Abstract
Inducible costimulator (ICOS) has been suggested to perform an important role in T helper cell type 2 (Th2) responses, germinal center formation, and isotype switching. The role of ICOS in chronic Th2 responses was studied in a nematode model with the filarial parasite, Brugia malayi. Contrary to expectations, we did not observe a significant defect in IL-4-producing Th2 cells in ICOS–/– mice or in eosinophil recruitment. We also found that ICOS was not required for the differentiation of alternatively activated macrophages (AAMΦ) that express Ym1 and Fizz1. Although the production of IgE was slightly reduced in ICOS–/– mice, this was not as significant as in CD28–/– mice. In contrast to live infection, the primary response of ICOS–/– mice immunized with soluble B. malayi antigen and complete Freund's adjuvant resulted in significantly fewer IL-4-producing cells in the lymph nodes. As previously reported, we observed a defect in antibody isotype switching toward the IgG1 isotype in ICOS–/– mice during live infection. Interestingly, there was a significant enhancement of parasite-specific IgG3 isotype antibodies. CD28–/– and MHC class II–/– mice also had enhanced parasite-specific IgG3 isotype antibodies. Our results suggest that ICOS is not required to maintain a chronic cellular Th2 response. The primary role of ICOS in a chronic helminth infection could be to drive antibodies toward type 2 isotypes. T-independent antibody response to the parasite could be enhanced in the absence of costimulation and T cell help.
Keywords: allergy, costimulation, helminth, IgE, inflammation
Many new costimulatory pathways have been discovered recently that influence the properties of T cell responses (1, 2). The third member of the CD28/CTLA-4 family to be identified is inducible costimulator (ICOS) (3). As its name suggests, ICOS is induced on activated T cells. ICOS specifically binds to B7-h (ICOSL, B7RP1, B7-H2, GL50, and LICOS) only (4). The expression of ICOS on activated T cells is consistent with observations that it plays a costimulatory role on activated T cells, enhancing the proliferation and secretion of cytokines (2, 5). Many studies have suggested that ICOS plays a particularly important role in T helper cell type 2 (Th2) responses. ICOS was found to be more highly expressed on Th2 cells, compared with Th1 cells (6). Although earlier studies suggest that the main role for ICOS was in Th2 responses (6–9), later studies showed that ICOS blockade or deficiency inhibits Th1- and Th2-associated responses (10, 11). In some models of experimental asthma, ICOS blockade or deficiency can help attenuate disease or reduce eosinophilia and IgE levels (7, 12, 13). Analysis of mice deficient in either ICOS (7, 14, 15) or B7h (13, 16, 17) also has highlighted a critical role for this costimulatory interaction in promoting antibody isotype switching, affinity maturation, and germinal center formation. Homozygous mutation of ICOS in human patients leads to an immunodeficiency syndrome characterized by the severe reduction in all Ig subclasses (18). More recently, CD28–/–ICOS–/– double-knockout mice were found to have defects in T-dependent antibody responses that extend beyond those observed in single knockouts (19).
Immunization of mice deficient for ICOS and B7h with protein antigens and adjuvants has highlighted a defect in IL-4 production (7, 8, 13, 16, 17). Parasitic nematodes are classic inducers of a strong Th2 response (20, 21). In previous studies of acute infection with the nematode parasites Nippostrongylus brasiliensis and Trichinella spiralis, ICOS blockade has been shown to reduce Th2 cytokine production (9, 22). However, the role of ICOS in a chronic response, which is typical of most human nematode infections, has not been examined. Brugia malayi is a parasitic nematode that can cause the human disease lymphatic filariasis. A key feature of infection with this parasite is the long period of coexistence between the adult stage of the parasite and the host, which is accompanied by a typical type 2 cytokine response as well as the presence of hyporesponsive, or anergic, T cells in the infected host (21, 23). Implantation of the adult stage of the filarial nematode, B. malayi, into mice results in similar characteristics to the chronic asymptomatic stage of this disease. The adult parasite induces a Th2 response that is characterized by elevated IgE and IgG1 production (20), an abundance of eosinophils (24), and alternatively activated macrophages (25) that express the IL-4-dependent genes Ym1 and Fizz1 (26). In this study, we have used this model to examine the role of ICOS in the Th2 response against a chronic nematode infection. To our surprise, we found that ICOS was not required to maintain a chronic cellular Th2 response.
