Abstract
The ank (progressive ankylosis) mutant mouse, which has a nonsense mutation in exon 12 of the inorganic pyrophosphate regulator gene (ank), exhibits aberrant joint ankylosis similar to human ankylosing spondylitis (AS). We previously performed family-based association analyses of 124 Caucasian AS families and showed that novel genetic markers in the 5' flanking region of ANKH (the human homolog of the murine ank gene) are modestly associated with AS. The objective of the present study was to conduct a more extensive evaluation of ANKH variants that are significantly associated with AS and to determine whether the association is gender specific. We genotyped 201 multiplex AS families with nine ANKH intragenetic and two flanking microsatellite markers, and performed family-based association analyses. We showed that ANKH variants located in two different regions of the ANKH gene were associated with AS. Results of haplotype analyses indicated that, after Bonferroni correction, the haplotype combination of rs26307 [C] and rs27356 [C] is significantly associated with AS in men (recessive/dominant model; P = 0.004), and the haplotype combination of rs28006 [C] and rs25957 [C] is significantly associated with AS in women (recessive/dominant model; P = 0.004). A test of interaction identified rs26307 (i.e. the region that was associated in men with AS) as showing a difference in the strength of the association by gender. The region associated with AS in women only showed significance in the test of interaction among the subset of families with affected individuals of both genders. These findings support the concept that ANKH plays a role in genetic susceptibility to AS and reveals a gender–genotype specificity in this interaction.
Introduction
Ankylosing spondylitis (AS) is a disorder that results in chronic joint and entheseal inflammation, and ankylosis of axial and peripheral joints. It affects approximately 0.1–0.8% of Caucasians [1]. The disease usually begins in young adulthood and can be associated with chronic pain and significant disability. AS is strongly associated with HLA-B27 [2], but analyses of recurrence risk among family members [3] suggest that at least three other genetic loci in addition to HLA-B27 are required to confer full susceptibility to AS. However, genome-wide linkage studies have detected very few strongly linked non-major histocompatibility complex (MHC) loci [4-6], implying that non-MHC susceptibility loci have small effects and/or that heterogeneous sets of loci combine with HLA-B27 to confer susceptibility to AS. This complexity highlights the strategic advantage of testing predetermined candidate genes. In addition, although several chromosomal regions showed potential linkage in several genome-wide linkage studies conducted in AS families [5,6], the identities of the predisposing genes in these regions remain largely unknown.
Normal osteogenesis depends critically on maintaining the physiological level of inorganic pyrophosphate (PPi). Abnormal PPi levels can be associated with aberrant bone formation. PPi export from the cell is regulated by the ANK protein [7], and mutant mice (ank/ank), which have a premature stop codon in the 3' end of the ank gene, develop severe ankylosis. As a first step in testing the hypothesis that specific polymorphisms in the ANKH gene might contribute to AS susceptibility, we previously reported the identification of two novel polymorphic sites, one in the 5' noncoding region (ANKH-OR) and the other in the promoter region (ANKH-TR), of ANKH [8]. These two marker alleles are in complete linkage disequilibrium (LD). Our results from a linkage analysis of 124 North American AS families [8] indicated that AS is genetically linked to ANKH, and the locus-specific sibling recurrence risk of ANKH to AS susceptibility (λS) is 1.9 (λS for HLA-B27 is 5.2). Our family-based association analysis on the same families [8] showed that AS is modestly associated with ANKH-OR allele 1 (additive model: P = 0.03). Because of insufficient numbers of informative families, our results did not allow us to distinguish between different modes of inheritance. In addition, our analyses were focused on the 5' end of the gene, using only two markers. For these reasons, we have now carried out fine mapping of the complete ANKH region, including not only the AS families used in the previous study but also an additional 77 multiplex AS families (a total of 201 multiplex AS families).
The prevalence of AS is 2.5 times higher in men than in women [9]. Extensive fusion of the spine is a phenotype of the mouse model ank. There has been a clinical impression that radiographic severity (e.g. the bamboo spine) may be relatively less common in affected women than in men [10-13]. It has also been observed that long-term outcome in AS is worse in men than in women [14,15], but the basis for this difference in severity of clinical expression remains unclear. It is unlikely that the major genetic factors that account for these differences are X-linked because there is no linkage of AS susceptibility with X-chromosome markers [16]. Gender also has a significant impact on heritability in AS. AS has a higher prevalence in the offspring of women than men with AS, and sons of men with AS are 2.5 times more likely than daughters to inherit the disease [17,18]. It remains unclear whether there is gender heterogeneity in non-MHC loci that confer susceptibility to AS. In the present study, we asked whether there is any gender difference in the association of ANKH with AS in multiplex families.
Materials and methods
Ankylosing spondylitis families
The study group comprised 201 Caucasian AS families (a total of 226 nuclear families; Tables 1 and 2). This group was recruited from the Toronto Western Spondylitis Clinic (23 families) and from other sites in the North American Spondylitis Consortium (178 families). All patients met modified New York criteria for the diagnosis of AS [19], which include radiographic evidence of sacroiliitis. Of the affected and unaffected individuals, 60% and 47% were men, respectively. The ages of the individuals ranged from 8 to 75 years. The study was approved by the University Health Network Research Ethics Board and the Committee for the Protection of Human Subjects at the University of Texas Health Science Center-Houston.
Table 1.
Characteristics of 226 nuclear families included in the family-based association studies
| Number of affected siblings | Number of unaffected siblings | Number of unaffected parents | Number of affected parents | Number of nuclear families |
| 2 | 0 | 0 | 0 | 71 |
| 2 | 0 | 2 | 0 | 33 |
| 2 | 0 | 1 | 0 | 14 |
| 2 | 0 | 1 | 1 | 5 |
| 2 | 1 | 0 | 0 | 4 |
| 2 | 2 | 0 | 0 | 3 |
| 2 | 1 | 0 | 1 | 3 |
| 2 | 1 | 2 | 0 | 2 |
| 2 | 2 | 2 | 0 | 1 |
| 3 | 0 | 0 | 0 | 9 |
| 3 | 0 | 2 | 0 | 6 |
| 3 | 0 | 1 | 0 | 4 |
| 3 | 0 | 1 | 1 | 2 |
| 3 | 1 | 0 | 0 | 1 |
| 3 | 1 | 1 | 0 | 1 |
| 4 | 0 | 0 | 0 | 1 |
| 4 | 0 | 2 | 0 | 2 |
| 4 | 0 | 1 | 0 | 1 |
| 4 | 1 | 2 | 0 | 1 |
| 1 | 0 | 2 | 0 | 25 |
| 1 | 0 | 1 | 1 | 8 |
| 1 | 0 | 1 | 0 | 2 |
| 1 | 0 | 0 | 1 | 7 |
| 1 | 1 | 0 | 0 | 3 |
| 1 | 1 | 0 | 1 | 3 |
| 1 | 1 | 2 | 0 | 1 |
| 1 | 1 | 1 | 1 | 1 |
| 1 | 2 | 0 | 0 | 1 |
| 1 | 2 | 0 | 1 | 1 |
| 1 | 2 | 2 | 0 | 1 |
| 1 | 3 | 0 | 0 | 1 |
| 1 | 3 | 2 | 0 | 1 |
| 0 | 2 | 0 | 0 | 1 |
| 0 | 2 | 1 | 1 | 2 |
| 0 | 1 | 0 | 1 | 3 |
Table 2.
