Abstract
Introduction
Pelvic fractures often result in life-threatening bleeding and hemodynamic instability. Resuscitative endovascular balloon occlusion of the aorta (REBOA) has emerged as a promising strategy for patients with severe pelvic fractures, facilitating subsequent hemostatic interventions. Transcatheter arterial embolization (TAE) is a well-established procedure for managing pelvic fractures accompanied by hemorrhage.
Ideally, an angiographic access point distinct from the initial REBOA placement is sought to maintain REBOA deflation without complete removal, thereby preventing hemodynamic instability during the procedure. However, in cases of extreme and severe pelvic trauma, gaining access for REBOA is already challenging, not to mention the additional difficulty posed by subsequent angiographic access.
This study aims to assess the challenges associated with gaining access in cases where successful TAE was ultimately performed, particularly in the context of severe pelvic trauma. We investigate the complexities surrounding access management and its implications for patient outcomes.
Methods
We conducted a retrospective analysis of patients who presented with pelvic fractures and underwent sequential REBOA and TAE procedures at our institution between 2017 and 2023. We excluded patients with Abbreviated Injury Scores (AIS) ≥3 in systems other than the pelvis, those who underwent TAE prior to REBOA, and cases of suboptimal REBOA insertion.
We collected demographic data, injury characteristics, details of the REBOA and TAE procedures, information on complications, and data on patient survival. The primary endpoints of our analysis included overall survival and the success of TAE (defined as post TAE mean arterial pressure (MAP) ≥65 mm Hg). Secondary endpoints encompassed the duration details of two interventions.
Results
Between 2017 and 2023, a total of 17 patients were included in this study. Among this cohort, 12 (70.6%) were male, with a median age of 51 years. Overall survival was 23.5%. Patients were grouped into angiography after REBOA deflation (AAD) or angiography after REBOA removal (AAR). AAR group was younger (39.0 vs 63.0, p=0.030) and had higher Shock Index at triage (2.30 vs 1.10, p=0.015). More patient whose post TAE MAP >=65 mm Hg was found in the AAR group, although no significant difference on overall survival (25.0% vs 22.2%, p=1.000). Angiographic cannulation times, pre-angiographic MAP, and amount of pre-angiographic transfusion of packed red blood cell were similar across groups.
Conclusion
Our findings provide empirical insights into vascular access selection and suggest that AAR in the management of severe pelvic fractures can be beneficial, particularly when pre-angiographic resuscitation is sufficient. Larger studies are required to validate these observations and assess long-term outcomes.
Level of evidence
III.
Keywords: Pelvic Floor, embolism, angiography, resuscitation
WHAT IS ALREADY KNOWN ON THIS TOPIC
Resuscitative endovascular balloon occlusion of the aorta (REBOA) is used to control hemorrhage in severe cases, and transcatheter arterial embolization (TAE) is an established procedure for managing hemorrhagic pelvic fractures.
However, securing vascular access for TAE after REBOA remains challenging.
WHAT THIS STUDY ADDS
This study highlights that angiography after REBOA removal (AAR) is associated with a higher rate of post-TAE mean arterial pressure ≥65 mm Hg and a shorter duration from angiography room arrival to successful cannulation compared with angiography after REBOA deflation.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The findings of this study indicate that AAR could be a preferable approach in severe pelvic fracture cases, potentially influencing clinical practice by providing a more efficient and effective method for vascular access in these critical situations.
Introduction
Pelvic trauma poses a significant challenge in trauma care, constituting 3% of skeletal injuries.1,3 Patients afflicted with pelvic fractures are typically young and present with elevated overall Injury Severity Scores (ISS) ranging from 25 to 48.4 Despite advancements in trauma care, mortality rates remain elevated, especially among those experiencing hemodynamic instability. The primary contributors to this heightened mortality include rapid exsanguination, the inherent difficulty in achieving hemostasis, and the presence of associated injuries.2 3 5
In response to this formidable challenge, resuscitative endovascular balloon occlusion of the aorta (REBOA) has emerged as a promising and innovative strategy for patients suffering from severe pelvic fractures.6 7 By temporarily occluding the aorta, REBOA provides a crucial window for subsequent hemostatic interventions, effectively managing hemorrhage in these high-stakes situations.8 Concurrently, transcatheter arterial embolization (TAE) stands as a well-established and effective procedure for addressing pelvic fractures associated with significant hemorrhage.6 9 10
The synergy between REBOA and TAE presents a comprehensive approach to managing severe pelvic trauma. However, navigating the intricacies of vascular access becomes particularly challenging in cases of extreme and severe pelvic trauma.11 12 The difficulty is compounded when considering the need for an angiographic access point distinct from the initial REBOA placement. This strategic requirement arises from the desire to maintain REBOA deflation without complete removal, a delicate balance crucial for preventing hemodynamic instability during subsequent angiographic procedures.
Although the integration of REBOA and TAE holds immense potential, the challenges regarding the route of vascular access and how TAE is ultimately performed remain a focal point of concern. The intricacies of securing access for REBOA, particularly in cases of severe pelvic trauma, significantly contribute to the complexity of treatment strategies. This study seeks to thoroughly examine the challenges of vascular access, specifically when TAE is required after REBOA in severe pelvic trauma cases. By diving deep into the complexities of access management, our goal is to clarify how these challenges affect patient outcomes and provide insights to refine clinical approaches in this critical area. This investigation aims to bridge a crucial knowledge gap, shedding light on the complexities of this integrated therapeutic approach and its impact on the overall care framework for patients with severe pelvic trauma.
