Abstract
Many studies have suggested that conflict monitoring involves the anterior cingulate cortex (ACC). We previously showed that a specific hypnotic suggestion reduces involuntary conflict and alters information processing in highly hypnotizable individuals. Hypothesizing that such conflict reduction would be associated with decreased ACC activation, we combined neuroimaging methods to provide high temporal and spatial resolution and studied highly and less-hypnotizable participants both with and without a suggestion to interpret visual words as nonsense strings. Functional MRI data revealed that under posthypnotic suggestion, both ACC and visual areas presented reduced activity in highly hypnotizable persons compared with either no-suggestion or less-hypnotizable controls. Scalp electrode recordings in highly hypnotizable subjects also showed reductions in posterior activation under suggestion, indicating visual system alterations. Our findings illuminate how suggestion affects cognitive control by modulating activity in specific brain areas, including early visual modules, and provide a more scientific account relating the neural effects of suggestion to placebo.
Keywords: anterior cingulate cortex, attention, hypnosis, neuroimaging, Stroop effect
General accounts of cognitive control identify the dorsal anterior cingulate cortex (ACC) as a key to the monitoring of conflict within a network of neural regions (1–3). In multiple tasks that involve a conflict between competing responses, functional MRI (fMRI) studies have measured higher signal levels at the ACC in conditions when conflict was present (1–4).
One conflict task showing reliable ACC activations requires proficient readers to name the ink color of a displayed word (5). Individuals are usually slower and less accurate indicating the ink color of an incompatible color word (e.g., responding “blue” when the word “RED” is displayed in blue ink) than identifying the ink color of a congruent color name (e.g., responding “red” when the word “RED” is inked in red). This difference in performance constitutes the Stroop conflict and is one of the most robust and well-studied phenomena in attentional research (6, 7). The dominant view regards reading as a largely automatic process whereby skilled readers cannot withhold activating a word's underlying meaning despite explicit instructions to attend only to its ink color. Indeed, the standard account maintains that semantic processing of words occurs involuntarily (6, 8) and that the Stroop is a benchmark experimental task of cognitive conflict (9). Nonetheless, independent researchers have challenged the robustness of the Stroop effect (10, 11), suggesting that rather than being inevitable, other factors such as attention may govern the process (12). Although critiqued (13), this approach has resulted in data showing either reduction or removal of Stroop interference (14, 15).
Recently, we used hypnotic suggestion as an attentional tool to manipulate conflict (16). Whereas earlier case reports (17, 18) and at least one esoteric study (19) reported promising preliminary findings by using hypnotic suggestions, we used an experimental design using a posthypnotic suggestion, a condition wherein a subject complies with a suggestion made during the hypnotic episode after termination of the hypnotic experience (16). Although subjects may not remember being told to adhere to a specific instruction, the posthypnotic suggestion is usually summoned on a prearranged signal and can be effective in highly hypnotizable individuals (16, 20–23). Posthypnotic suggestions, therefore, unlike hypnotic suggestions, take effect in a conventionally behaving person during common wakefulness (16). Earlier, we used this system in a laboratory setting and presented behavioral findings showing elimination of Stroop interference (20). We then replicated our results by using appropriate control for visual accommodation as well as eye movements (21). Together with other findings (24), these data led us to conclude that a top-down neural process, rather than optical degradation of the input stimuli, is responsible for this effect (25).
In the present study, we used neuroimaging to extend our behavioral findings and illuminate the underlying brain mechanisms responsible for such vigorous modulation. Complementing the excellent spatial resolution of fMRI with the high temporal resolution afforded by electrical scalp recording of event-related potentials (ERP), we unraveled the neural substrates by which suggestion moderates conflict. We show that suggestion decreases conflict by strongly modulating both early occipital cortex activity and later ACC activation.
Methods
Participants. Sixteen neurologically healthy participants with normal color vision, eight highly hypnotizable and eight lesshypnotizable (22, 23), volunteered for a combined fMRI–ERP experiment, which was approved by the Weill Medical College of Cornell University institutional review board for the rights of human subjects in research. Participants were right-handed proficient readers of English aged 20–35 years (mean = 27 years). All participants were recruited from a pool of 95 volunteers who had been screened for suggestibility in a hypnotic context by using the Harvard Group Scale (Form A) (23) and then individually by using the Stanford Hypnotic Susceptibility Scale (Form C) excluding the anosmia to ammonia challenge (22). The eight highly hypnotizable participants (four female and four male) scored in the highly susceptible range (10–11 of a possible 11), whereas the eight control participants (four female and four male) scored in the less-susceptible range (1–2 of a possible 11). Preceding the experiment, an experimenter notified the participants that the purpose of the study was to investigate the effects of suggestion on cognitive performance. Participants were told that hypnotic inductions and suggestions would be administered at certain points during the experiment and that they would be asked to play a computer game (i.e., the Stroop task). After receiving an explanation of the procedures, participants provided written informed consent.