Gender information for affected individuals in the 201 ankylosing spondylitis families
| Number of affected men/women in a family | Number of families |
| Families with both affected men and women | 94 |
| 1/1 | 60 |
| 2/1 | 17 |
| 3/1 | 1 |
| 1/2 | 8 |
| 2/2 | 3 |
| 3/2 | 1 |
| 1/3 | 2 |
| 2/3 | 2 |
| Families with only affected men | 74 |
| 1/0 | 5 |
| 2/0 | 54 |
| 3/0 | 12 |
| 4/0 | 2 |
| 5/0 | 1 |
| Families with only affected women | 33 |
| 0/1 | 2 |
| 0/2 | 26 |
| 0/3 | 4 |
| 0/4 | 1 |
Genotyping
DNA from the affected and unaffected family members was prepared from peripheral blood lymphocytes using standard techniques.
Microsatellite markers
Genotyping was performed using three microsatellite markers flanking ANKH on chromosome 5p: D5S1953, D5S1991 and D5S1954. Polymerase chain reaction fragments were run on native polyacrylamide gel, stained with ethidium bromide and visualized using an imager (Bio-Rad, Hercules, CA, USA).
Single nucleotide polymorphisms
Genotyping was performed using seven intronic single nucleotide polymorphisms (SNPs; rs26307 [C/T], rs27356 [C/T], 3088132 [G/C], rs153929 [A/G], rs258215 [A/T], rs28006 [C/T] and rs25957 [C/G]). Optimized allelic discrimination assays for SNPs were purchased from Applied Biosystems (Foster City, CA, USA). The plates were read on an ABI PRISM 7900 sequence detection system (Applied Biosystems).
Statistical analysis
Error checking
To minimize data errors, extensive error checking procedures were used. For microsatellite markers, allele assignment was checked manually for all genotypes by two independent individuals. Size data were converted into discrete allele numbers; samples not following Mendelian patterns of inheritance were identified using Pedmanager (available online at ftp://ftp-genome.wi.mit.edu/distribution/software/pedmanager), and these samples were subjected to repeat genotyping.
Family-based association analyses
The transmission disequilibrium test (TDT) was used to test for transmission of specific alleles from heterozygous parents to affected offspring [20]. We computed the test statistics using the empirical variance option of family-based association testing (FBAT) software, version 1.5.5 (available online at http://www.biostat.harvard.edu/~fbat/default.html) [21]. This option is used when testing for associations in an area of known linkage (the null hypothesis assumes no association but linkage) with multiple affected siblings in a family or when multiple nuclear families in a pedigree are considered. This program uses data from nuclear families, sibships, pedigrees or any combination, and provides unbiased tests with or without founder genotypes. Biallelic tests were performed using additive, dominant/recessive genetic models. Haplotype analyses were carried out using the haplotype-based association testing (HBAT) empirical variance (-e) option in the FBAT program. For Bonferroni correction, because eight tests (four haplotypes and two models) were carried out in the HBAT-e analyses, P < 0.00625 (0.05/8) was considered statistically significant.
For analysis of affected men/women, the FBAT command 'setafftrait' was used. The unaffected siblings and parents from the families were coded as unknown (0) phenotype, the affected men were coded as 2, and the affected women as 1. FBAT-e analyses using the setafftrait 1 0 0 command were used to test specifically for affected men, and analyses using the setafftrait 0 -1 0 command were used to test specifically for affected women. To test for differences between family-based association for affected men and women, the setafftrait 1 -1 0 command was used.
TDT was used to estimate the frequency of transmission to the affected men or women of the haplotypes of interest. Findings in one affected individual, randomly selected from each of the multiplex families, were used in the calculations.
Results
Association between specific ANKH variants and ankylosing spondilitis
The ANKH gene encodes for ANKH transcripts with different lengths at the 3' untranslated region. The longer transcript (3928 bp; AB046801) is derived from 12 exons, whereas the shorter transcript (2426 bp; AK001799, which contains the last 1721 bp of this transcript) is derived from 13 exons. We fine-mapped the ANKH gene using 11 markers (Fig. 1): three microsatellite markers (D5S1954, D5S1991 and D5S1953), one 5' untranslated region variant (ANKH-OR), and seven intronic SNPs (rs25957 and rs28006 in intron 1, rs258215 in intron 2, rs153929 in intron 7, 3088132 and rs27356 in intron 8, and rs26307 in intron 12).
Figure 1.
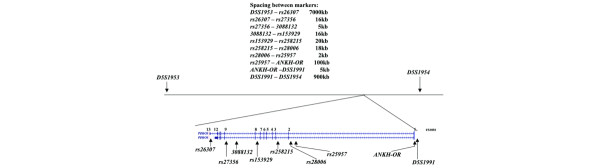
Locations and spacings of genetic markers used for genotyping. D5S1991 and ANKH-OR are located at the 5' flanking region of ANKH. All seven single nucleotide polymorphisms used are located in the introns of ANKH.
As an extension to our previous study [8], we included a total of 201 multiplex AS families in a family-based association analysis (77 additional multiplex AS families were included, in addition to the 124 AS families considered in the first study). All of the families were genotyped with 11 markers in the ANKH region (D5S1953, rs26307, rs27356, 3088132, rs153929, rs258215, rs28006, rs25957, ANKH-OR, D5S1991 and D5S1954). FBAT analyses showed two regions in the ANKH gene where associations between ANKH variants and AS were detected. Using both additive and recessive models, rs27356 [C] was significantly associated with AS (additive model: Z score = 2.54, P = 0.011; recessive model: Z score = 2.32, P = 0.020). However, depending on the model used for the analysis, two different ANKH markers were also associated with AS. Using an additive model, an intron 1 SNP, namely rs25957 [C], was associated with AS (Z score = 2.02, P = 0.043). Using a dominant model, ANKH-OR allele 1 was associated with AS (Z score = 2.20, P = 0.027). The results are summarized in Table 3. However, these markers are located in different haplotype or LD blocks (see below), implying that there is more than one susceptibility locus in the ANKH gene.