Patients and methods
Study design and population
We conducted a single-center, retrospective cohort study of patients with pelvic fractures and subsequently underwent sequential REBOA and TAE at our institution from January 2017 to December 2023. Patients were included if they received both REBOA and TAE as part of their management. Those with significant concurrent injuries, defined as an Abbreviated Injury Score (AIS) of 3 or greater in any body system other than the pelvis, were excluded. Patients who underwent TAE prior to REBOA and those with suboptimal REBOA insertion, as determined by post-procedural imaging or surgical notes, were also excluded.
Retrospective data were collected through a comprehensive review of electronic medical records, radiologic images, and procedural reports. The collected data encompassed demographic information, type and severity of pelvic injuries, details of REBOA procedure, details of TAE procedure and findings, complications, amounts of transfusions and patient outcome.
The study’s primary outcome measures are overall survival and TAE successful rate. A successful TAE was defined as optimal embolization and achieving a mean arterial pressure (MAP) ≥65 mmHg after TAE. Secondary outcomes include the duration for which the REBOA device is inflated and taking effect, as well as the time span from patient’s arrival at the emergency department (ED) to the successful cannulation in the angiography suite.
Study grouping
Patients were divided into two groups based on the timing of angiographic cannulation relative to the REBOA procedure: angiography after REBOA deflation (AAD) and angiography after REBOA removal (AAR). In the AAD group, angiographic cannulation occurs after REBOA is partially or fully deflated but not removed, necessitating an additional arterial access, typically established contralaterally, for subsequent TAE. Conversely, in the AAR group, patients undergo angiography after the REBOA is removed. Thus, angiographic cannulation can be directly inserted into the sheath used for the preceding REBOA, expediting the procedure.
Statistical analysis
Descriptive statistics were used to summarize demographic and clinical characteristics. Continuous variables were expressed as medians and IQRs, as appropriate. Categorical variables were summarized as counts and percentages. Non-parametric comparisons were conducted using the Mann-Whitney U test, whereas univariate analysis for identifying risk factors associated with mortality employed logistic regression. A p value <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS V.25 for Windows.
Ethical considerations
This study was conducted in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments. This study was approved by the Institutional Review Board (IRB) of Chang Gung Memorial Hospital, Taoyuan city, Taiwan on August 1, 2022 (approval number: 201902275B0). Due to the retrospective nature of the study, the requirement for informed consent was waived.
Results
During the 6-year period from 2017 to 2023, after excluding 10 patients who was not fit for our study, we enrolled 17 patients with pelvic fractures who underwent both REBOA and TAE procedures (see figure 1). Among 10 excluded patients, 2 of them was due to failed REBOA attempts, resulting from the kinking of the catheters during REBOA placement. The majority of enrolled patients were male (12 patients, 70.6%), with a median age of 51 years (IQR 37 years). Motor vehicle collisions were the leading cause of injury, accounting for 76.5% of cases (13 patients). Prior to REBOA, 29.4% of patients experienced cardiac arrest. Overall, the survival rate was 23.5%, with only 4 patients surviving to hospital discharge. Notably, 17.6% of patients arrived at the emergency department in a traumatic out-of-hospital cardiac arrest (OHCA) status. Among the factors analyzed for mortality risk, only the body temperature at ED arrival was found to be significant (OR=0.308, p=0.039) (see table 1).
Figure 1. Study population and protocol of the current study. AIS, Abbreviated Injury Scores; MAP, mean arterial pressure; OS, overall survival; REBOA, resuscitative endovascular balloon occlusion of the aorta; TAE, transcatheter arterial embolization.
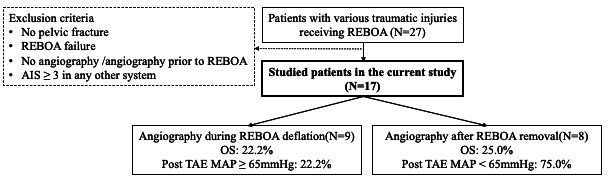
Table 1. Demographic data and univariate analysis of risk factors affecting OS of patients with traumatic pelvic injury underwent REBOA followed by TAE (n=17).
