genotypes were mostly focal, with cells still retaining epithelial morphology. Immunohistochemistry using phospho-specific antibodies against Akt and S6 showed strong Akt phosphorylation and membrane localization, as well as elevated S6 phosphorylation in PIN of all four genotypes (Fig. 2A). Using the cellular localization (nuclear vs. cytoplasmic) of FKHR to quantify the extent of Akt activation, we found that PIN from PTEN+/-p85β-/- mice harbored fewer cells with cytoplasmic FKHR staining and more cells with nuclear FKHR staining compared to PIN from PTEN+/- littermates (Fig. 2B), indicating that Akt was less activated in the PIN of PTEN+/- p85β-/- mice. Although this difference failed to reach statistical significance, it was associated with a statistically significant reduction in Ki-67-positive cells in the PIN lesions from PTEN+/-p85β-/- mice (Fig. 2C). Taken together, these results suggest that the loss of p85β may attenuate Akt signaling and cell proliferation in PIN of PTEN+/- mice, although such difference was not sufficient to affect the incidence of the PIN phenotype. Curiously, the PTEN+/-p85α+/-p85β-/- mice had a similar ratio of cytoplasmic to nuclear FKHR and a similar fraction of Ki-67-positive cells compared to PTEN+/- littermates, indicating that the heterozygous loss of p85α alleviated the suppression of cell proliferation observed in the p85β-/- background.
Table 2. Incidence of PIN in 5- to 10-month-old PTEN+/- males with various p85 alleles.
| PIN grade, %
|
||||||
|---|---|---|---|---|---|---|
| Genotype | Normal | <25 | 25–50 | >50 | Total | Incidence rate, % |
| PTEN+/– | 4 | 7 | 1 | 1 | 9 (13) | 69 |
| PTEN+/–p85β-/- | 2 | 10 | 0 | 0 | 10 (12) | 83 |
| PTEN+/–p85α+/– | 2 | 9 | 0 | 0 | 9 (11) | 82 |
| PTEN+/–p85α+/–p85β-/- | 1 | 7 | 2 | 1 | 10 (11) | 91 |
The numbers of mice harboring PIN versus the total number of mice analyzed (in parentheses) per genotype are shown. The extent of PIN in individual animals was graded based on the percentage area of the total prostate epithelium that manifested neoplasia.
Fig. 2.
Loss of p85β reduces cell proliferation in PIN of PTEN+/- mice. (A) Immunohistochemistry of prostate PIN from 5- to 10-month-old males revealed elevated Akt phosphorylation, cytoplasmic localization of FKHR, and elevated S6 phosphorylation. For each genotype, representative staining of adjacent sections from the same lesion are shown. (B) The relative distribution of FKHR staining of cells in PIN. Results shown are mean ± SEM (n = 9 independent lesions from three mice per genotype). (C) The percentage of Ki-67-positive cells in PIN. Results shown are mean ± SEM (n = 9 independent lesions from three mice per genotype; *, P < 0.05 compared to PTEN+/- mice, two-tailed t test).
We also compared the incidence and morphology of endometrial neoplasia among 5- to 10-month-old female mice and found no difference in incidence rates or lesion morphology among PTEN+/-, PTEN+/-p85β-/-, PTEN+/-p85α+/-, and PTEN+/-p85α+/-p85β-/- mice. Endometrial lesions from all four genotypes showed hyperactivation of Akt and elevated S6 phosphorylation, and the percentages of Ki-67-positive cells in the lesions were also comparable among the four genotypes (Table 3 and Fig. 4, which are published as supporting information on the PNAS web site).
Reduction of p85α Levels Attenuates Akt Activation in T Cells but Does Not Prevent Lymphoid Hyperplasia in PTEN+/- Mice. Because lymphoid hyperplasia is of high penetrance in PTEN+/- mice (9, 10), we also investigated the effect of p85 allele dosage on lymphoid hyperplasia. Analysis of lymphocytes and splenocytes from PTEN+/- mice revealed selective accumulation of CD4+CD44high T cells in the spleen of these animals (Fig. 3A), whereas no accumulation of B cells or change in B cell surface markers were detected (data not shown). This finding suggests that the lymphoid hyperplasia in PTEN+/- mice on this genetic background is likely to be due to the accumulation of activated T cells. T and B cells from control p85β-/-, p85α+/-, or p85α+/-p85β-/- mice appeared normal and did not show changes in population distribution or surface marker density in comparison to wild-type animals, and these mice did not display any lymphoid or splenic abnormalities (data not shown).