Table 3.
FBAT-e analyses conducted in 226 ankylosing spondylitis nuclear families (201 pedigrees, 894 persons)
| Marker | Allele | Allele frequency | Number of informative families | Z score | P |
| Additive model: biallelic test | |||||
| D5S1953 | 2 | 0.45 | 54 | 0.57 | 0.569 |
| rs26307 | C | 0.81 | 35 | 1.73 | 0.084 |
| rs27356 | C | 0.80 | 39 | 2.54 | 0.011* |
| 3088132 | G | 0.79 | 28 | 1.28 | 0.198 |
| rs153929 | A | 0.76 | 48 | 1.61 | 0.107 |
| rs258215 | A | 0.59 | 43 | 1.58 | 0.113 |
| rs28006 | C | 0.74 | 34 | 1.62 | 0.104 |
| rs25957 | C | 0.76 | 36 | 2.02 | 0.043* |
| ANKH-OR | 1 | 0.47 | 59 | 1.62 | 0.105 |
| D5S1991 | 2 | 0.48 | 58 | 1.33 | 0.181 |
| D5S1954 | 1 | 0.64 | 54 | 0.65 | 0.515 |
| Recessive model: biallelic test | |||||
| D5S1953 | 2 | 0.43 | 48 | 0.45 | 0.683 |
| rs26307 | C | 0.80 | 40 | 1.49 | 0.137 |
| rs27356 | C | 0.79 | 44 | 2.32 | 0.020* |
| 3088132 | G | 0.79 | 31 | 1.19 | 0.233 |
| rs153929 | A | 0.76 | 50 | 1.86 | 0.062 |
| rs258215 | A | 0.58 | 32 | 1.55 | 0.120 |
| rs28006 | C | 0.74 | 37 | 1.46 | 0.142 |
| rs25957 | C | 0.76 | 38 | 1.65 | 0.098 |
| ANKH-OR | 2 | 0.52 | 37 | -2.20 | 0.027* |
| D5S1991 | 1 | 0.52 | 45 | -1.96 | 0.050* |
| D5S1954 | 1 | 0.64 | 54 | 0.65 | 0.517 |
*Statistically significant findings. FBAT, family-based association testing.
Thus, our analyses of 201 multiplex AS families showed that ANKH variants found in two different regions of the ANKH gene are modestly associated with AS. Our working hypothesis was that there are two subsets of AS patients, each with a different predisposing polymorphism in the ANKH locus. Because ANKH has been shown to be an androgen responsive gene [22-24], we considered whether there are gender differences between family-based associations of ANKH variants to AS.
Men with ankylosing spondilitis differ from affected women for association with different ANKH variants
Radiographic features of AS vary between men and women, with extensive spinal ankylosis being relatively infrequent in women with AS [10]. Table 2 summarizes gender information for the affected individuals in the 201 North American multiplex AS families. There were 94 families with both affected men and women in each family, 74 families with affected men only, and 33 families with affected women only. In this cohort of North American multiplex AS families, men have a significantly earlier age at diagnosis than that for women (mean [± standard deviation] age of diagnosis for affected men = 28 ± 11 years [n = 213]; mean age of diagnosis for affected women = 30 ± 11 years [n = 149]; including family as an independent variable [using SAS PROC GLM; SAS Institute Inc., Cary, NC, USA]: F = 5.10, P = 0.025; Fig. 2). In addition, analysis of age at AS diagnosis in affected men did not reveal a normal distribution; rather the distribution was skewed toward an earlier onset.
Figure 2.
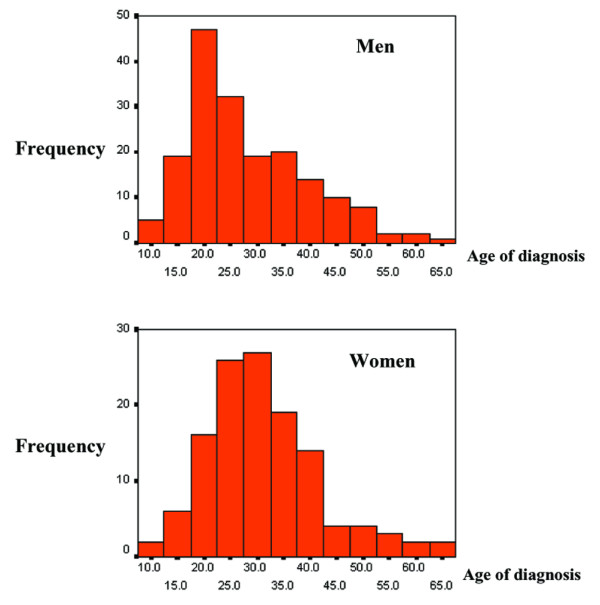
Age of diagnosis for (a) men and (b) women in the North American multiplex ankylosing spondilitis families.
In view of these gender differences, we re-analyzed our genotyping results along gender lines in two separate FBAT analyses using the setafftrait command. FBAT analysis of transmission of alleles to affected women showed that both rs25957 [G] and rs28006 [T] were associated with AS (additive model and biallelic test: rs25957 [G], Z score = 2.82, P = 0.004; rs28006 [T], Z score = 2.82, P = 0.004; Table 4). These results indicate that only ANKH variants at the 5' end, and not those at the 3' end, of ANKH are associated with AS in affected women. This also suggested that ANKH variants at the 3' end of the gene might be associated with AS only in affected men.
Table 4.