| Variables | Numbers/median | %/IQR | OR | P value |
| Male | 12 | 70.6 | 1.333 | 0.825 |
| Age (year) | 51 | 37 | 1.008 | 0.760 |
| Mechanism | ||||
| MVA | 13 | 76.5 | 0.000 | 0.999 |
| Falling | 4 | 23.5 | – | |
| Traumatic OHCA | 3 | 17.6 | 0.000 | 0.999 |
| Temperature at ED arrival (°C) | 35.0 | 2.8 | 0.308 | 0.039* |
| HR at ED arrival (bpm) | 113.0 | 57.0 | 1.002 | 0.878 |
| Shock index at ED arrival | 1.5 | 1.4 | 1.236 | 0.660 |
| MAP at ED arrival (mm Hg) | 46.7 | 50.0 | 0.996 | 0.806 |
| Pre-REBOA MAP (mm Hg) | 42.0 | 22.3 | 1.016 | 0.647 |
| Post-REBOA MAP (mm Hg) | 59.0 | 25.9 | 0.986 | 0.731 |
| Pre-angiography MAP (mm Hg) | 69.0 | 29.4 | 1.012 | 0.714 |
| Intra-angiography lowest MAP (mm Hg) | 68.0 | 46.0 | 1.030 | 0.336 |
| (Intra-angiography lowest MAP – Pre-angiography MAP)/Pre-angiography MAP (%) | 22.0 | 36.5 | 4.938 | 0.490 |
| Post-TAE MAP (mm Hg) | 64.3 | 49.9 | 1.026 | 0.194 |
| Post-TAE MAP ≥65 mm Hg | 8 | 47.1 | 0.208 | 0.223 |
| GCS at ED arrival | 3.0 | 7.0 | 0.028 | 0.999 |
| ISS | 45.0 | 13.0 | 0.948 | 0.355 |
| Open pelvic fracture | 3 | 17.6 | 1.833 | 0.662 |
| Associated abdominal injury | 7 | 41.2 | 6.750 | 0.142 |
| Y-B classification of pelvic fracture | – | |||
| APC I | 1 | 5.9 | ||
| APC II | 3 | 17.6 | ||
| PC III | 7 | 41.2 | ||
| LC II | 2 | 11.8 | ||
| LC III | 4 | 23.5 | ||
| PT-INR | 1.70 | 0.70 | 0.714 | 0.798 |
| Hb (g/dL) | 9.60 | 2.75 | 0.130 | 0.623 |
| SBE (mmol/L) | −13.55 | −7.45 | 0.976 | 0.783 |
| Lactate (mg/dL) | 87.55 | 70.28 | 0.967 | 0.153 |
| Procedure time of REBOA placement (min) | 9.0 | 5.5 | 0.466 | 0.153 |
| PRBC transfusion before TAE (U) | 12.0 | 16.0 | 0.925 | 0.324 |
| PRBC transfusion 24 hours after TAE (U) | 18.0 | 16.0 | 1.045 | 0.268 |
| Cardiac arrest before REBOA | 5 | 29.4 | 0.000 | 0.999 |
| Zone of REBOA placement | – | |||
| Zone 1 | 4 | 23.5 | ||
| Zone 3 | 13 | 76.5 | ||
| REBOA access by cutdown | 2 | 11.8 | 0.000 | 0.999 |
| Duration of REBOA inflation (min) | 107.0 | 83.5 | 0.516 | 0.994 |
| Duration from ED arrival to cannulation (min) | 147.0 | 80.5 | 0.979 | 0.170 |
| Duration from angio-room arrival to cannulation (min) | 7.0 | 12.0 | 0.966 | 0.569 |
| Duration of angiographic cannulation (min) | 67.0 | 34.5 | 1.001 | 0.952 |
| Type of angiographic cannulation | ||||
| AAD | 9 | 52.9 | 1.120 | 0.857 |
| AAR | 8 | 47.1 | – | |
| Contrast medium during angiography (mL) | 60.0 | 45.0 | 0.993 | 0.770 |
| PPP | 2 | 11.8 | 4.000 | 0.373 |
| Iatrogenic vascular injury | 1 | 5.9 | – | |
| LOS (day) | 10 | 39.0 | – | |
| Survival 72 hours after TAE | 7 | 41.2 | – | |
| OS | 4 | 23.5 | – |
P value <0.05.
AADangiography after REBOA deflationAARangiography after REBOA removalAPCanterior-posterior compressionCEcontrast extravasationCTcomputed tomographyEDemergent departmentEDemergency departmentGCSGlasgow Coma ScaleHbhemoglobinHRheart rateIQRinterquartile rangeISSInjury Severity ScoreLClateral compressionLOSlength of stayMAPmean arterial pressureMVAmotor vehicle accidentOHCAout-of-hospital cardiac arrestORodds ratioOSoverall survivalPPPpre-peritoneal pelvic packingPRBCpacked red blood cellPT-INRinternational normalized ratio derived from prothrombin timeREBOAresuscitative endovascular balloon occlusion of the aortaSBEstandard base excessTAEtranscatheter arterial embolizationY-B classificationYoung-Burgess classification
The AAR group had a significantly lower median age (39.0 years vs 63.0 years, p=0.030) and a significantly higher Shock Index at triage (2.30 vs 1.10, p=0.015) compared with the AAD group (see table 2). However, no statistical disparities were found between the groups regarding trauma mechanism, ratio of traumatic OHCA (12.5% vs 22.2%, p=1.000), temperature (35.40°C vs 34.00°C, p=0.193), Glasgow Coma Scale (GCS) (3.8 vs 9.5, p=0.663), MAP at ED arrival (32.20 mm Hg vs 80.15 mm Hg, p=0.630), Injury Severity Score (ISS) (13.0 vs 15.0, p=0.697), ratio of associated abdominal injury (25.0% vs 55.6%, p=0.335), pre-angiography MAP (61.00 mm Hg vs 79.30 mm Hg, p=0.149), arterial contrast leakage (62.5% vs 77.8%, p=0.620), laboratory parameters, or PRBC transfusion before TAE (10.0 IU vs 12.0 IU, p=0.883).
Table 2. Comparisons of characteristics between angiography after REBOA deflation (AAD) and angiography after REBOA removal (AAR) in patients with traumatic pelvic injury underwent REBOA followed by TAE (n=17).