Stimuli. Stimuli consisted of an English word written in one of four ink colors (red, blue, green, or yellow) appearing at the center of the computer screen where a black fixation cross was visible. All characters were displayed in uppercase font against a white background, and the stimuli subtended visual angles of 0.5° vertically and 1.3–1.9° horizontally (depending on word length). A congruent condition consisted of a color word inked in its own color, whereas an incongruent condition consisted of a color word inked in any of the three colors other than its own. During each trial, subjects were asked to indicate the ink color in which a word was written by depressing one of four keys on a response pad by using the index and middle fingers of each hand.
Participants were exhorted to focus their eyes on a fixation cross at the center of the screen. Then, a stimulus would appear on the screen replacing the crosshair. The stimulus remained on the screen for a maximum of 2 sec or until participants responded. After a response, the fixation cross was redisplayed at the center for a variable duration contingent on the subject's reaction time (RT). At this point, a new stimulus appeared on the screen, again replacing the fixation cross and beginning the next trial.
Posthypnotic Suggestion. Subsequent to a standard hypnotic induction (22), the following posthypnotic suggestion was verbally presented to all participants: “Very soon you will be playing the computer game. Every time you will hear my voice talking to you over the intercom system, you will immediately realize that meaningless symbols are going to appear in the middle of the screen. They will feel like characters of a foreign language that you do not know, and you will not attempt to attribute any meaning to them. This gibberish will be printed in one of four ink colors: red, blue, green, or yellow. Although you will only be able to attend to the symbols' ink color, you will look straight at the scrambled signs and crisply see all of them. Your job is to quickly and accurately depress the key that corresponds to the ink color shown. You will find that you can play this game easily and effortlessly. As soon as the scanning noise stops, you will relax back to your regular reading self.” In half the blocks, this posthypnotic suggestion was triggered by talking to the subjects via the intercom. In the remaining blocks, when posthypnotic suggestion was absent, conventional Stroop instructions appeared on the screen: “Please respond as quickly and as accurately to the ink color in which the stimuli are written.” Block administration order was counterbalanced across participants in each group.
Training. At least one full-length practice block preceded the fMRI scan for each subject. Practice was part of a simulation-and-acclimation procedure run on a mock scanner before the actual scan. The sham scan, performed on a replica of the actual scanner, confirmed that participants were able to prepare for and understand the task, proficiently map the four colors to the appropriate response keys, and respond quickly and accurately. As part of this training session, participants completed at least 176 experimental trials.
Neuroimaging. When we previously asked highly and less-suggestible participants to see Stroop words as meaningless symbols, only the highly suggestible individuals were influenced by the suggestion (20, 21). Our current fMRI data further corroborate this point. Accordingly, whereas we scanned both highly and less-suggestible participants by using fMRI, we separately acquired ERP data from the highly suggestible individuals only. Behavioral data were collected during all neuroimaging sessions while the same participants performed the identical Stroop task tailored to either ERP or fMRI measurements.
fMRI. A 3.0-tesla General Electric Signa scanner acquired blood oxygenation level-dependent images. For the functional part, image volumes were collected continuously by using a T2*-weighted gradient echo planar imaging sequence [echo time (TE) = 35 msec; repetition time (TR) = 2,000 msec; flip angle = 80°] with an in-plane resolution of 3.44 × 3.44 mm (64 × 64 matrix; 220-mm field of view). To cover the whole brain, 24 5-mm slices (with 1-mm skip between slices) were acquired along the anterior commissure–posterior commissure plane as determined by the midsagittal section. For the structural part, we used a T1-weighted sequence in the same orientation as the functional sequences to provide detailed anatomical images aligned to the functional scans. High-resolution structural images were also acquired for the purpose of cross-subject registration. Scanning consisted of an event-related design with jittered intertrial intervals randomly chosen from an exponential distribution ranging from 3,000 to 15,000 msec (mean = 6,000 msec). Each session consisted of eight 38-trial blocks with the first 2 trials of each block serving as buffer trials. Task order was counterbalanced across subjects. We used statistical parametric mapping software (spm 99, www.fil.ion.ucl.ac.uk/spm) to analyze the fMRI data. The imaging time series was realigned, spatially normalized to stereotactic Montreal Neurological Institute space, and smoothed with a 8 × 8 × 12-mm full-width at half-maximum Gaussian kernel. The general linear model identified voxels activated during the experimental conditions. High-pass filtering removed participant-specific low-frequency drift in signal while proportional scaling removed global changes. A statistical threshold of P < 0.05 was used (uncorrected and thresholded at 50 voxels, with voxel size of 2 × 2 × 2 mm3).