Materials and Methods
Animals. C57BL/6 mice were obtained from The Jackson Laboratory. ICOS–/– mice (8) and CD28–/– (27), both on the C57BL/6 background, were bred in-house. 4GET mice (28), which have a bicistronic knock-in IL-4 gene linked via an internal ribosomal entry site with enhanced green fluorescent protein, were backcrossed five generations onto the C57BL/6 background before being crossed onto the ICOS–/– background. Littermate ICOS–/– and ICOS+/– mice heterozygous for the 4GET allele were used in this study. The ICOS–/– phenotype was determined by FACS using anti-ICOS antibody.
Parasite Model and Antigen Preparation and Immunization. B. malayi adult parasites were obtained from infected gerbils purchased from TRS Laboratories (Athens, GA). Adult worms were removed from the peritoneal cavity of gerbils and washed in RPMI medium 1640, and five live adult B. malayi females were surgically implanted into the peritoneal cavity of mice. After 3–6 weeks, mice were killed by cardiac puncture, and peritoneal exudate cells (PEC) were harvested by thorough washing of the peritoneal cavity with RPMI medium 1640. PBS-soluble extracts of mixed adult B. malayi antigen (BMA) were prepared by homogenizing each worm population in sterile ice-cold PBS by using a glass homogenizer. The homogenates were centrifuged, and the supernatants containing PBS-soluble antigens were collected and frozen for subsequent use. Mice were immunized with BMA in complete Freund's adjuvant (CFA) (GIBCO) or with PBS in CFA. Each mouse received a total dose of 10 μg of nematode extract emulsified with CFA in a 1:1 mixture and in a final volume of 200 μl (50 ml per site). Ten days after immunization, the popliteal and inguinal lymph nodes were removed and analyzed by FACS.
Real-Time PCR. Total RNA was isolated by using TRI reagent (Sigma), and cDNA was synthesized by using SuperScript II (Invitrogen). Relative quantification of the genes of interest was measured by real-time PCR using the GeneAmp 5700 sequence detection system (Applied Biosystems). Serial 1:10 dilutions of a positive control were used for a standard curve. Data were converted into arbitrary units, and the expression level was standardized by comparison to GAPDH and expressed as a ratio. The sequences of the primers used are available upon request.
FACS and Cytospin Analysis. α-F4/80 was from Caltag (Burlingame, CA), and α-Ly6G, α-CD4, and α-B220 were from eBioscience (San Diego). For FACS analysis, 2–5 × 105 cells per well) were preincubated with unlabeled α-CD16/32 (24G2) and then incubated with the relevant antibodies. Cytocentrifuge preparations of 1 × 105 cells were made by using a Cytospin 3 (Shandon, Pittsburgh). Cytospins were air-dried, fixed in methanol, stained with Giemsa (Sigma), and examined with a microscope. More than 300 cells per cytospin were counted from randomly selected fields.
Cellular Proliferation and Antigen Presentation Assays. For splenocyte assays, single-cell suspensions were prepared from individual mice in complete RPMI medium 1640 and 5 × 105 cells per well were stimulated with 5 μg/ml BMA for 72 h, after which supernatants were collected and analyzed by ELISA. Antigen-specific cytokine production was determined by subtracting control culture values from antigen cultures. The results were normalized between experiments by conversion to a percentage of the mean of WT control mice (at 100%) for each experiment.