| Variables | AAD (n=9) | AAR (n=8) | P value |
| Male (n/%) | 6 (66.7) | 7 (87.5) | 0.294 |
| Age (median year/IQR) | 63.0 (20.5) | 39.0 (27.8) | 0.030* |
| Mechanism (n/%) | 1.000 | ||
| MVA | 7 (77.8) | 6 (75.0) | |
| Falling | 2 (22.2) | 2 (25.0) | |
| Traumatic OHCA (n/%) | 2 (22.2) | 1 (12.5) | 1.000 |
| Temperature at ED arrival (median °C/IQR) | 34.00 (2.45) | 35.40 (3.55) | 0.193 |
| HR at ED arrival (median bpm/IQR) | 84.0 (28.5) | 134.0 (19.0) | 0.009* |
| Shock index at ED arrival (median/IQR) | 1.10 (0.80) | 2.30 (2.55) | 0.015* |
| MAP at ED arrival (mm Hg/IQR) | 52.00 (80.15) | 37.00 (32.20) | 0.630 |
| Pre-REBOA MAP (mm Hg/IQR) | 42.70 (47.70) | 40.65 (22.50) | 0.499 |
| Post-REBOA MAP (mm Hg/IQR) | 66.70 (27.50) | 57.85 (26.15) | 0.847 |
| Pre-angiography MAP (mm Hg/IQR) | 79.30 (34.00) | 61.00 (30.93) | 0.149 |
| Intra-angiography lowest MAP (mm Hg/IQR) | 52.7 (35.65) | 56.65 (37.28) | 0.700 |
| (Pre-angiography MAP − Intra-angiography lowest MAP)/Pre-angiography MAP (%/IQR) | 24.0 (38.0) | 2.0 (56.7) | 0.092 |
| Post-TAE MAP (mm Hg/IQR) | 62.30 (22.00) | 89.15 (66.73) | 0.136 |
| Post-TAE MAP ≥65 mm Hg (n/%) | 2 (22.2) | 6 (75.0) | 0.044* |
| GCS at ED arrival (median/IQR) | 3.0 (9.5) | 3.0 (3.8) | 0.663 |
| ISS (median/IQR) | 45.0 (15.0) | 43.0 (13.0) | 0.697 |
| Open pelvic fracture (n/%) | 1 (11.1) | 2 (25.0) | 0.576 |
| Associated abdominal injury (n/%) | 5 (55.6) | 2 (25.0) | 0.335 |
| Y-B classification of pelvic fracture (n/%) | – | ||
| APC I | 1 (11.1) | 0 (0.0) | |
| APC II | 1 (11.1) | 2 (25.0) | |
| APC III | 3 (33.3) | 4 (50.0) | |
| LC II | 1 (11.1) | 1 (12.5) | |
| LC III | 3 (33.3) | 1 (12.5) | |
| PT-INR (median/IQR) | 1.70 (0.85) | 1.70 (0.70) | 0.710 |
| Hb (median g/dL/IQR) | 9.60 (3.25) | 9.50 (3.18) | 0.700 |
| SBE (median mmol/L/IQR) | −12.40 (6.25) | −13.90 (18.75) | 0.560 |
| Lactate (median mg/dL/IQR) | 93.25 (61.68) | 78.55 (72.72) | 0.834 |
| Procedure time of REBOA placement (median minute/IQR) | 9.0 (3.5) | 10.0 (8.3) | 0.734 |
| PRBC transfusion before TAE (median U/IQR) | 12.0 (9.0) | 10.0 (25.5) | 0.883 |
| PRBC transfusion 24 hours after TAE (median U/IQR) | 18.0 (13.0) | 14.0 (28.5) | 0.529 |
| Cardiac arrest before REBOA (n/%) | 4 (44.4) | 1 (12.5) | 0.294 |
| Zone of REBOA placement (n/%) | 0.082 | ||
| Zone 1 | 4 (44.4) | 0 (0.0) | |
| Zone 3 | 5 (55.6) | 8 (100.0) | |
| REBOA access by cutdown (n/%) | 0 (0.0) | 2 (25.0) | 0.206 |
| Duration of REBOA inflation (median minute/IQR) | |||
| Total | 112.0 (85.5) | 77.0 (127.3) | 0.178 |
| Zone 1 | 130.5 (119.5) | – | – |
| Zone 3 | 75.0 (62.5) | 77.0 (127.3) | 1.000 |
| Duration from ED arrival to cannulation(median minute/IQR) | 135.0 (87.0) | 153.5 (87.3) | 0.563 |
| Duration from angio-room arrival to cannulation (median minute/IQR) | 16.0 (18.5) | 5.5 (10.8) | 0.038* |
| Duration of angiographic cannulation(median minute/IQR) | 67.0 (65.5) | 66.5 (18.8) | 0.810 |
| Iatrogenic vascular injury (n/%) | 0 (0.0) | 1 (12.5) | 0.471 |
| LOS (median day/IQR) | 9.0 (29.8) | 22.0 (50.5) | 0.226 |
| Survival 72 hours after TAE (n/%) | 4 (44.4) | 3 (37.5) | 0.968 |
| Hemostatic intervention (n/%) before TAE | |||
| PPP | 1 (11.1) | 1 (12.5) | – |
| Laparotomy | 1 (11.1) | 0 (0.0) | – |
| No | 7 (77.8) | 7 (87.5) | – |
| OS (n/%) | 2 (22.2) | 2 (25.0) | 1.000 |
APCanterior-posterior compressionCEcontrast extravasationCTcomputed tomographyEDemergency departmentGCSGlasgow Coma ScaleHbhemoglobinHRheart rateIQRinterquartile rangeISSInjury Severity ScoreLClateral compressionLOSlength of stayMAPmean arterial pressureMVAmotor vehicle accidentOHCAout-of-hospital cardiac arrestOSoverall survivalPPPpre-peritoneal pelvic packingPRBCpacked red blood cellPT-INRinternational normalized ratio derived from prothrombin timeREBOAresuscitative endovascular balloon occlusion of the aortaSBEstandard base excessTAEtranscatheter arterial embolizationY-B classificationYoung-Burgess classification
Although more patients in the AAR group achieved a post-TAE MAP ≥65 mm Hg compared with the AAD group (75.0% vs 22.2%, p=0.044), there were no significant differences in OS (25.0% vs 22.2%, p=1.000) or survival 72 hours after TAE (37.5% vs 44.4%, p=0.968). Additionally, the AAR group exhibited a significantly shorter duration from angio-room arrival to successful cannulation (5.5 min vs 16.0 min, p=0.038) compared with the AAD group. However, TAE duration, REBOA placement procedure duration, and PRBC transfusion volume 24 hours after TAE were comparable across both groups.