FBAT-e analyses using setafftrait 0 -1 0, testing specifically for affected women
| Marker | Allele | Allele frequency | Number of informative families | Z score | P |
| Additive model: biallelic test | |||||
| D5S1953 | 1 | 0.56 | 47 | 0.47 | 0.635 |
| rs26307 | T | 0.19 | 22 | 0.32 | 0.751 |
| rs27356 | T | 0.20 | 24 | 1.03 | 0.302 |
| 3088132 | C | 0.21 | 20 | 0.37 | 0.712 |
| rs153929 | G | 0.24 | 46 | 1.31 | 0.191 |
| rs258215 | T | 0.41 | 30 | 1.54 | 0.123 |
| rs28006 | T | 0.26 | 31 | 2.82 | 0.004* |
| rs25957 | G | 0.23 | 23 | 2.82 | 0.004* |
| ANKH-OR | 2 | 0.52 | 59 | 0.94 | 0.347 |
| D5S1991 | 1 | 0.52 | 53 | 1.10 | 0.270 |
| D5S1954 | 2 | 0.36 | 45 | 1.38 | 0.168 |
| Recessive model: biallelic test | |||||
| D5S1953 | 2 | 0.44 | 39 | -1.21 | 0.227 |
| rs26307 | C | 0.81 | 26 | 0.52 | 0.606 |
| rs27356 | T | 0.20 | 10 | 1.81 | 0.069 |
| 3088132 | G | 0.79 | 22 | 0.08 | 0.930 |
| rs153929 | A | 0.76 | 44 | -1.39 | 0.162 |
| rs258215 | A | 0.58 | 21 | -1.76 | 0.077 |
| rs28006 | C | 0.74 | 28 | -2.49 | 0.012* |
| rs25957 | C | 0.76 | 23 | -2.25 | 0.024* |
| ANKH-OR | 2 | 0.52 | 34 | 1.14 | 0.254 |
| D5S1991 | 1 | 0.51 | 33 | 1.52 | 0.127 |
| D5S1954 | 1 | 0.64 | 45 | -1.54 | 0.122 |
*Statistically significant findings. FBAT, family-based association testing.
To test this hypothesis, we analyzed transmission of alleles to affected men. FBAT analysis of transmission of alleles to affected men using the setafftrait command showed that two neighbouring ANKH variants at the 3' end of the gene, namely rs26307 [C] and rs27356 [C] (16 kb apart), were associated with AS in affected men as was predicted (additive model: rs26307 [C], Z score = 2.06, P = 0.039; rs27356 [C], Z score = 2.63, P = 0.008; recessive model: rs26307 [C], Z score = 2.51, P = 0.012; rs27356 [C], Z score = 2.99, P = 0.002; Table 5).
Table 5.
FBAT-e analyses using setafftrait 1 0 0, testing specifically for affected men
| Marker | Allele | Allele frequency | Number of informative families | Z score | P |
| Additive model: biallelic test | |||||
| D5S1953 | 2 | 0.44 | 47 | 0.25 | 0.805 |
| rs26307 | C | 0.80 | 22 | 2.06 | 0.039* |
| rs27356 | C | 0.79 | 25 | 2.63 | 0.008* |
| 3088132 | G | 0.79 | 18 | 1.23 | 0.216 |
| rs153929 | A | 0.76 | 36 | 0.49 | 0.619 |
| rs258215 | A | 0.58 | 32 | 0.81 | 0.419 |
| rs28006 | T | 0.26 | 37 | 0.15 | 0.877 |
| rs25957 | C | 0.77 | 33 | 0.76 | 0.446 |
| ANKH-OR | 1 | 0.48 | 64 | 1.04 | 0.297 |
| D5S1991 | 2 | 0.49 | 64 | 0.39 | 0.696 |
| D5S1954 | 2 | 0.36 | 49 | 0.33 | 0.736 |
| Recessive model: biallelic test | |||||
| D5S1953 | 1 | 0.56 | 40 | -0.83 | 0.406 |
| rs26307 | C | 0.81 | 25 | 2.51 | 0.012* |
| rs27356 | C | 0.80 | 28 | 2.99 | 0.002* |
| 3088132 | G | 0.79 | 22 | 1.44 | 0.149 |
| rs153929 | A | 0.76 | 39 | 0.71 | 0.477 |
| rs258215 | A | 0.59 | 23 | 0.60 | 0.543 |
| rs28006 | T | 0.26 | 16 | 0.33 | 0.739 |
| rs25957 | C | 0.76 | 29 | 0.64 | 0.518 |
| ANKH-OR | 2 | 0.52 | 44 | -1.26 | 0.205 |
| D5S1991 | 1 | 0.51 | 46 | -0.71 | 0.472 |
| D5S1954 | 1 | 0.64 | 43 | -0.65 | 0.510 |
*Statistically significant findings. FBAT, family-based association testing.
Identification of ANKH haplotypes that are associated with ankylosinig spondylitis
Where the aetiological variant is not typed, haplotype-based analysis is more powerful for association studies in which there is significant LD in the region of interest. We took advantage of the data from the HapMap project (12 October 2004 release 12; http://www.hapmap.org[25]). The markers we used for genotyping are located in four different haplotype blocks (block 1: rs26307, rs27356; block 2: 3088132 and rs153929; block 3: rs28006 and rs25957; block 4: ANKH-OR and D5S1991).
We carried out haplotype analyses based on this information, using the HBAT empirical variance option in the FBAT program, and the results are summarized in Table 6. For HBAT analyses considering all 226 AS nuclear families, in each of three different haplotype blocks (blocks 1, 2 and 4) there was one haplotype with a significant P value, suggesting that there is heterogeneity in this locus. When HBAT analyses were carried out specifically for affected women, a haplotype with a significant P value was found in haplotype block 3 located at the 5' end of the gene. When HBAT analyses were conducted specifically for affected men, one haplotype with a significant P value was present in block 1, which is located at the 3' end of the gene. These results are consistent with those from single-marker tests in the FBAT analyses. Furthermore, after Bonferroni correction for the number of haplotypes and models (n = 8), the haplotype combination of rs26307 [C] and rs27356 [C] remained significantly associated with AS in men (recessive/dominant model: P = 0.004), and the haplotype combination of rs28006 [C] and rs25957 [C] was significantly associated with AS in women (recessive/dominant model: P = 0.004).
Table 6.
HBAT-e analyses using ANKH markers in four haplotype blocks defined in HapMap
| Markers in the haplotype block | AS nuclear families (n = 226) | Testing specifically for affected women | Testing specifically for affected men |
| Additive model | |||
| rs26307, rs27356 | [C,C]; 0.78; 39; 0.04 | NS | [C,C]; 0.79; 24; 0.014 |
| 3088132, rs153929 | NS | NS | NS |
| rs28006, rs25957 | NS | [T,G]; 0.25; 19; 0.007 | NS |
| ANKH-OR, D5S1991 | [1,2]; 0.43; 57; 0.013 | NS | NS |
| Recessive/dominant model | |||
| rs26307, rs27356 | [C,C]; 0.78; 40; 0.02 | NS | [C,C]; 0.79; 25; 0.004* |
| 3088132, rs153929 | [G,A]; 0.70; 32; 0.02 | NS | NS |
| rs28006, rs25957 | NS | [C,C]; 0.71; 18; 0.004* | NS |
| ANKH-OR, D5S1991 | NS | NS | NS |
Data are expressed as [allele]; allele frequency; number of informative families; P value.