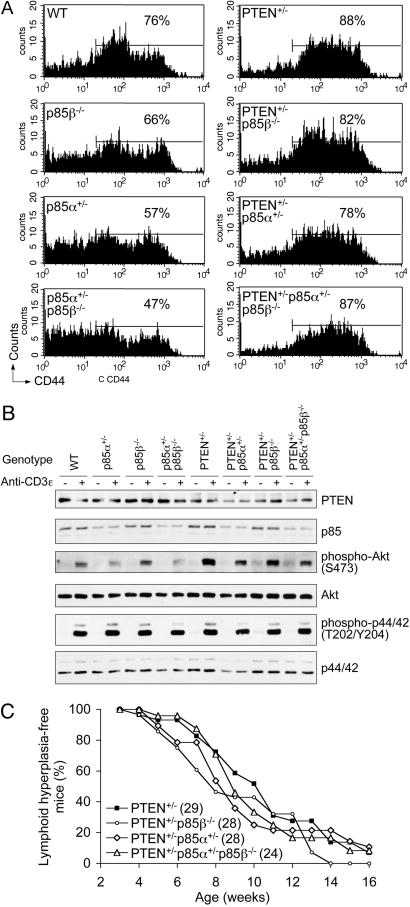
Fig. 3.
Loss of one p85α allele reduces Akt activation in T cells from PTEN+/- mice. (A) The levels of CD44 in splenic CD4+ T cells were profiled in 8- to 10-week-old mice, and the percentage of CD44high cells was calculated for each profile by using the same gate. Representative profiles of four experiments are shown. (B) Heterozygosity of the p85α allele led to reduced Akt activation in T cells in vitro. T cells from 6- to 8-week-old animals were stimulated with anti-CD3ε antibody and the phosphorylation of Akt and p44/42 Erk were assessed by Western blot. Representative blots of four independent experiments from four groups of mice are shown. (C) Heterozygosity of the p85α allele did not significantly attenuate the onset of lymphoid hyperplasia in PTEN+/- mice. Kaplan-Meier curve of s.c. lymphoid hyperplasia-free mice over time is shown. The total number of mice analyzed are shown in parentheses.
We next assessed PI3K signaling downstream of T cell receptor (TCR) in primary T cells in vitro. As expected, in response to CD3 ligation, PTEN+/- T cells exhibited enhanced Akt activation compared to wild-type T cells, whereas Akt activation is attenuated in p85α+/- and p85α+/-p85β-/- T cells but not in p85β-/- T cells (Fig. 3B). This finding suggests that the p85α isoforms are likely to be the major regulatory subunits of class IA PI3K downstream of TCR. On the PTEN+/- background, the loss of one p85α allele significantly attenuated Akt activation, whereas the loss of both p85β alleles resulted in a slight reduction in Akt activation. However, even the most severe reduction in p85 subunits was insufficient to counter the haplo-insufficiency of PTEN; hence, Akt activity in PTEN+/-p85α+/-p85β-/- T cells was, albeit significantly lower than that in PTEN+/- T cells, still higher than that in wild-type T cells (Fig. 3B). TCR-mediated Erk activation was comparable in T cells of all genotypes.
When we examined T cells from PTEN+/- mice that also carry the various p85 alleles, we found accumulation of splenic CD4+CD44high T cells in PTEN+/-p85β-/-, PTEN+/-p85α+/-, and PTEN+/-p85α+/-p85β-/- animals similar to that observed in PTEN+/- animals (Fig. 3A). Consequently, when we followed the onset of s.c. lymph node hyperplasia, we found that the introduction of various p85 alleles in the PTEN+/- background did not significantly alter the rate at which these mice develop lymphoid hyperplasia (Fig. 3C). Taken together, these results suggest that a reduction in p85 (particularly p85α) levels attenuates Akt signaling in T cells, although it is insufficient to abrogate the hyperactivation of Akt due to PTEN haplo-insufficiency and attenuate lymphoid hyperplasia in PTEN+/- mice.