As shown in table 3, there was one case in the AAD group where preperitoneal pelvic packing was performed after REBOA placement, and the patient underwent TAE after preperitoneal packing. Due to severe inguinal crushing injury, arterial access on the contralateral side for angiography is not feasible. Furthermore, the inability to remove the REBOA arises from its severe compression by preperitoneal packing. Consequently, a novel femoral arterial access point proximal to the previous REBOA sheath becomes imperative to facilitate successful completion of TAE. During angiography, confirmation of contralateral external iliac traumatic transection further underscores the complexity and critical nature of the vascular injury management in this context. Additionally, there was another case in the AAR group where the patient received preperitoneal packing prior to TAE and subsequently had a favorable outcome, being discharged without major complications. In contrast, none of our patients received vasopressors or underwent pelvic fixation before TAE.
Table 3. Demographics and outcome characteristics of patients with traumatic pelvic injury underwent REBOA followed by TAE (n=17).
| Demographics | Trauma characteristics | REBOA details | Angiography details | Outcomes | |||||||||||||
| No. | Age | S/I at arrival | ISS | Associated injury | MAP ≥65 mm Hg after REBOA | C/A ever | Zone | Access method | Inflation duration | Access duration | Embolizer | Angioduration | Post TAEMAP ≥65 mm Hg | Post TAE survival in 72 hours | LOS | OS | Remark |
| AAD | |||||||||||||||||
| 1 | 70s | 0.7 | 29 | Tibia fx, L-spine fx | Y | N | 3 | P/C | 154 | 5 | Gelatin | 34 | N | N | 2 | N | |
| 2 | 50s | N/A | 50 | Pneumothorax, mesocolon injury | N | Y | 1 | P/C | 149 | 58 | Gelatin+Coils | 113 | N | N | 0 | N | PPP causing REBOA removal failure, contralateral iliac artery transaction |
| 3 | 60s | 1.3 | 57 | Spleen injury, mesentery injury | Y | N | 1 | P/C | 112 | 19 | Gelatin | 67 | N | Y | 22 | Y | |
| 4 | 70s | N/A | 45 | Hemothorax, lung contusion | Y | Y | 1 | P/C | 112 | 7 | Gelatin | 37 | N | N | 0 | N | CPCR at OR without ROSC |
| 5 | 60s | 0.8 | 41 | Pneumo-hemothorax | Y | Y | 3 | P/C | 79 | 6 | Gelatin+Coils | 50 | N | N | N/A | N | REBOA adjustment at ED |
| 6 | 30s | 1.5 | 41 | Mesentery injury | N | Y | 1 | P/C | 259 | 30 | Gelatin+Coils | 105 | N | N | 2 | N | Manpower shortage leading to delayed angiography |
| 7 | 60s | 0.7 | 48 | Pneumo-hemothorax | Y | N | 3 | P/C | 75 | 18 | Gelatin | 156 | N | Y | 32 | N | Agitation requiring sedation during angiography |
| 8 | 80s | 1.1 | 20 | Liver laceration | N | N | 3 | P/C | 51 | 6 | Gelatin | 99 | Y | Y | 29 | Y | |
| 9 | 20s | 1.9 | 50 | SDH, spleen injury, lung contusion | N | N | 3 | P/C | 57 | 16 | Gelatin | 55 | Y | Y | 20 | N | Expired due to brain stem failure |
| AAR | |||||||||||||||||
| 10 | 30s | 4.0 | 41 | Lung contusion, SDH | N | N | 3 | P/C | 137 | 15 | Gelatin | 63 | Y | N | N/A | N | Altered access due to iatrogenic external iliac artery injury |
| 11 | 30s | 3.9 | 25 | Femoral fx, urethral injury | N | N | 3 | P/C | 107 | 5 | Gelatin | 54 | Y | Y | 12 | Y | PPP performed |
| 12 | 10s | 1.2 | 50 | No | N | N | 3 | P/C | 8 | 3 | Gelatin+Coils | 76 | Y | Y | 4 | N | Reinflation of REBOA after TAE due to shock |
| 13 | 40s | 4.2 | 38 | Heart contusion, femoral fx | Y | Y | 3 | Cut-down | 15 | 1 | Gelatin | 73 | Y | N | N/A | N | |
| 14 | 50s | 2.1 | 36 | Spleen injury | Y | N | 3 | P/C | 138 | 14 | Gelatin | 52 | N | N | N/A | N | Avoiding any BT, as Jehovah’s Witness |
| 15 | 20s | 0.9 | 45 | Hemothorax, aortic injury | Y | N | 3 | P/C | 147 | 1 | Gelatin | 55 | Y | N | 1 | N | Contralateral internal iliac artery traumatic occlusion |
| 16 | 60s | 2.3 | 48 | SAH, leg traumatic amputation | N | N | 3 | Cut-down | 9 | 6 | Gelatin | 70 | N | N | N/A | N | Angiography paused due to shock, retrying after REBOA completed |
| 17 | 20s | 2.3 | 50 | EDH, perineal laceration | N | N | 3 | P/C | 47 | 7 | Gelatin+Coils | 73 | Y | Y | 55 | Y | Contralateral internal iliac artery traumatic dissection |
AADangiography after REBOA deflationAARangiography after REBOA removalBTblood transfusionC/Acardiac arrestCPCRcardiopulmonary cerebral resuscitationEDemergency departmentEDHepidural hemorrhageFfemalefxfractureISSInjury Severity ScoreLOSlength of stayMmaleMVAmotor vehicle accidentNnoN/Anot associatedN/Dnot detectedN/Rnot recordedORoperation roomOSoverall survivalP/CpercutaneousPPPpre-peritoneal packingREBOAresuscitative endovascular balloon occlusion of the aortaROSCreturn of spontaneous circulationSAHsubarachnoid hemorrhageSDHsubdural hemorrhageS/IShock IndexTAEtranscatheter arterial embolismYyes
The most common indication for choosing AAR over AAD was injury to the contralateral internal iliac artery, observed in 50% of AAR patients (four patients), either due to initial trauma or iatrogenic injury. The findings of angiography in our study were listed below: bilateral internal iliac artery and associated branches bleeding (n=4), unilateral internal iliac artery and associated artery bleeding (n=8), unilateral internal iliac artery pseudoaneurysm (n=1), and no evidence of active bleeding (n=4). All of the relevant procedures were non-selective embolization over bilateral internal iliac arteries. Among all AAD patients, two of them underwent reinflation of the balloon due to severe hemodynamic compromise during TAE, whereas one of these two patients had transient left leg hypoperfusion but recovered without complications.