*Significant P value after Bonferroni correction. (Because eight tests [four haplotypes and two models] were carried out in the haplotype-based association testing [HBAT]-e analyses, P < 0.00625 [0.05/8] is considered statistically significant.) AS, ankylosing spondylitis; NS, not significant.
A direct test for differences between family-based association with affected men and women
In order to conclude that there are gender differences in ANKH variants associated with AS, one must show significant heterogeneity between affected men and women. For this purpose, we used the setafftrait 1 -1 0 command to conduct the FBAT-e analyses. We coded unaffected siblings and parents from the families as unknown phenotype (0), affected men as phenotype 2, and affected women as phenotype 1. The setafftrait 1 -1 0 command converted affect status to trait 1 (affected men), -1 (affected women) and 0 (unaffected siblings and parents), and the results are summarized in Table 7. The only marker with a significant P value was rs26307 [C] (dominant/recessive model: P = 0.03), suggesting that this marker was significantly associated with AS only in affected men.
Table 7.
FBAT-e analyses considering 226 ankylosing spondylitis nuclear families (201 pedigrees, 894 persons): summary of results using setafftrait 1 -1 0
| Marker | Allele | Allele frequency | Number of informative families | Z score | P |
| Additive model: biallelic test | |||||
| D5S1953 | 1 | 0.56 | 63 | 0.15 | 0.879 |
| rs26307 | C | 0.81 | 35 | 1.29 | 0.195 |
| rs27356 | C | 0.80 | 39 | 1.12 | 0.261 |
| 3088132 | G | 0.79 | 29 | 0.69 | 0.489 |
| rs153929 | G | 0.24 | 52 | 0.61 | 0.541 |
| rs258215 | T | 0.41 | 41 | 0.46 | 0.638 |
| rs28006 | T | 0.26 | 44 | 1.69 | 0.090 |
| rs25957 | G | 0.23 | 39 | 0.90 | 0.366 |
| ANKH-OR | 1 | 0.48 | 80 | 0.20 | 0.841 |
| D5S1991 | 1 | 0.52 | 80 | 0.29 | 0.769 |
| D5S1954 | 2 | 0.36 | 63 | 1.21 | 0.227 |
| Recessive model: biallelic test | |||||
| D5S1953 | 1 | 0.56 | 46 | -0.89 | 0.370 |
| rs26307 | C | 0.81 | 37 | 2.17 | 0.030* |
| rs27356 | C | 0.80 | 41 | 1.91 | 0.055 |
| 3088132 | G | 0.79 | 29 | 1.17 | 0.240 |
| rs153929 | G | 0.24 | 18 | 0.55 | 0.582 |
| rs258215 | A | 0.59 | 29 | -0.75 | 0.453 |
| rs28006 | C | 0.74 | 40 | -1.13 | 0.186 |
| rs25957 | C | 0.77 | 36 | -0.66 | 0.507 |
| ANKH-OR | 2 | 0.52 | 50 | -0.34 | 0.733 |
| D5S1991 | 2 | 0.49 | 52 | -0.28 | 0.779 |
| D5S1954 | 1 | 0.64 | 61 | -1.53 | 0.126 |
*Statistically significant findings. FBAT, family-based association testing.
In view of this finding, we considered whether there is a subset of AS multiplex families in which ANKH variants were significantly associated with AS only in affected women. As summarized in Table 2, there were two types of families in our cohort of multiplex AS families: families with affected individuals of both genders; and families with only one gender of affected individuals (either affected men or affected women).
To assess whether there was significant heterogeneity between affected men and women in the families of the first family type (with affected men and women in each family), we used the setafftrait 1 -1 0 command to conduct the FBAT-e analyses. The results are summarized in Table 8. Two markers (rs28006 [T] and rs25957 [G]) exhibited significant P values (additive model: P = 0.004 for rs28006 and P = 0.017 for rs25957), suggesting that these two markers were associated with AS only in affected women in the subset of AS families with affected individuals of both genders.
Table 8.
FBAT-e analyses considering 108 ankylosing spondylitis nuclear families (94 pedigrees, 425 persons) in which both affected men and women are present in each family: summary of the results using setafftrait 1 -1 0
| Marker | Allele | Allele frequency | Number of informative families | Z score | P |
| Additive model: biallelic test | |||||
| D5S1953 | 1 | 0.56 | 39 | 0.77 | 0.437 |
| rs26307 | C | 0.90 | 10 | 0.50 | 0.617 |
| rs27356 | C | 0.77 | 17 | 0.15 | 0.875 |
| 3088132 | C | 0.21 | 13 | 0.63 | 0.527 |
| rs153929 | G | 0.23 | 32 | 0.81 | 0.418 |
| rs258215 | T | 0.49 | 21 | 1.62 | 0.104 |
| rs28006 | T | 0.29 | 27 | 2.81 | 0.004* |
| rs25957 | G | 0.32 | 22 | 2.37 | 0.017* |
| ANKH-OR | 1 | 0.48 | 51 | 0.25 | 0.801 |
| D5S1991 | 2 | 0.50 | 48 | 0.13 | 0.891 |
| D5S1954 | 2 | 0.33 | 31 | 1.13 | 0.254 |
| Recessive model: biallelic test | |||||
| D5S1953 | 2 | 0.44 | 31 | -1.34 | 0.177 |
| rs26307 | C | 0.79 | 19 | 1.00 | 0.314 |
| rs27356 | C | 0.80 | 20 | 1.17 | 0.239 |
| 3088132 | G | 0.79 | 14 | -0.26 | 0.788 |
| rs153929 | G | 0.23 | 10 | 1.06 | 0.288 |
| rs258215 | A | 0.51 | 12 | -2.49 | 0.012* |
| rs28006 | C | 0.71 | 20 | -2.344 | 0.019* |
| rs25957 | C | 0.67 | 15 | -1.97 | 0.048* |
| ANKH-OR | 1 | 0.48 | 27 | 0.83 | 0.406 |
| D5S1991 | 2 | 0.49 | 29 | 0.98 | 0.322 |
| D5S1954 | 1 | 0.66 | 31 | -1.67 | 0.093 |
*Statistically significant findings. FBAT, family-based association testing.