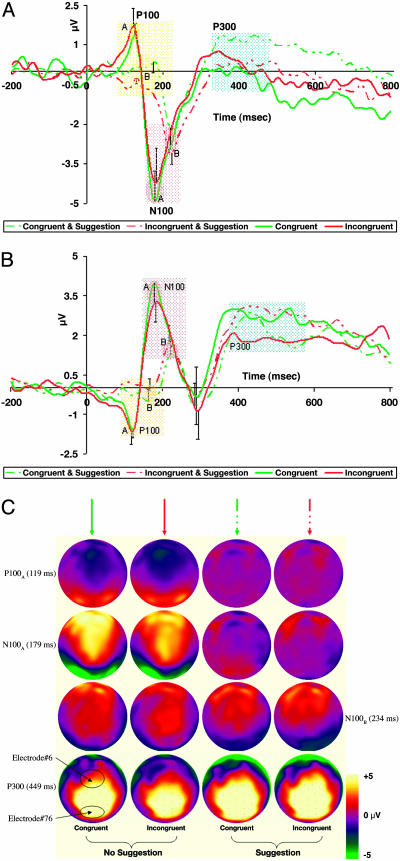
ERP. Sampled at 250 Hz with 128-electrode dense-array geodesic sensor net referenced to the vertex (26), we recorded ERPs from highly suggestible persons. Trials were averaged in a stimulus-locked fashion, digitally transformed to an average reference, band-pass filtered (15 Hz), and corrected for baseline over a 200-msec window before stimulus onset. However, trials with incorrect responses, voltages exceeding ±100 μV, transients exceeding ±50 μV, electro-oculogram activity exceeding ±70 μV, and RTs either below or above 2SD of subject's mean latency were excluded from analysis. Comparisons between subjects and conditions were calculated during the temporal range, which included the experimental effect seen in the waveforms (roughly 120–250 msec). Grand-mean peak differences between conditions recorded over the above-mentioned window at the midfrontal (electrode no. 6) and midoccipital (electrode no. 76) sites, respectively, were validated by computing repeated-measures analysis of variance examining both amplitude and latency as a function of Suggestion (Absent, Present) and Condition (Congruent, Incongruent) with a criterion of P < 0.05. Fig. 2C shows representative snapshots from a video animation based on two-dimensional maps of scalp voltages, which were constructed by spherical spline interpolation mapping across the highly hypnotizable individuals on both the incongruent and congruent trials as a function of suggestion (see Movie 1, which is published as supporting information on the PNAS web site).
Fig. 2.
Mean ERP and derived source waveforms from eight highly suggestible individuals (±1 SE). (A and B) Midoccipital (A) and midfrontal (B) activity under both no-suggestion and a specific posthypnotic suggestion to view Stroop words as meaningless symbols. These two electrode locations roughly corresponded with the locations of the posterior and anterior fMRI activations, respectively. (C) Representative snapshots of global cortical activity at peak times. The two leftmost and rightmost images of each quartet indicate electrical activity with and without suggestion, respectively (see Movie 1). Brain activity in posterior and anterior regions was delayed and largely reduced, supporting the notion that the posthypnotic suggestion affected processing of the entire visual stream and was not specific to visual words.
Results
Behavioral data collected during ERP sessions showed elimination of the Stroop effect (i.e., a significant Incongruent minus Congruent difference) as evidenced by both accuracy and RT measures in highly hypnotizable individuals as a function of suggestion (Table 1). Behavioral results acquired throughout fMRI scans revealed a reduction in Stroop conflict for the highly hypnotizable persons as a function of suggestion (Table 1) but showed no performance differences for the less-hypnotizable individuals (Table 2). Compared with highly suggestible individuals, less-suggestible participants were ≈10% slower overall.