Cytokine and Serum Antibody Assays. Cytokine capture ELISAs were performed with 11B11 (anti-IL-4) and R46A2 (anti-IFN-γ) as capture antibodies and biotin-labeled antibodies (Pharmingen) detected with Streptavidin-HRP (Jackson ImmunoResearch) with reference to standard curves of known amounts of IFN-γ and recombinant IL-4 (Genzyme). Isotype-specific antibody ELISAs against parasite antigen were performed as described in ref. 29 with 5 μg of BMA as the coating antigen. Horseradish peroxidase-conjugated goat anti-mouse IgG1, IgG2a, IgG2b, and IgG3 polyclonal antibodies (Southern Biotechnology Associates) were used for detection. To measure serum IgE, we used a sandwich ELISA with clone R35-72 (BD Pharmingen) as capture antibody and biotinylated clone R35-92 for detection and purified IgEa (clone C48-2) as a standard.
Statistical Analysis. Unless otherwise stated, data between groups were compared with a two-tailed unpaired Student t test by using prism 3.0 software (GraphPad, San Diego) with a normality test. ***, P < 0.0005; **, P < 0.005. Error bars always show variation between individual mice.
Results
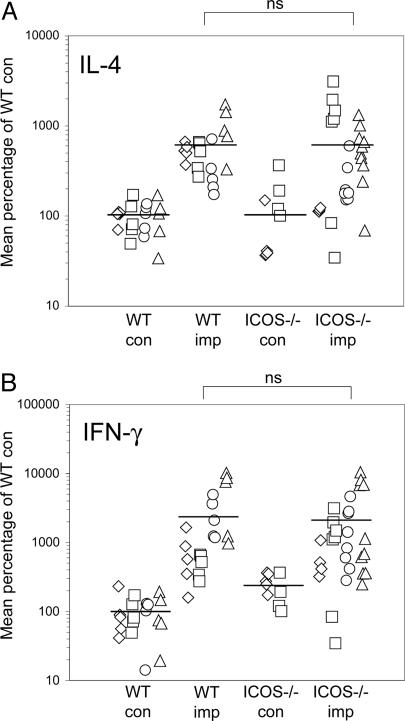
ICOS and Effector Th2 Function. To investigate the role of ICOS in a chronic Th2 response, we implanted the filarial nematode parasite B. malayi into the peritoneal cavity of ICOS–/– mice and compared their response with that of WT C57BL/6 mice. The parasites can survive in the peritoneal cavity of the asymptomatic host for as long as 6 months. We terminated the experiments after 4–6 weeks when the response had settled into a steady-state characteristic of an asymptomatic Th2 response, with the recruitment of eosinophils (24) and alternatively activated macrophages (25) into the peritoneal cavity and the production of Th2 cytokines in splenocyte assays (30). Surprisingly, analysis of multiple experiments showed that there were no consistently significant differences in the production of IL-4 by ICOS–/–-infected mice, compared with WT mice (Fig. 1A). There were some mice among the ICOS–/– groups that did not produce any IL-4 at all in response to stimulation of splenocytes with soluble BMA; however, the majority of ICOS–/– mice produced as much IL-4 as did WT mice. Assays for IFN-γ also showed no significant differences between WT and ICOS–/– mice (Fig. 1B).
Fig. 1.
Normal cytokine responses in chronically infected ICOS–/– mice. (A) B. malayi parasites were implanted into ICOS–/– mice and C57BL/6 mice for 4–6 weeks, and splenocyte assays were analyzed for production of IL-4 (A) and IFN-γ (B). The data shown are compiled from four separate experiments and are indicated by different symbols for separate experiments. Results were normalized between experiments by conversion to a percentage of the mean of WT control mice (at 100%).