Discussion
The role of REBOA in the management of pelvic fracture
The introduction of REBOA presents a pivotal shift in the paradigm of managing complex pelvic fractures.3 This study highlights REBOA’s integral role in stabilizing patients with severe pelvic trauma by providing temporary hemorrhagic control, which is crucial for facilitating subsequent definitive hemostatic interventions. Our findings found that the median MAP of post-REBOA is increased by 17.0 from pre-REBOA, aligning with previous research indicating REBOA’s efficacy in hemodynamic stability among patients with traumatic hemorrhage when employed judiciously.13
The synergistic application of REBOA with TAE offers a comprehensive approach that addresses both immediate hemorrhage control and definitive bleeding source management, as suggested by Harfouche et al.14 However, the challenges in achieving vascular access for TAE after REBOA deployment, particularly in the context of severe trauma, underscore the need for advanced planning and procedural proficiency.
Our study is among the first to comprehensively discuss the technical and clinical considerations regarding vascular access route selection in patients with severe pelvic injury. Theoretically, the indication for inclusion into AAR or AAD was the presence of an injury. Typically, the decision would be based on physiology. Patients who are extremely labile would be better served in the AAD, whereas those who are stable could be in the AAR group. Deflation of REBOA without removal of REBOA catheter has the potential benefit that the REBOA can be reinflated whenever the patient’s hemodynamics become unstable during TAE. The TAE procedure can be even performed with the partially inflated balloon. Therefore, the hemodynamic stability can be maintained more easily compared with those patients who had their REBOA catheter been removed before TAE.
Interestingly, our results revealed that there was no statistical difference between the AAD and AAR groups regarding their pre-TAE MAP (79.3 mm Hg vs 61.0 mm Hg, p=0.149) and the amount of PRBC transfusion before TAE (12.0 vs 10.0, p=0.883). This suggests that as long as patients can be adequately resuscitated to maintain hemodynamic stability, both AAR and AAD could be viable options for surgeons.
Furthermore, the severity of MAP drops during TAE, represented by the ratio of (Intra-angiography lowest MAP – Pre-angiography MAP)/Pre-angiography MAP, showed no significant difference between the two groups. Interestingly, there was even a trend toward a higher ratio in the AAD group then the AAR group (24.0% vs 2.0%, p=0.092).
These findings suggest that as long as adequate resuscitation can be achieved before TAE, the potential advantages of REBOA deflation might be mitigated, thereby making alternative arterial access methods feasible in clinical practice.
On the other hand, removal of REBOA before starting TAE was believed to be a way to accelerate vascular access and facilitate definitive hemostasis. In our study, we found that the duration from angio-room arrival to starting cannulation was significantly shorter in the AAR group than in the AAD group, whereas the time required for successful cannulation was similar between the two groups. Such results might be attributed to the combined effect of several factors including experiences of individual radiologists.
In addition to the AAR and AAD techniques, there are alternative approaches for performing TAE after REBOA under extreme conditions, such as partial REBOA or intermittent REBOA.15 16 Although these methods were not used or detailed in our study, we think they represent viable options at the discretion of the radiologist, depending on the clinical scenario and patient-specific factors.
A recent prospective randomized controlled trial17 involving 1388 patients undergoing coronary angiography and percutaneous coronary intervention demonstrated that ultrasound-guided puncture significantly enhanced the efficiency and overall success rate of arterial access. Similar to the above-mentioned study, we routinely employ ultrasound-guided puncture for every patient requiring arterial access for angiography. This approach helps mitigate operator bias and ensures consistency of successful arterial puncture in a short period of time across all cases.
One retrospective study reported by Awwad et al18 revealed that for the management of severe pelvic injuries, selective embolization had a superior overall survival rate compared with non-selective embolization. Additionally, the use of gelfoam as the sole embolizer had a greater survival benefit compared with those using a combination of gelfoam and coils. However, such differences were not observed in our cohort possibly due to the limited number of cases in the current study.
Outcome and complications of REBOA utilization in severe pelvic fracture
Although REBOA is a promising technique, it is not without complications. Complications associated with the use of REBOA in the management of pelvic fractures include access site complications like hematoma and pseudoaneurysm,12 19 which may result in lower limb ischemia and consequently lead to amputation of the limbs in severe cases.20 Technical difficulties in the insertion of the catheter and the potential for iatrogenic injury during the procedure are also reported.11 In our study, one patient experienced an iatrogenic injury to the external iliac artery, which necessitated TAE of the injured iliac artery after the removal of the REBOA catheter.