Discussion
In this study, we used genetic approaches to study the roles of different regulatory subunits of class IA PI3K in the development of neoplastic and hyperplastic lesions in mice that are heterozygous for the PIP3 phosphatase PTEN. In the particular genetic background of the mice used in this study (mixed 129Sv/C57BL6), we found lymphoid hyperplasia and neoplasia of the intestinal, prostatic, and endometrial epithelia to be the most common lesions in PTEN+/- animals (data not shown). The additional introduction of either p85α+/- or p85β-/- alleles or a combination of both did not significantly reduce the overall incidence rates of these lesions, suggesting that one p85α allele alone was sufficient to support the development of such lesions in PTEN+/- mice. This finding is not entirely surprising, as there is evidence that the neoplastic/hyperplastic lesions in PTEN+/- mice frequently show loss-of-heterozygosity of the remaining PTEN allele (7, 8, 10). The perinatal lethality of p85α-/- mice (24) prevented us from testing whether the loss of both p85α alleles would attenuate hyperplasia/neoplasia development in PTEN+/- mice. Generation of mice that allow tissue-specific deletion of both PTEN and p85α would allow such genetic test in the tissue of interest.
In the intestinal epithelium, we found the loss of one p85α allele led to an increased incidence of polyps in the PTEN+/- background (Table 1). In the PTEN+/-p85α+/-p85β-/- animals, this higher incidence rate was also associated with elevated cell proliferation and increased cytoplasmic localization of FKHR (Fig. 1). Thus, the intestinal epithelium appeared to be analogous to insulin-sensitive tissues with regard to class IA PI3K signaling in the sense that in these cells there might exist a significant amount of free p85 in excess over p110 that acts to suppress PI3K signaling (23, 31). The effect of deleting some of the p85 alleles would be a net reduction in the ratio of p85 monomers to p85/p110 dimers, thus giving rise to improved PI3K signaling by eliminating the dominant negative effect of monomeric p85. So far, the negative regulation of PI3K signaling by monomeric p85 has only been implicated downstream of the insulin and IGF-1 receptors and appears to be mediated by the ability of p85 to sequester IRS-1 into a nonsignaling complex (35). It is possible that IGF-1 is a major growth factor that contributes to PI3K signaling in the intestinal polyps of PTEN+/- mice. Alternatively, PI3K signaling downstream of other growth factor receptors in these polyps might be subjected to negative regulation by monomeric p85.
The development of prostate neoplasia in mice is exquisitely sensitive to PI3K signaling. Mice expressing a myristolated form of Akt in the prostate develop severe PIN (33). Whereas mice with germ-line heterozygosity in the PTEN gene develop noninvasive PIN, mice with homozygous deletion of PTEN in the prostate develop metastatic carcinoma (11, 13). We found that the loss of p85β, albeit insufficient to prevent PIN in PTEN+/- mice, attenuated Akt activation and reduced cell proliferation in these lesions (Fig. 2). Curiously, this suppressive effect was not seen in PTEN+/-p85α+/-p85β-/- animals. This finding indicates that there might be more complex interplay between the balance of the p85α and p85β isoforms in the prostate epithelium. Deletion of p85β versus p85α results in distinct effects on IRS-2 phosphorylation (26), and this might result in opposing effects on IGF-1 signaling.
The lymphoid hyperplasia in PTEN+/- mice is largely due to the accumulation of activated T cells (9). In support of this, mice with T cell-specific deletion of PTEN develop lymphomas (12). Unlike the insulin-sensitive tissues where p85α heterozygosity results in an enhanced PI3K signaling (29), in T cells, p85α heterozygosity led to a reduction in Akt activation downstream of the T cell receptor. This finding suggests that, in T cells, there might be very little free p85 and most p85 is bound to p110; therefore, a reduction in p85 level leads to a net reduction in the level of the p85/p110 dimer. Although Akt activation in vitro is significantly attenuated in p85a+/- T cells, such attenuation was insufficient to prevent the accumulation of activated T cells in PTEN+/-p85α+/- or PTEN+/-p85α+/-p85β-/- animals. Given that T cell development and proliferation appear to be normal in the complete absence of p85α isoforms (25), it is possible that only a small amount of PI3K activity is normally required in T cells, and the presence of one p85α allele may provide a sufficient level of PI3K signaling for aberrant T cell survival in the PTEN+/- background. It is also possible that the hyperproliferation of T cells is not mediated by class IA PI3K signaling, but by class IB PI3K that is activated by G protein-coupled receptors (34).
Taken together, our results suggest that the p85α and p85β isoforms of class IA PI3K play different roles in mediating PI3K signaling in different hyperplastic/neoplastic lesions in PTEN+/- mice, and therapeutic approaches that target the PI3K pathway in cancer may exploit such differences between these isoforms.