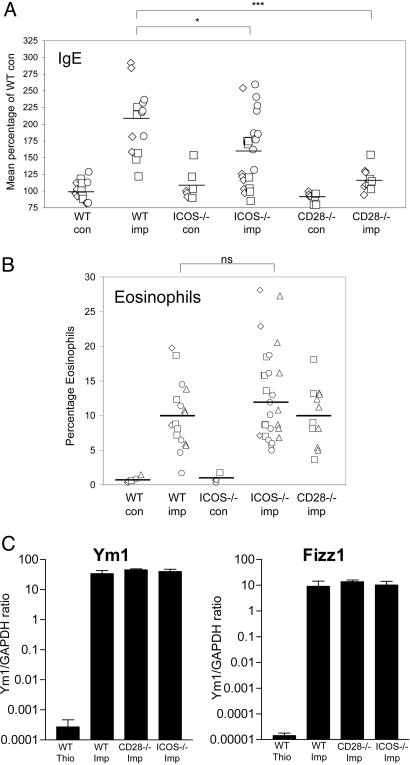
Previous studies have shown that either ICOS or B7h deficiency has an effect on production of IgE and eosinophil recruitment (7, 13). It has been shown that implantation with adult B. malayi induces a robust IgE response and the recruitment of eosinophils into the peritoneal cavity (24). When we examined the total levels of IgE in serum, we found that there was a slight decrease in parasite-implanted ICOS–/– mice, compared with WT mice (Fig. 2A). However, this decrease was not as pronounced as the IgE defect that we observed in parasite-implanted CD28–/– mice (Fig. 2A). Similar to our analysis of cytokine production, there seemed to be some ICOS–/– mice that produced control levels of IgE; however, a significant number of parasite-implanted ICOS–/– mice also produced IgE at levels similar to implanted WT mice. When we examined the number of eosinophils that had been recruited into the peritoneal cavity, we observed no differences between the parasite-implanted WT mice, compared with either ICOS–/– or CD28–/– mice (Fig. 2B).
Fig. 2.
Serum IgE, eosinophil recruitment, and alternative macrophage activation in ICOS–/– mice and CD28–/– mice. (A) Total serum IgE was measured by ELISA from mice that had been implanted with B. malayi parasites. (B) The percentage of eosinophils among the peritoneal exudates cells was determined by cytospin analysis. (C) Expression of AAMΦ marker genes Ym1 and Fizz1 was determined by real-time PCR analysis of adherent macrophages from parasite-implanted PEC. Control mice were injected with 4% thioglycollate. The data shown are representative of three separate experiments. The error bars show the standard deviation between individual mice.
We have previously shown that the recruitment of alternatively activated macrophages is a key feature of chronic infection (25). We asked whether the costimulatory molecules CD28 and ICOS were required to provide help for AAMΦ differentiation by characterizing macrophages recruited in ICOS–/– and CD28–/– mice after infection (Fig. 2C). Real-time PCR was used to look for the expression of AAMΦ marker genes such as Ym1 and Fizz1, which we and others have previously shown to serve as useful genetic markers to identify AAMΦ (26, 31). Interestingly, macrophages recruited by parasites in CD28- and ICOS-deficient mice are indistinguishable from macrophages recruited in control B6 mice in terms of expressing Ym1 and Fizz1 (Fig. 2C).
Peripheral Effector T Cells and ICOS. The observation that expression of B7h can be enhanced by inflammatory cytokines on endothelial cells led to the hypothesis that ICOS could play a role in activating effector T cells as they migrate into peripheral inflamed tissues (32). Because there were no differences in the Th2 response in the spleen, we asked whether there are fewer Th2 cells that are recruited into peripheral tissues where the parasite resides in ICOS–/– mice. We crossed the ICOS–/– to IL-4 GFP reporter mice (4GET mice) for a sensitive way of identifying IL-4-producing T cells in the periphery. There were very few IL-4-producing CD4+ cells found in the spleen (<0.1%) when the animals were killed (4–6 weeks) (data not shown). Most of the IL-4-producing CD4+ cells appear to have migrated to the peritoneal cavity.