We also conducted FBAT-e analysis using setafftrait command 1 -1 0 in families with only one gender of affected individuals (data not shown). However, there were few informative families (<20 families from which we could track the transmission of alleles), and so the results might not be reliable.
Selective transmission of haplotypes of interest to the affected men/women
In order to estimate the magnitude of the effect, we calculated the frequency at which the haplotypes of interest were transmitted to the affected men or women using TDT. For the haplotype rs28006 [C] rs25957 [C], the frequency of transmission was 74% (17/23) to affected women and 40% (12/30) to affected men. Thus, the 'odds ratio' for increased risk is 1.85 (0.74/0.4). More dramatic proportions were seen in the subset of families with affected individuals of both genders. In these families, this haplotype was transmitted to affected women 79% of the time (15/19) but to affected men only 27% of the time (3/11). In this case, the 'odds ratio' for increased risk approaches 3.0 (0.79/0.27 = 2.92).
For the haplotype rs26307 [C] rs27356 [C], the frequency of transmission was 70% (21/30) to affected men and 43% (13/30) to affected women (an 'odds ratio' for increased risk of 1.75). In the subset of families with only affected men, 94% (16/17) of the time this haplotype was transmitted to affected men. There were too few informative families with only affected women with this variant (n = 6), and so we do not have a reliable assessment of the frequency at which this haplotype was transmitted to affected women in this subset for comparison.
Discussion
In this study of the association of ANKH genetic markers with AS, including 201 AS multiplex families, we found that ANKH variants located in two different regions of the ANKH gene were associated with AS. A more striking finding was that the genetic association for men and women with AS differed. In men, AS was associated with genetic markers at the 3' end of the ANKH gene, whereas in women AS appeared to be associated with genetic markers at the 5' end of the ANKH gene. As expected, when the genders of AS patients were analyzed separately, we observed more than one SNP in each region (within the same haplotype block) showing significant association with AS. Haplotype analyses appeared to confirm the results of the single-marker tests (FBAT analyses), indicating that the predisposing polymorphism(s) for men with AS probably lies at the 3' end of the ANKH gene, whereas those for affected women are probably at the 5' end of the gene. After Bonferroni correction for the number of haplotypes and models, the haplotype combination of rs26307 [C] and rs27356 [C] was significantly associated with men with AS; and the haplotype combination of rs28006 [C] and rs25957 [C] was significantly associated with women with AS. However, in both cases, the significance level was modest. We attribute this to the fact that we have not identified the aetiological variants in the men/women with AS. Despite the modest P values (which are a function of sample size), the calculated 'odds ratios' for increased risk (which provide estimates of the magnitude of the effect) were close to 2 for the transmission of rs26307 [C] rs27356 [C] to affected men, and close to 3 for the transmission of rs28006 [C] rs25957 [C] to affected women in the subset of families with affected individuals of both genders.
A test of interaction identified the region that was associated in men with AS (rs26307) as showing a difference in the strength of the association by gender. The region associated with AS in women only showed significance of the test of interaction among the subset of families with affected individuals of both genders. Our current efforts are to identify and analyze more common SNPs in these two regions, ultimately finding the predisposing polymorphisms in men and women.
The rationale for studying multiplex AS families is to enhance the chances of identifying the genes involved. There are very few studies that directly compare familial versus sporadic AS. In one study [26], familial versus sporadic Dutch AS patients exhibited no difference in age at disease onset, age at diagnosis, or prevalence of peripheral arthritis and acute anterior uveitis. In another study, familial AS disease was significantly milder than sporadic disease, as assessed by spinal mobility score, Arthritis Impact Measurement Scales (AIMS) overall impact score, AIMS physical activity score, AIMS social function score and AIMS pain score [27]. Thus, findings from multiplex families might not be directly applicable to individuals affected with sporadic AS. Most studies assessing the impact gender has on age at AS onset or diagnosis have been conducted without addressing whether the individuals had familial or sporadic disease [28-30]; these studies showed that the age at disease onset is similar between genders. However, in our cohort of AS multiplex families, men had a significantly earlier age at diagnosis compared with that for women (for men 28 ± 11 years [n = 213]; and for women 30 ± 11 years [n = 149]). Because these are AS multiplex families, it is unlikely that there is a bias leading physicians to delay diagnosis in affected women. The misconception that AS is exclusively a male disease may yet be a confounding factor. In the subset of families with affected individuals of both genders, men have an even earlier age at diagnosis (27.8 ± 11 years [n = 94]) compared with that for women (32.6 ± 11 years [n = 101]), whereas both men and women have similar ages of diagnosis in the subsets of families with affected individuals of only one gender (for men 28.6 ± 12 years [n = 130]; for women 29.9 ± 12 years [n = 53]). This finding suggests that there is heterogeneity even in multiplex AS families.
The ANKH variants that were significantly associated with AS are located in introns 1, 8 and 12. It is likely that the predisposing polymorphisms affect gender-specific regulation of ANKH expression. Very little is known regarding the molecular mechanisms that underlie the regulation of ANKH expression. One study [31] reported that ANKH is a growth factor responsive gene. Three recent reports [22-24] showed that ANKH is an androgen responsive gene. In androgen-treated prostate cancer cell lines, the abundance of ANKH transcripts was sixfold higher than in the untreated cells. In the ANKH promoter, there is a sequence at position -1015 (AGAACAcacTtTcCT) with 83% match to an androgen response element (ARE) consensus sequence [22]. It remains unclear whether this ARE-like motif is functional. In view of the locations of the ANKH variants associated with AS, it remains unclear whether this ARE-like motif at the promoter region can directly contribute to the regulation of ANKH expression by the predisposing polymorphisms. It is also unknown whether there is a different mode of ANKH regulation in women.
A report recently concluded that ANKH did not significantly contribute to susceptibility or specific disease expression in AS patients from the UK [32]. In that report, a case–control study was conducted using five ANKH SNPs within the coding region and flanking splice sites and three known promoter variants. There was no association between these polymorphisms and AS or the clinical pattern of the disease. In addition, using 185 affected sib-pair AS families, no linkage between ANKH and AS was observed. However, the exact linkage results were not shown. Using multipoint exclusion mapping of the ANKH region, the presence of a gene contributing more than 10% of the recurrence risk to AS (λS = 1.4) was excluded. Using λS of 1.4 as the cutoff may exclude genes with modest effects. In that report, the LD between markers was not shown. In situations where the aetiological variant is not typed, haplotype-based analysis may be a more powerful analytical method when there is significant LD.