Supplementary Material
Acknowledgments
We thank C. Quigley for assistance with immunohistochemistry; J. Tigges for assistance with flow cytometry; and Drs. P. K. Majumder, W. R. Sellers, D. Di Vizio, D. W. Abbott, J. A. Engelman, T. M. Roberts, J. Blenis, and J. Yuan for discussion and advice. This work is supported by a Howard Hughes Medical Institute predoctoral fellowship (to J.L.) and National Institutes of Health Grant P01-CA089021 (to L.C.C., R.A.D., and M.L.).
Author contributions: J.L. and L.C.C. designed research; J.L., C.L.S., N.M.L., J.M.W., E.S., Y.S., and L.P. performed research; J.L., S.S., F.O., M.L., R.A.D., B.G.N., and L.C.C. analyzed data; and J.L. and L.C.C. wrote the paper.
Abbreviations: PI3K, phosphoinositide 3-kinase; PIP3, phosphatidylinositiol-3,4,5-trisphosphate; PIN, prostate intraepithelial neoplasia
References
- 1.Cantley, L. C. (2002) Science 296, 1655-1657. [DOI] [PubMed] [Google Scholar]
- 2.Vivanco, I. & Sawyers, C. L. (2002) Nat. Rev. Cancer 2, 489-501. [DOI] [PubMed] [Google Scholar]
- 3.Sarbassov, D. D., Guertin, D. A., Ali, S. M. & Sabatini, D. M. (2005) Science 307, 1098-1101. [DOI] [PubMed] [Google Scholar]
- 4.Burgering, B. M. & Medema, R. H. (2003) J. Leukoc. Biol. 73, 689-701. [DOI] [PubMed] [Google Scholar]
- 5.Fingar, D. C. & Blenis, J. (2004) Oncogene 23, 3151-3171. [DOI] [PubMed] [Google Scholar]
- 6.Sansal, I. & Sellers, W. R. (2004) J. Clin. Oncol. 22, 2954-2963. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki, A., de la Pompa, J. L., Stambolic, V., Elia, A. J., Sasaki, T., del Barco Barrantes, I., Ho, A., Wakeham, A., Itie, A., Khoo, W., et al. (1998) Curr. Biol. 8, 1169-1178. [DOI] [PubMed] [Google Scholar]
- 8.Stambolic, V., Tsao, M. S., Macpherson, D., Suzuki, A., Chapman, W. B. & Mak, T. W. (2000) Cancer Res. 60, 3605-3611. [PubMed] [Google Scholar]
- 9.Di Cristofano, A., Kotsi, P., Peng, Y. F., Cordon-Cardo, C., Elkon, K. B. & Pandolfi, P. P. (1999) Science 285, 2122-2125. [DOI] [PubMed] [Google Scholar]
- 10.Podsypanina, K., Ellenson, L. H., Nemes, A., Gu, J., Tamura, M., Yamada, K. M., Cordon-Cardo, C., Catoretti, G., Fisher, P. E. & Parsons, R. (1999) Proc. Natl. Acad. Sci. USA 96, 1563-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trotman, L. C., Niki, M., Dotan, Z. A., Koutcher, J. A., Di Cristofano, A., Xiao, A., Khoo, A. S., Roy-Burman, P., Greenberg, N. M., Van Dyke, T., et al. (2003) PloS. Biol. 1, E59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki, A., Yamaguchi, M. T., Ohteki, T., Sasaki, T., Kaisho, T., Kimura, Y., Yoshida, R., Wakeham, A., Higuchi, T., Fukumoto, M., et al. (2001) Immunity 14, 523-534. [DOI] [PubMed] [Google Scholar]
- 13.Wang, S., Gao, J., Lei, Q., Rozengurt, N., Pritchard, C., Jiao, J., Thomas, G. V., Li, G., Roy-Burman, P., Nelson, P. S., et al. (2003) Cancer Cell 4, 209-221. [DOI] [PubMed] [Google Scholar]
- 14.Yu, J., Zhang, Y., McIlroy, J., Rordorf-Nikolic, T., Orr, G. A. & Backer, J. M. (1998) Mol. Cell. Biol. 18, 1379-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fruman, D. A., Meyers, R. E. & Cantley, L. C. (1998) Annu. Rev. Biochem. 67, 481-507. [DOI] [PubMed] [Google Scholar]
- 16.Luo, J., Manning, B. D. & Cantley, L. C. (2003) Cancer Cell 4, 257-262. [DOI] [PubMed] [Google Scholar]
- 17.Campbell, I. G., Russell, S. E., Choong, D. Y., Montgomery, K. G., Ciavarella, M. L., Hooi, C. S., Cristiano, B. E., Pearson, R. B. & Phillips, W. A. (2004) Cancer Res. 64, 7678-7681. [DOI] [PubMed] [Google Scholar]