Table 1. Behavioral data from the highly hypnotizable individuals.
| fMRI
|
ERP
|
|||
|---|---|---|---|---|
| Suggestion | RTI-C, msec | AccuracyI-C, % | RTI-C msec | AccuracyI-C, % |
| Absent | 139 (14.15)* | -1.3 (0.39)* | 90 (7.89)* | -0.8 (0.14)* |
| Present | 41 (14.22)† | -0.2 (0.29) | 9 (7.88) | -0.1 (0.10) |
Standard error is shown in parentheses. RT data include only correct responses and exclude RT exceeding ±2 SD around the mean score for each subject in each condition. Repeated-measures analyses of Group (highly hypnotizable or less-hypnotizable) × Suggestion (absent or present) × Congruency (congruent, incongruent, or neutral) were calculated by using the linear [proc mixed in sas (SAS Institute, Cary, NC)] and binary (proc genmod in sas) mixed models for the RT and accuracy data, respectively. Whereas RT got significantly faster as a function of posthypnotic suggestion for both fMRI [F(1,68) = 23.34, P < 0.01] and ERP [F(1,14) = 52.31, P < 0.01], a significant increase in accuracy occurred for the ERP (χ2(1) = 3.92, P < .05) but not for the fMRI (χ2(1) = 3.37, P = .066). Notably, although suggestion significantly reduced the Stroop effect in the fMRI setting, it effectively removed the Stroop effect in the ERP sessions. *, P < 0.01; †, P < 0.05.
Table 2. RT and accuracy data from the fMRI sessions.
| Posthypnotic suggestion congruency | RT (SD), msec | Accuracy (SD), % |
|---|---|---|
| Highly hypnotizable | ||
| Absent | ||
| Congruent | 725 (157) | 98.2 (13.4) |
| Incongruent | 865 (245) | 93.0 (25.6) |
| Neutral | 755 (176) | 97.7 (15.1) |
| Present | ||
| Congruent | 685 (163) | 94.3 (23.3) |
| Incongruent | 721 (230) | 93.2 (25.2) |
| Neutral | 718 (192) | 94.8 (22.2) |
| Less-hypnotizable | ||
| Absent | ||
| Congruent | 861 (220) | 98.2 (13.4) |
| Incongruent | 933 (252) | 96.1 (19.4) |
| Neutral | 854 (196) | 96.6 (18.1) |
| Present | ||
| Congruent | 809 (207) | 97.1 (16.7) |
| Incongruent | 860 (210) | 95.1 (21.7) |
| Neutral | 807 (200) | 97.4 (15.9) |
Significant RT main effects (P < 0.005) and interactions (P < 0.01) were calculated for Group, Suggestion, Congruency, Group × Congruency and Suggestion × Congruency. A main effect for Congruency (P < 0.05) was the only significant result obtained for accuracy. Although suggestion removed RT interference (i.e., incongruent minus neutral) and reduced conflict (i.e., incongruent minus congruent) for the highly hypnotizable persons, it affected neither for the less-hypnotizable individuals.
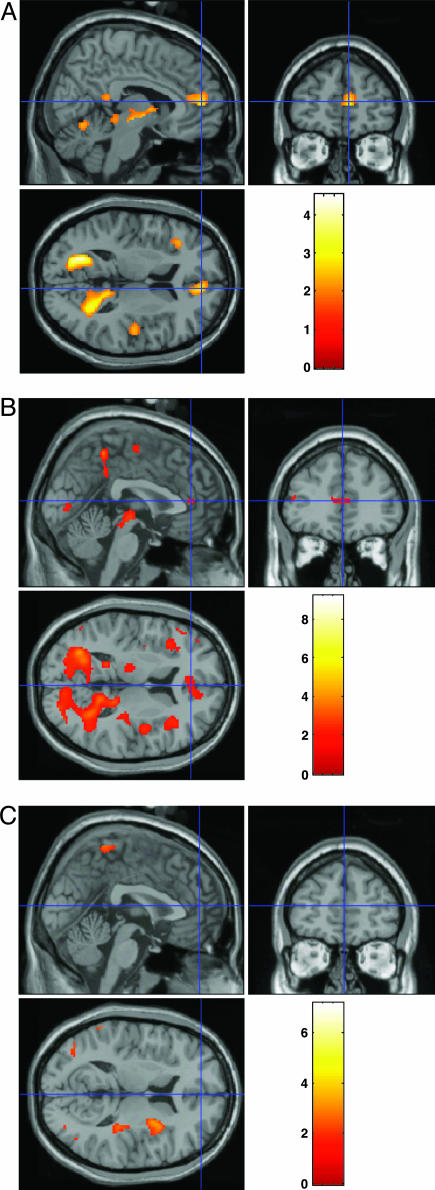
Investigating the Stroop conflict by using fMRI, we compared brain activity with and without posthypnotic suggestion at the ACC both between and within groups. Fig. 1A shows significant interaction between group (highly vs. less-hypnotizable persons) and suggestion (absent vs. present) for Stroop conflict (see also Table 3). Further comparisons revealed that whereas for the less-suggestible controls ACC activation was not reduced upon suggestion, within the highly hypnotizable group, suggestion elicited a significant reduction in ACC activation (see the ACC in Fig. 1 B and C). In fact, although fMRI data from less-suggestible individuals showed a significant increase in activation on incongruent trials, no difference in brain activity between congruent and incongruent trials appeared in highly hypnotizable persons given the posthypnotic suggestion (Fig. 1C). In addition to ACC activity reduction, we found fMRI signal reduction in posterior brain activity within an extrastriate visual area (Fig. 1 A).