The risks associated with prolonged occlusion, such as reperfusion injury and limb ischemia, must be carefully weighed against the benefits, especially in cases where delays to definitive care are anticipated.21 The balance between maintaining aortic occlusion to ensure hemodynamic stability and mitigating the risks associated with ischemia is a delicate one that requires further investigation. In our study, although the median aortic occlusion time (overall: 107.0 min; Zone 1: 130.5 min; Zone 3: 75.0 min) exceeded the optimal durations suggested in the studies by Norii et al, Matsumura et al, and Sadeghi et al (30–45 min for Zone 1 and 60–90 min for Zone 3),22,24 none of the patients developed limb ischemia or required subsequent amputation. This may be due to the timely provision of definitive treatment at our trauma center.
Limitations
The limitations of our study, including its single-center retrospective design and small sample size of 17 patients over 6 years, restrict the generalizability and statistical power of our findings. We acknowledge that the small sample size of 17 cases limits the reliability of using logistic regression analysis, as it increases the risk of overfitting and instability in the results. Consequently, we have chosen to address potential confounders qualitatively in our discussion. Moreover, the primary objective of this study was to share our experiences with the application of both AAR and AAD methods for patients requiring TAE after REBOA, rather than to establish the superiority of one method over the other. Given this focus, we determined that logistic regression analysis was not essential for this study. Furthermore, the study timeframe is 6 years and the resuscitation protocols as well as the equipment available may change overtime, which could have influenced outcomes. Variations in the REBOA and TAE procedures, such as the partial or intermittent REBOA method, arterial access sites, and embolic materials, contribute to data heterogeneity, complicating result interpretation. These factors collectively underscore the need for caution when extrapolating our conclusions to broader clinical practice.
Conclusion
In summary, this cohort study compared the outcomes of different vascular access methods in the combined REBOA-TAE approach for patients with severe pelvic fractures. Our findings suggest that both AAR and AAD are viable options for patients requiring TAE after REBOA. The choice between these techniques should be guided by the clinical scenario, patient-specific factors, and the discretion of the radiologist. Further research with larger cohorts is essential to validate these findings and explore long-term outcomes.
Acknowledgements
The authors gratefully acknowledge that all team members dedicate their best efforts to care for patients with acute abdominal diseases.
There was no confilct of interest
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Patient consent for publication: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
References
- 1.Arvieux C, Thony F, Broux C, Ageron F-X, Rancurel E, Abba J, Faucheron J-L, Rambeaud J-J, Tonetti J. Current management of severe pelvic and perineal trauma. J Visc Surg. 2012;149:e227–38. doi: 10.1016/j.jviscsurg.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Cullinane DC, Schiller HJ, Zielinski MD, Bilaniuk JW, Collier BR, Como J, Holevar M, Sabater EA, Sems SA, Vassy WM, et al. Eastern Association for the Surgery of Trauma practice management guidelines for hemorrhage in pelvic fracture--update and systematic review. J Trauma. 2011;71:1850–68. doi: 10.1097/TA.0b013e31823dca9a. [DOI] [PubMed] [Google Scholar]
- 3.Magnone S, Coccolini F, Manfredi R, Piazzalunga D, Agazzi R, Arici C, Barozzi M, Bellanova G, Belluati A, Berlot G, et al. Management of hemodynamically unstable pelvic trauma: results of the first Italian consensus conference (cooperative guidelines of the Italian Society of Surgery, the Italian Association of Hospital Surgeons, the Multi-specialist Italian Society of Young Surgeons, the Italian Society of Emergency Surgery and Trauma, the Italian Society of Anesthesia, Analgesia, Resuscitation and Intensive Care, the Italian Society of Orthopaedics and Traumatology, the Italian Society of Emergency Medicine, the Italian Society of Medical Radiology -Section of Vascular and Interventional Radiology- and the World Society of Emergency Surgery) World J Emerg Surg. 2014;9:18. doi: 10.1186/1749-7922-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grotz MRW, Allami MK, Harwood P, Pape HC, Krettek C, Giannoudis PV. Open pelvic fractures: epidemiology, current concepts of management and outcome. Injury. 2005;36:1–13. doi: 10.1016/j.injury.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 5.Perkins ZB, Maytham GD, Koers L, Bates P, Brohi K, Tai NRM. Impact on outcome of a targeted performance improvement programme in haemodynamically unstable patients with a pelvic fracture. Bone Joint J. 2014;96-B:1090–7. doi: 10.1302/0301-620X.96B8.33383. [DOI] [PubMed] [Google Scholar]