The basis for the discrepancy between the UK results [32] and ours is not entirely clear, but there are several possible explanations. First, the UK group focused on analyzing exonic variants, variants near splice junctions and in the promoter region. Their analysis did not include any ANKH variants in the 3' region, where we detected association with AS in men. Second, although the UK group included a gender breakdown of their patients (63.5% men and 36.5% women), the analysis did not include a breakdown of AS patients by gender, and variants with modest gender-specific effects might have been missed. Third, it is possible that there are some intrinsic differences between the two populations (UK versus North American). Genome-wide linkage scans performed in the two groups revealed some similar susceptibility regions, such as on chromosomes 6p (the MHC), 5q and 10q [5,6]. However, the linkage identified on chromosome 11q23 in the North American Spondylitis Consortium families was not seen in the UK study. In addition, the linkage identified on chromosome 2q in the UK study was not seen in the North American Spondylitis Consortium study. The intrinsic differences could reflect clinical differences in the patient population recruited, or they could be due to population-specific mechanisms of genetic susceptibility. Finally, because both groups analyzed about 200 AS families, there might not be sufficient power to detect genes with 'small effects' consistently, leading to discrepancies between results.
In our cohort of North American multiplex AS families, the age at diagnosis was significantly younger in men than in women. However, FBAT analyses using the offset option (-o; an option which works for both quantitative and qualitative traits) did not show any significant association of age at diagnosis in the men or women with AS, even in subsets of families, using the ANKH markers (data not shown), suggesting that ANKH variants are responsible for disease susceptibility. Our finding of gender-specific polymorphisms in the ANKH gene conferring differential susceptibility to AS might shed light on the biological basis of these clinical observations.
In view of the difficulty in locating susceptibility loci with modest effects in recent genome-wide linkage studies conducted in AS families, it will be of interest to assess whether gender subsetting in the analyses of genome-wide linkage studies might yield further insights into the genetic basis of rheumatic diseases, many of which have a strong gender predilection.
Conclusion
Taken together, our findings showed that, after Bonferroni correction, two intronic markers at the 3' end of the ANKH gene were significantly associated with AS only in affected men, and two intronic markers at the 5' end of the ANKH gene were significantly associated with AS only in affected women. This may partly account for the gender difference in the prevalence of AS.
Abbreviations
AIMS = Arthritis Impact Measurement Scales; ARE = androgen response element; AS = ankylosing spondylitis; bp = base pairs; FBAT = family-based association testing; HBAT = haplotype-based association testing; LD = linkage disequilibrium; MHC = major histocompatibility complex; PPi = inorganic pyrophosphate; SNP = single nucleotide polymorphism; TDT = transmission disequilibrium test.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
HWT conducted all of the genotyping and analyzed the data. RDI conceived the study, provided some of the patients' blood/cells for extracting DNA and reviewed the manuscript. ADP designed the study, supervised the statistical analyses and revised the manuscript. JDR coordinated the recruitment of individuals from AS families, provided most of the DNA samples and reviewed the manuscript. FWLT conceived, designed and coordinated the study, analyzed and interpreted the data, performed statistical analyses, and drafted and revised the manuscript. All authors read and approved the final manuscript.
Figure 3.
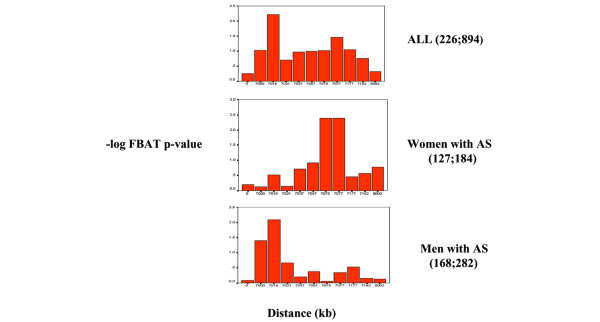
A summary of family-based association analyses (FBAT; additive model and biallelic tests). Each column in the charts represents the -log FBAT P value for each marker located at a distance relative to one another. D5S1953 is positioned at 0 distance. Chart ALL shows analysis of 226 ankylosing spondylitis (AS) nuclear families with 894 persons. Chart Women shows analysis of 127 AS families with 184 affected women. Chart Men shows analysis of 168 AS families with 282 affected men.
Acknowledgments
Acknowledgements
We thank Dr Cathy Barr for making the ABI sequence detection system available, Karen Wigg for reading the plates, and Dr Celia Greenwood for review of the manuscript and helpful suggestions on the statistical analyses. We also thank the Ontario Spondylitis Association and the Spondylitis Association of America for their assistance in recruiting the families included in this study.
This work was supported by grants from the Canadian Institutes of Health Research, the Arthritis Center of Excellence, Genome Canada, and by the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01-AR-46208 to Dr Reveille). Dr Paterson is a Canada Research Chair.
Contributor Information
Hing Wo Tsui, Email: htsui@uhnres.utoronto.ca.
Robert D Inman, Email: Robert.Inman@uhn.on.ca.
Andrew D Paterson, Email: andrew.paterson@utoronto.ca.
John D Reveille, Email: john.d.reveille@uth.tmc.edu.
Florence WL Tsui, Email: ftsui@uhnres.utoronto.ca.