Fig. 1.
Stroop conflict (incongruent minus congruent) fMRI data. (A) Interaction between group (highly hypnotizable, less-hypnotizable) and suggestion (absent, present). Compared with the less-hypnotizable controls, conflict reduction (i.e., activation decrease) was significant in the highly hypnotizable individuals (Tables 1, 2, 3). (B and C) Interpretation of highly suggestible fMRI absent posthypnotic suggestion (B) and under posthypnotic suggestion (C) to construe the stimuli as nonsense strings proposes that no difference was detected between incongruent and congruent trials. Whereas prefrontal activations (e.g., crosshair at the ACC) probably correlated with cognitive control, posterior activations might relate to early occipital modulation or aspects of visual word recognition.
Table 3. Brain regions showing significant fMRI signal.
| Anatomical structure | Brodmann's area | x | y | z | Z-score | P | Voxels |
|---|---|---|---|---|---|---|---|
| Cuneus | 17 | -20 | -67 | 14 | 3.49 | 0.000 | 4,416 |
| Left middle frontal gyrus | 6 | -28 | 17 | 38 | 2.93 | 0.002 | 257 |
| Right cingulate gyrus | 32 | 8 | 49 | 7 | 2.43 | 0.008 | 258 |
| Left insula | -34 | -12 | 1 | 2.33 | 0.010 | 188 | |
| Left inferior frontal gyrus | 46 | -36 | 28 | 10 | 2.23 | 0.013 | 163 |
| Right superior temporal gyrus | 22 | 50 | -10 | 4 | 2.23 | 0.013 | 423 |
The coordinates (x,y,z) are converted from MNI to Talairach space. These are the brain areas shown in Fig. 1A for the interaction (less-hypnotizable minus highly hypnotizable, no suggestion minus suggestion, and incongruent minus congruent). The maximally activated voxel in each of the regions and their Z-values are shown to the right.
The higher temporal resolution afforded by scalp ERP showed that in highly hypnotizable individuals, the common early visual effect appeared both delayed and diminished under suggestion (Fig. 2). Significant main effects based on the presence of suggestion indicate its influence on electrophysiological activity across both congruent and incongruent conditions. This phenomenon is observed as early as 119 msec after word onset. Analyses of ERP data from both midoccipital (Fig. 2 A) and midfrontal (Fig. 2B) locations show significant differences for peak amplitude as well as for amplitude latency for both the P100 and N100 (100 ms to peak; P, positive; N, negative). Analyses of the midfrontal ERP (Fig. 2B) reveal differences for peak amplitude [P100AB: congruent t(7) = –1.50, P = 0.152; incongruent t(7) = –2.62, P < 0.05; N100AB: congruent t(7) = 2.86, P < 0.01; incongruent t(7) = 2.02, P = 0.053] and latency [P100AB: congruent t(7) =–4.13, P < 0.001; incongruent t(7) = –4.99, P < 0.001; N100AB: congruent t(7) = –5.10, P < 0.001; incongruent t(7) = –3.97, P < 0.001]. Similar analyses of midoccipital ERP (Fig. 2 A) show differences for amplitude [P100AB: congruent t(7) = 1.48, P = 0.150; incongruent t(7) = 3.32, P < 0.01; N100AB: congruent t(7) = –2.22, P < 0.05; incongruent t(7) = –1.08, P = 0.290] and latency [P100AB: congruent t(7) =–2.65, P < 0.05; incongruent t(7) =–1.80, P = 0.083; N100AB: congruent t(7) =–5.96, P < 0.001; incongruent t(7) = –6.58, P < 0.001].