- 18.Levine, D. A., Bogomolniy, F., Yee, C. J., Lash, A., Barakat, R. R., Borgen, P. I. & Boyd, J. (2005) Clin. Cancer Res. 11, 2875-2878. [DOI] [PubMed] [Google Scholar]
- 19.Lee, J. W., Soung, Y. H., Kim, S. Y., Lee, H. W., Park, W. S., Nam, S. W., Kim, S. H., Lee, J. Y., Yoo, N. J. & Lee, S. H. (2005) Oncogene 24, 1477-1480. [DOI] [PubMed] [Google Scholar]
- 20.Samuels, Y., Wang, Z., Bardelli, A., Silliman, N., Ptak, J., Szabo, S., Yan, H., Gazdar, A., Powell, S. M., Riggins, G. J., et al. (2004) Science 304, 554. [DOI] [PubMed] [Google Scholar]
- 21.Kang, S., Bader, A. G. & Vogt, P. K. (2005) Proc. Natl. Acad. Sci. USA 102, 802-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bi, L., Okabe, I., Bernard, D. J., Wynshaw-Boris, A. & Nussbaum, R. L. (1999) J. Biol. Chem. 274, 10963-10968. [DOI] [PubMed] [Google Scholar]
- 23.Brachmann, S. M., Ueki, K., Engelman, J. A., Kahn, R. C. & Cantley, L. C. (2005) Mol. Cell. Biol. 25, 1596-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fruman, D. A., Mauvais-Jarvis, F., Pollard, D. A., Yballe, C. M., Brazil, D., Bronson, R. T., Kahn, C. R. & Cantley, L. C. (2000) Nat. Genet. 26, 379-382. [DOI] [PubMed] [Google Scholar]
- 25.Fruman, D. A., Snapper, S. B., Yballe, C. M., Davidson, L., Yu, J. Y., Alt, F. W. & Cantley, L. C. (1999) Science 283, 393-397. [DOI] [PubMed] [Google Scholar]
- 26.Ueki, K., Yballe, C. M., Brachmann, S. M., Vicent, D., Watt, J. M., Kahn, C. R. & Cantley, L. C. (2002) Proc. Natl. Acad. Sci. USA 99, 419-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brachmann, S. M., Yballe, C. M., Innocenti, M., Deane, J. A., Fruman, D. A., Thomas, S. M. & Cantley, L. C. (2005) Mol. Cell. Biol. 25, 2593-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen, D., Mauvais-Jarvis, F., Bluher, M., Fisher, S. J., Jozsi, A., Goodyear, L. J., Ueki, K. & Kahn, C. R. (2004) Mol. Cell. Biol. 24, 320-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mauvais-Jarvis, F., Ueki, K., Fruman, D. A., Hirshman, M. F., Sakamoto, K., Goodyear, L. J., Iannacone, M., Accili, D., Cantley, L. C. & Kahn, C. R. (2002) J. Clin. Invest. 109, 141-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terauchi, Y., Tsuji, Y., Satoh, S., Minoura, H., Murakami, K., Okuno, A., Inukai, K., Asano, T., Kaburagi, Y., Ueki, K., et al. (1999) Nat. Genet. 21, 230-235. [DOI] [PubMed] [Google Scholar]
- 31.Ueki, K., Fruman, D. A., Brachmann, S. M., Tseng, Y. H., Cantley, L. C. & Kahn, C. R. (2002) Mol. Cell. Biol. 22, 965-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Cristofano, A., Pesce, B., Cordon-Cardo, C. & Pandolfi, P. P. (1998) Nat. Genet. 19, 348-355. [DOI] [PubMed] [Google Scholar]
- 33.Majumder, P. K., Yeh, J. J., George, D. J., Febbo, P. G., Kum, J., Xue, Q., Bikoff, R., Ma, H., Kantoff, P. W., Golub, T. R., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 7841-7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wymann, M. P., Bjorklof, K., Calvez, R., Finan, P., Thomast, M., Trifilieff, A., Barbier, M., Altruda, F., Hirsch, E. & Laffargue, M. (2003) Biochem. Soc. Trans. 31, 275-280. [DOI] [PubMed] [Google Scholar]
- 35.Luo, J., Field, S. J., Lee, J. Y., Engleman, J. A. & Cantley, L. C. (2005) Gen. Cell Biol., in press.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.