References
- Calin A. Ankylosing spondylitis. In: Maddison PJ, Isenberg DA, Woo P, Glass DN, editor. Oxford Textbook of Rheumatology. Vol. 2. Oxford: Oxford University Press; 1998. pp. 1058–1070. [Google Scholar]
- Braun J, Bollow M, Remlinger G, Eggens U, Rudwaleit M, Distler A, Sieper J. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum. 1998;41:58–67. doi: 10.1002/1529-0131(199801)41:1<58::AID-ART8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Brown MA, Laval SH, Brophy S, Calin A. Recurrence risk modeling of the genetic susceptibility to ankylosing spondylitis. Ann Rheum Dis. 2000;59:883–886. doi: 10.1136/ard.59.11.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MA, Pile KD, Kennedy LG, Campbell D, Andrew L, March R, Shatford JL, Weeks DE, Calin A, Wordsworth BP, et al. A genome-wide screen for susceptibility loci in ankylosing spondylitis. Arthritis Rheum. 1998;41:588–595. doi: 10.1002/1529-0131(199804)41:4<588::AID-ART5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Laval SH, Timms A, Edwards L, Bradbury L, Brophy S, Milicic A, Rubin L, Siminovitch KA, Weeks DE, Calin A, et al. Whole-genome screening in ankylosing spondylitis: evidence of non-MHC genetic-susceptibility loci. Am J Hum Genet. 2001;68:918–926. doi: 10.1086/319509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Luo J, Bruckel J, Weisman MA, Schumacher HR, Kahn MA, Inman RD, Mahowald M, Maksymowych WP, Martin TM, et al. Genetic studies in familial ankylosing spondylitis susceptibility. Arthritis Rheum. 2004;50:2246–2254. doi: 10.1002/art.20308. [DOI] [PubMed] [Google Scholar]
- Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- Tsui FWL, Tsui HW, Cheng EY, Stone M, Payne U, Reveille JD, Paterson AD, Inman RD. Novel genetic markers in the 5'-flanking region of ANKH are associated with ankylosing spondylitis. Arthritis Rheum. 2003;48:791–797. doi: 10.1002/art.10844. [DOI] [PubMed] [Google Scholar]
- Calin A. Ankylosing spondylitis. Clin Rheum Dis. 1985;11:41–60. [PubMed] [Google Scholar]
- Eustace S, Coughlan RJ, McCarthy C. Ankylosing spondylitis. A comparison of clinical and radiographic features in men and women. Ir Med J. 1993;86:120–122. [PubMed] [Google Scholar]
- Gran JT, Husby G, Hordvik M, Stormer J, Romberg-Andersen O. Radiological changes in men and women with ankylosing spondylitis. Ann Rheum Dis. 1984;43:570–575. doi: 10.1136/ard.43.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will R, Edmunds L, Elswood J, Calin A. Is there sexual inequality in ankylosing spondylitis? A study of 498 women and 1202 men. J Rheumatol. 1990;17:1649–1652. [PubMed] [Google Scholar]
- Kidd B, Mullee M, Frank A, Cawley M. Disease expression of ankylosing spondylitis in males and females. J Rheumatol. 1988;15:1407–1409. [PubMed] [Google Scholar]
- Guillemin F, Briancon S, Pourel J, Gaucher A. Longterm disability and prolonged sick leave as outcome measures in ankylosing spondylitis. Arthritis Rheum. 1990;33:1001–1006. doi: 10.1002/art.1780330712. [DOI] [PubMed] [Google Scholar]
- Doran MF, Brophy S, MacKay K, Taylor G, Calin A. Predictors of longterm outcome in ankylosing spondylitis. J Rheum. 2003;30:316–320. [PubMed] [Google Scholar]
- Hoyle E, Laval SH, Calin A, Wordsworth BP, Brown MA. The X-chromosome and susceptibility to ankylosing spondylitis. Arthritis Rheum. 2000;43:1353–1355. doi: 10.1002/1529-0131(200006)43:6<1353::AID-ANR19>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Calin A, Brophy S, Blake D. Impact of sex on inheritance of ankylosing spondylitis: a cohort study. Lancet. 1999;354:1687–1690. doi: 10.1016/S0140-6736(99)03219-5. [DOI] [PubMed] [Google Scholar]
- Brophy S, Taylor G, Blake D, Calin A. The interrelationship between sex, susceptibility factors, and outcome in ankylosing spondylitis and its associated disorders including inflammatory bowel disease, psoriasis and iritis. J Rheumatol. 2003;30:2054–2058. [PubMed] [Google Scholar]
- Van der Linden S, Valkenburg H, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- Spielman RS, McGinnis RE, Ewens WJ. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM) Am J Hum Genet. 1993;52:506–516. [PMC free article] [PubMed] [Google Scholar]
- Lake SL, Blacker D, Laird NM. Family-based tests of association in the presence of linkage. Am J Hum Genet. 1993;52:506–516. doi: 10.1086/316895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PS, Clegg N, Arnold H, Ferguson C, Bonham M, White J, Hood L, Lin B. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci USA. 2002;99:11890–11895. doi: 10.1073/pnas.182376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePrimo SE, Diehn M, Belson JB, Reiter RE, Matese J, Fero M, Tibshirani R, Brown PO, Brooks JD. Transcriptional programs activated by exposure of human prostate cancer cells to androgen. Genome Biol. 2002;3:32.1–32.12. doi: 10.1186/gb-2002-3-7-research0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa T, Nau ME, Xu LL, Chilukuri RN, Makaren M, Zhang W, Petrovics G, Sesterhenn IA, McLeod DG, Moul JW, et al. Androgen-induced expression of endoplasmic reticulum (ER) stress response genes in prostate cancer cells. Oncogene. 2002;21:8749–8758. doi: 10.1038/sj.onc.1205992. [DOI] [PubMed] [Google Scholar]
- The international HapMap Consortium The international HapMap project. Nature. 2003;426:789–795. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- Paardt M, Dijkmans B, Giltay E, vander Horst-Bruinsma I. Dutch patients with familial and sporadic ankylosing spondylitis do not differ in disease phenotype. J Rheumatol. 2002;29:2583–2584. [PubMed] [Google Scholar]
- Calin A, Kennedy LG, Edmunds L, Will R. Familial versus sporadic ankylosing spondylitis. Two different diseases? Arthritis Rheum. 1993;36:676–681. doi: 10.1002/art.1780360515. [DOI] [PubMed] [Google Scholar]
- Van der Linden SM, Valkenburg HA, de Jongh BM, Cats A. The risk of developing ankylosing spondylitis in HLA-B27 positive individuals. Arthritis Rheum. 1984;27:241–249. doi: 10.1002/art.1780270301. [DOI] [PubMed] [Google Scholar]
- Gran JT, Ostensen M, Husby G. A clinical comparison between males and females with ankylosing spondylitis. J Rheumatol. 1985;12:126–129. [PubMed] [Google Scholar]
- Feldtkeller E, Khan MA, van der Heijde Désirée, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23:61–66. doi: 10.1007/s00296-002-0237-4. [DOI] [PubMed] [Google Scholar]
- Guo Y, Hsu DKW, Feng SY, Richards CM, Winkles JA. Polypeptide growth factors and phorbol ester induce progressive ankylosis (ank) gene expression in murine and human fibroblasts. J Cell Biochem. 2001;84:27–38. doi: 10.1002/jcb.1263. [DOI] [PubMed] [Google Scholar]
- Timms AE, Zhang Y, Bradbury L, Wordsworth BP, Brown MA. Investigation of the role of ANKH in ankylosing spondylitis. Arthritis Rheum. 2003;48:2898–2902. doi: 10.1002/art.11258. [DOI] [PubMed] [Google Scholar]