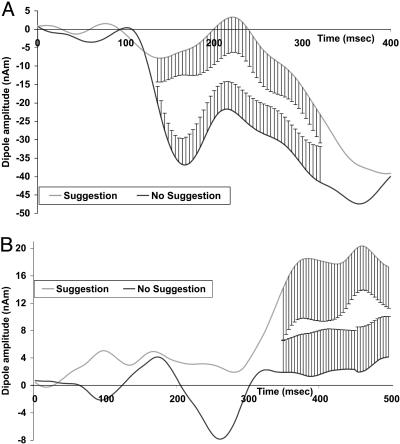
Dipole models were generated by using besa (27) (Version 5.0). Six fixed dipoles were placed at locations suggested by the fMRI data (Table 3). Significant results were obtained (low cut-off fixed time constant 0.3 sec; high cut-off 15 Hz) for both the suggestion-present (residual variance, 4.54%; best, 2.44%) and suggestion-absent conditions (residual variance, 5.42%; best, 2.21%). Fig. 3 shows the activity differences at both the cuneus and ACC.
Fig. 3.
Dipole model analysis from six fixed dipoles placed at locations suggested by the fMRI data (Table 3). Matching dipole orientation and strength to ERP difference (incongruent minus congruent) at these locations, besa indicated independent alterations at the cuneus (A) preceding changes in the ACC (B). Standard error is plotted where the two curves differ.
The source-localization algorithm provided evidence consistent with independent generators at both the cuneus and ACC (Fig. 3). Whereas the derived source waveforms from the cuneus seem to relate to the early visual alteration (Fig. 3A), differences at the ACC reached significance only 350 msec after target onset and appear to relate to subsequent conflict processing (Fig. 3B).
Discussion
We have provided evidence that, at least for highly hypnotizable individuals, a specific posthypnotic suggestion to perceive words as nonsense strings produces a strong modulation of activity in both the early occipital cortex (Figs. 2 A and 3A) and in the ACC (Figs. 2B and 3B). The behavioral results demonstrate elimination and reduction of Stroop conflict in the ERP and fMRI, respectively (Table 1). Behavioral data from the ERP sessions were comparable with our previously reported findings concerning Stroop removal (20, 21). But the present behavioral data from the fMRI scans may shed light on the neural mechanisms involved in a gradual decrease of this effect (Table 2).
The reduced fMRI signal seen in some parts of the prestriate area (Fig. 1 A) might be related to reading visual words (28), but interpretation of activations in these areas remains controversial (29, 30). Nonetheless, the reduced visual activity is in line with positron emission tomography data showing that hypnotic suggestion to see a color pattern as gray-scale reduced activity in color-related visual areas (24).
Positron emission tomography assays of pain show that specific modulatory hypnotic suggestions affect activation of different brain structures: whereas suggesting a drop in pain unpleasantness reduces specific activity in ACC (31), suggesting decreased pain intensity produces activity reduction in somatosensory cortex (32). A recent fMRI study extended these findings to illuminate the role of placebo in the context of pain (33). These collective accounts underline the influence that attention and suggestion can impart to conf lict situations, top-down cognitive organization, self-regulation, and effortful control (16, 34, 35).
Consonant with reports showing left and right lateralization for orthographic and nonorthographic stimuli, respectively (36), our ERP data show that in the absence of suggestion (e.g., Fig. 2C at 179 msec), posterior brain activity was more left-lateralized (i.e., in line with orthography), whereas the presence of suggestion reversed this trend (e.g., Fig. 2C at 234 msec). Furthermore, the ERP findings show that suggestion likely influences attention-sensitive electrophysiological components (37). These results seem to indicate that suggestion wields a general dampening-down effect on early visual activity as indexed by electrophysiological components (i.e., P100 and N100), showing both a shift and a reduction in amplitude. Representative snapshots, captured from a time-course video showing cortical electrophysiological activity across the entire brain (Movie 1), illustrate these effects at their respective peaks (Fig. 2C). Notably, whereas suggestion attenuated earlier components, the P300 remained unaffected.
Suggestion may instigate lowered visual system activation by reducing attention either to specific visual stimuli (e.g., words) or to the actual input stream (e.g., dampening down all visual stimuli). The paucity of fMRI signal differences between incongruent and congruent trials (Fig. 1C) together with the ERP data of the highly hypnotizable individuals under suggestion (Fig. 2) seem to support the latter possibility. Hence, despite explicit instructions to construe the input stimuli as nonsense strings, in highly suggestible persons this suggestion appears to have elicited a general alteration in early visual processing, not a language-specific filter, consequently resulting in a diminished Stroop effect.
We related the fMRI with the ERP data using besa to explore the time course of the fMRI generators. By using dipole source modeling and coregistration with both positron emission tomography and fMRI data, the neural generators of the attention-sensitive P100 and N100 components have been previously localized to specific zones of extrastriate visual-cortical areas. Additionally, earlier magnetoencephalographic data provided evidence that the discriminative processing associated with the N100 component was localized to the inferior occipito-temporal cortex of the ventral stream beginning after ≈150 msec (37).