- 6.Coccolini F, Stahel PF, Montori G, Biffl W, Horer TM, Catena F, Kluger Y, Moore EE, Peitzman AB, Ivatury R, et al. Pelvic trauma: WSES classification and guidelines. World J Emerg Surg. 2017;12:5. doi: 10.1186/s13017-017-0117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JV, Yuan S. Traumatic Injuries of the Pelvis. Emerg Med Clin North Am. 2020;38:125–42. doi: 10.1016/j.emc.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Jarvis S, Kelly M, Mains C, Corrigan C, Patel N, Carrick M, Lieser M, Banton K, Bar-Or D. A descriptive survey on the use of resuscitative endovascular balloon occlusion of the aorta (REBOA) for pelvic fractures at US level I trauma centers. Pat Saf Surg. 2019;13:43. doi: 10.1186/s13037-019-0223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metsemakers W-J, Vanderschot P, Jennes E, Nijs S, Heye S, Maleux G. Transcatheter embolotherapy after external surgical stabilization is a valuable treatment algorithm for patients with persistent haemorrhage from unstable pelvic fractures: outcomes of a single centre experience. Injury. 2013;44:964–8. doi: 10.1016/j.injury.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Broadwell SR, Ray CE. Transcatheter Embolization in Pelvic Trauma. Semin intervent Radiol. 2004;21:23–35. doi: 10.1055/s-2004-831402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Özkurtul O, Staab H, Osterhoff G, Ondruschka B, Höch A, Josten C, Fakler JKM. Technical limitations of REBOA in a patient with exsanguinating pelvic crush trauma: a case report. Pat Saf Surg. 2019;13:25. doi: 10.1186/s13037-019-0204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manzano-Nunez R, Orlas CP, Herrera-Escobar JP, Galvagno S, DuBose J, Melendez JJ, Serna JJ, Salcedo A, Peña CA, Angamarca E, et al. A meta-analysis of the incidence of complications associated with groin access after the use of resuscitative endovascular balloon occlusion of the aorta in trauma patients. J Trauma Acute Care Surg. 2018;85:626–34. doi: 10.1097/TA.0000000000001978. [DOI] [PubMed] [Google Scholar]
- 13.Hurley S, Erdogan M, Kureshi N, Casey P, Smith M, Green RS. Comparison of clinical and anatomical criteria for resuscitative endovascular balloon occlusion of the aorta (REBOA) among major trauma patients in Nova Scotia. CJEM. 2021;23:528–36. doi: 10.1007/s43678-021-00100-3. [DOI] [PubMed] [Google Scholar]
- 14.Harfouche M, Inaba K, Cannon J, Seamon M, Moore E, Scalea T, DuBose J. Patterns and outcomes of zone 3 REBOA use in the management of severe pelvic fractures: Results from the AAST Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery database. J Trauma Acute Care Surg. 2021;90:659–65. doi: 10.1097/TA.0000000000003053. [DOI] [PubMed] [Google Scholar]
- 15.Russo RM, Williams TK, Grayson JK, Lamb CM, Cannon JW, Clement NF, Galante JM, Neff LP. Extending the golden hour: Partial resuscitative endovascular balloon occlusion of the aorta in a highly lethal swine liver injury model. J Trauma Acute Care Surg. 2016;80:372–8. doi: 10.1097/TA.0000000000000940. [DOI] [PubMed] [Google Scholar]
- 16.Kuckelman J, Derickson M, Barron M, Phillips CJ, Moe D, Levine T, Kononchik JP, Marko ST, Eckert M, Martin MJ. Efficacy of intermittent versus standard resuscitative endovascular balloon occlusion of the aorta in a lethal solid organ injury model. J Trauma Acute Care Surg. 2019;87:9–17. doi: 10.1097/TA.0000000000002307. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen P, Makris A, Hennessy A, Jayanti S, Wang A, Park K, Chen V, Nguyen T, Lo S, Xuan W, et al. Standard versus ultrasound-guided radial and femoral access in coronary angiography and intervention (SURF): a randomised controlled trial. EuroIntervention. 2019;15:e522–30. doi: 10.4244/EIJ-D-19-00336. [DOI] [PubMed] [Google Scholar]
- 18.Awwad A, Dhillon PS, Ramjas G, Habib SB, Al-Obaydi W. Trans-arterial embolisation (TAE) in haemorrhagic pelvic injury: review of management and mid-term outcome of a major trauma centre. CVIR Endovasc. 2018;1:32. doi: 10.1186/s42155-018-0031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borger van der Burg BLS, van Dongen TTCF, Morrison JJ, Hedeman Joosten PPA, DuBose JJ, Hörer TM, Hoencamp R. A systematic review and meta-analysis of the use of resuscitative endovascular balloon occlusion of the aorta in the management of major exsanguination. Eur J Trauma Emerg Surg. 2018;44:535–50. doi: 10.1007/s00068-018-0959-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumura Y, Matsumoto J, Kondo H, Idoguchi K, Ishida T, Kon Y, Tomita K, Ishida K, Hirose T, Umakoshi K, et al. Fewer REBOA complications with smaller devices and partial occlusion: evidence from a multicentre registry in Japan. Emerg Med J. 2017;34:793–9. doi: 10.1136/emermed-2016-206383. [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro Junior MAF, Feng CYD, Nguyen ATM, Rodrigues VC, Bechara GEK, de-Moura RR, Brenner M. The complications associated with Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) World J Emerg Surg. 2018;13:20. doi: 10.1186/s13017-018-0181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score-adjusted untreated patients. J Trauma Acute Care Surg. 2015;78:721–8. doi: 10.1097/TA.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 23.Matsumura Y, Matsumoto J, Kondo H, Idoguchi K, Ishida T, Okada Y, Kon Y, Oka K, Ishida K, Toyoda Y, et al. Early arterial access for resuscitative endovascular balloon occlusion of the aorta is related to survival outcome in trauma. J Trauma Acute Care Surg. 2018;85:507–11. doi: 10.1097/TA.0000000000002004. [DOI] [PubMed] [Google Scholar]
- 24.Sadeghi M, Dogan EM, Karlsson C, Jansson K, Seilitz J, Skoog P, Hörer TM, Nilsson KF. Total resuscitative endovascular balloon occlusion of the aorta causes inflammatory activation and organ damage within 30 minutes of occlusion in normovolemic pigs. BMC Surg. 2020;20:43. doi: 10.1186/s12893-020-00700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.