Exploration of the behavioral data shows that, similar to our previous findings (20, 21), Stroop interference after suggestion was completely removed during the ERP sessions. However, findings from highly hypnotizable individuals during fMRI reveal a significant reduction, but not a removal, of Stroop interference. One way to account for these results relates to the different experimental environments in which these disparate neuroimaging measurements occur. Whereas our earlier behavioral studies, as well as the current ERP experiment, require participants to perform while sitting upright in front of display devices, fMRI obliges participants to perform while lying supine and motionless inside a narrow bore. We recently outlined how the ergonomic factors associated with current fMRI technology may skew cognitive processing and influence hemodynamic measurements (38). It is plausible, therefore, that the psychological and physical stressors, which are part of the fMRI procedures, may have provided for a suboptimal hypnotic experience and consequently a less forcible influence of suggestion. In this regard, individual and group differences (e.g., overall performance of the highly suggestible participants was ≈100 msec faster than that of the less-suggestible persons) may be important to consider (39).
Our results show that in highly hypnotizable persons, a specific posthypnotic suggestion to construe Stroop words as nonsense strings reduced conflict, as indicated by both behavioral data and ACC activity reduction. Evidence of reduced ERP under suggestion proposes strong modulation of early occipital cortex activity. This altered visual processing probably affected downstream cognitive activity, including ACC activation. Our results highlight the role of posthypnotic suggestions in altering cognitive processes. This knowledge may pave the road toward illuminating the neural correlates of other suggestion-based interventions. For example, a greater importance has been placed recently on trying to understand the placebo effect (33). It is important to compare hypnotic suggestions with other methods for modulating cognitive control, including placebo.
Supplementary Material
Acknowledgments
A.R. was supported in part by the DeWitt Wallace–Reader's Digest Research Fellowship in Psychiatry while at the Sackler Institute for Developmental Psychobiology, Weill Medical College of Cornell University.
Author contributions: A.R., J.F., and M.I.P. designed research; A.R. performed research; A.R. and J.F. analyzed data; and A.R. and M.I.P. wrote the paper.
Abbreviations: ACC, anterior cingulate cortex; fMRI, functional MRI; ERP, event-related potentials; RT, reaction time.
References
- 1.Kerns, J. G., Cohen, J. D., MacDonald, A. W., III, Cho, R. Y., Stenger, V. A. & Carter, C. S. (2004) Science 303, 1023–1026. [DOI] [PubMed] [Google Scholar]
- 2.Bush, G., Luu, P. & Posner, M. I. (2000) Trends Cognit. Sci. 4, 215–222. [DOI] [PubMed] [Google Scholar]
- 3.Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S. & Cohen, J. D. (2001) Psychol. Rev. 108, 624–652. [DOI] [PubMed] [Google Scholar]
- 4.Fan, J., Fossella, J., Sommer, T., Wu, Y. & Posner, M. I. (2003) Proc. Natl. Acad. Sci. USA 100, 7406–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stroop, J. R. (1935) J. Exp. Psychol. 18, 643–661. [Google Scholar]
- 6.MacLeod, C. M. (1991) Psychol. Bull. 109, 163–203. [DOI] [PubMed] [Google Scholar]
- 7.MacLeod, C. M. & MacDonald, P. A. (2000) Trends Cognit. Sci. 4, 383–391. [DOI] [PubMed] [Google Scholar]
- 8.Neely, J. H. (1991) in Basic Processes in Reading: Visual Word Recognition, eds. Besner, D. & Humphreys, G. W. (Erlbaum, Hillsdale, NJ), pp. 264–336.
- 9.MacLeod, C. M. (1992) J. Exp. Psychol. 121, 12–14. [Google Scholar]
- 10.Besner, D. (2001) Psychonomic Bull. Rev. 8, 324–330. [DOI] [PubMed] [Google Scholar]
- 11.Dishon-Berkovits, M. & Algom, D. (2000) Mem. Cognit. 28, 1437–1449. [DOI] [PubMed] [Google Scholar]
- 12.Melara, R. D. & Algom, D. (2003) Psychol. Rev. 110, 422–471. [DOI] [PubMed] [Google Scholar]
- 13.Neely, J. H. & Kahan, T. (2000) in The Nature of Remembering: Essays in Honor of Robert G. Crowder, eds. Roediger, H. L., Nairne, J. S., Neath, I. & Surprenant, A. M. (Am. Psychological Assoc., Washington, DC).
- 14.Kuhl, J. & Kazén, M. (1999) J. Exp. Psychol. 128, 382–399. [Google Scholar]
- 15.Long, D. L. & Prat, C. S. (2002) Mem. Cognit. 30, 294–301. [DOI] [PubMed] [Google Scholar]
- 16.Raz, A. & Shapiro, T. (2002) Arch. Gen. Psychiatry 59, 85–90. [DOI] [PubMed] [Google Scholar]
- 17.MacLeod, C. M. & Sheehan, P. W. (2003) Conscious Cognit. 12, 347–353. [DOI] [PubMed] [Google Scholar]
- 18.Schatzman, M. (1980) The Story of Ruth (Putnam, New York).
- 19.Sun, S. (1994) Psychol. Sci. 17, 287–290. [Google Scholar]
- 20.Raz, A., Shapiro, T., Fan, J. & Posner, M. I. (2002) Arch. Gen. Psychiatry 59, 1155–1161. [DOI] [PubMed] [Google Scholar]
- 21.Raz, A., Landzberg, K. S., Schweizer, H. R., Zephrani, Z. R., Shapiro, T., Fan, J. & Posner, M. I. (2003) Conscious Cognit. 12, 332–346. [DOI] [PubMed] [Google Scholar]
- 22.Weitzenhoffer, A. M. & Hilgard, E. R. (1962) Stanford Hypnotic Susceptibility Scale: Form C (Consulting Psychologists Press, Palo Alto, CA).
- 23.Shor, R. & Orne, E. C. (1962) Harvard Group Scale of Hypnotic Susceptibility (Consulting Psychologists Press, Palo Alto, CA).
- 24.Kosslyn, S. M., Thompson, W. L., Costantini-Ferrando, M. F., Alpert, N. M. & Spiegel, D. (2000) Am. J. Psychiatry 157, 1279–1284. [DOI] [PubMed] [Google Scholar]
- 25.Raz, A. (2004) in Cognitive Neuroscience of Attention, ed. Posner, M. I. (Guilford, New York), pp. 420–429.
- 26.Tucker, D. M. (1993) Electroencephalogr. Clin. Neurophysiol. 87, 154–163. [DOI] [PubMed] [Google Scholar]
- 27.Scherg, M. & Berg, P. (1990) BESA: Brain Electric Source Analysis Handbook (Max-Planck Institute for Psychiatry, Munich).
- 28.Cohen, L. & Dehaene, S. (2004) NeuroImage 22, 466–476. [DOI] [PubMed] [Google Scholar]
- 29.Dehaene, S., Jobert, A., Naccache, L., Ciuciu, P., Poline, J. B., Le Bihan, D. & Cohen, L. (2004) Psychol. Sci. 15, 307–313. [DOI] [PubMed] [Google Scholar]
- 30.Price, C. J. & Devlin, J. T. (2003) NeuroImage 19, 473–481. [DOI] [PubMed] [Google Scholar]
- 31.Rainville, P., Duncan, G. H., Price, D. D., Carrier, B. & Bushnell, M. C. (1997) Science 277, 968–971. [DOI] [PubMed] [Google Scholar]
- 32.Hofbauer, R. K., Rainville, P., Duncan, G. H. & Bushnell, M. C. (2001) J. Neurophysiol. 86, 402–411. [DOI] [PubMed] [Google Scholar]
- 33.Wager, T. D., Rilling, J. K., Smith, E. E., Sokolik, A., Casey, K. L., Davidson, R. J., Kosslyn, S. M., Rose, R. M. & Cohen, J. D. (2004) Science 303, 1162–1167. [DOI] [PubMed] [Google Scholar]
- 34.Posner, M. I. & Rothbart, M. K. (1998) Philos. Trans. R. Soc. London B 353, 1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baumeister, R. F. (2004) Handbook of Self-Regulation: Research, Theory, and Applications (Guilford, New York).
- 36.Bentin, S., Mouchetant-Rostaing, Y., Giard, M. H., Echallier, J. F. & Pernier, J. (1999) J. Cognit. Neurosci. 11, 235–260. [DOI] [PubMed] [Google Scholar]
- 37.Hopfinger, J. B., Luck, S. J. & Hillyard, S. A. (2005) in The New Cognitive Neurosciences, ed. Gazzaniga, M. S. (MIT Press, Cambridge, MA), in press.
- 38.Raz, A., Lieber, B., Soliman, F., Buhle, J., Posner, J., Peterson, B. S. & Posner, M. I. (2005) NeuroImage 25, 1–7. [DOI] [PubMed] [Google Scholar]
- 39.Horton, J. E., Crawford, H. J., Harrington, G. & Downs, J. H., III (2004) Brain 127, 1741–1747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.