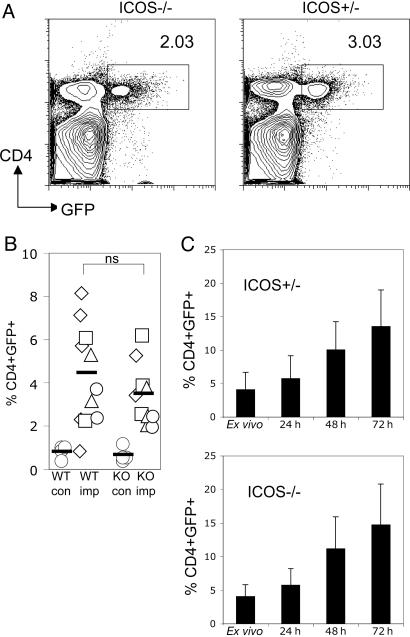
Ex vivo analysis of PEC from B. malayi-implanted 4GET/ICOS+/– mice vs. 4GET/ICOS–/– mice revealed very similar proportions of IL-4+CD4+ cells between these two groups (Fig. 3 A and B). Gating specifically on CD4+ cells in the peritoneal cavity showed that a similar (if not increased) percentage of CD4+ cells in the ICOS–/– mice, compared with the ICOS+/– mice expressed the IL-4 transcript (data not shown). When we cultured the PEC from implanted mice by incubating them with 5 μg/ml soluble BMA in vitro, we observed a similar expansion of CD4+IL-4+ cells in ICOS+/– vs. ICOS–/– mice (Fig. 3C). These results show that ICOS deficiency did not influence the recruitment of IL-4-producing Th2 cells into peripheral tissues or affect their ability to expand upon reencountering parasite antigen.
Fig. 3.
Normal recruitment of effector Th2 cells in peripheral tissues of 4GET/ICOS–/– mice. (A) Representative FACS plot showing the percentage of IL-4/GFP+CD4+ cells recruited in the peritoneal cavity 4–6 weeks after parasite implantation. (B) Compiled data from three experiments for the proportion of IL-4/GFP+CD4+ cells that are present in the peritoneal cavity after parasite implantation. (C) PEC from parasite-implanted mice were cultured with soluble antigen (BMA) and collected at separate time points to examine the expansion of peripheral effector IL-4/GFP+CD4+ cells after restimulation with parasite antigen. The data shown are representative of two separate experiments with similar results, and the error bar represents variation between individual mice of each group.
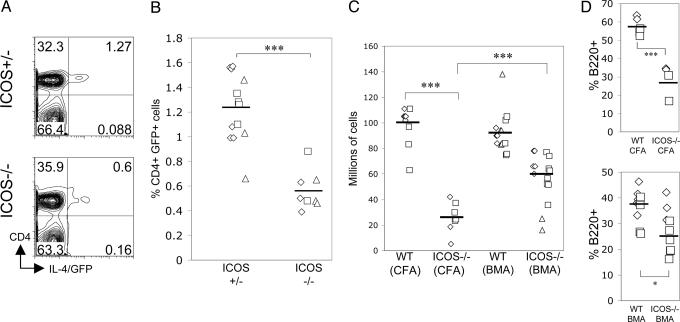
ICOS Deficiency Affects Primary Expansion of Th2 Cells. To determine whether the normal IL-4 response that we observe in ICOS–/– mice in response to B. malayi was due to the chronic nature of our parasite nematode model or due to the complex nature of the parasite antigen, we immunized WT mice and ICOS–/– mice with soluble BMA with CFA. It has been shown that immunization with BMA and CFA can induce a very robust expansion of IL-4-producing Th2 cells 10 days after immunization (33). In these experiments, we observed that 4GET/ICOS–/– mice had a significantly lower percentage of IL-4/GFP+CD4+ cells in the lymph node 10 days after immunization (Fig. 4 A and B). Combined with the fewer overall numbers of lymph node cells in total (Fig. 4C), this result suggested that ICOS promotes the expansion of IL-4-producing cells in the lymph nodes during the primary expansion phase after immunization.
Fig. 4.
Reduced expansion of IL-4+ Th2 cells and B cells in the primary response of ICOS–/– mice to soluble BMA. (A) 4GET/ICOS+/– and 4GET/ICOS–/– mice were immunized in the footpad with 10 μg of BMA in CFA, and the popliteal lymph node cells were removed 10 days after immunization and analyzed by FACS. Representative FACS plot from two individual mice are shown. (B) Data compiled from three separate experiments showing the percentage of IL-4/GFP+CD4+ cells in the popliteal lymph node 10 days after immunization. (C) WT mice and ICOS–/– mice were immunized with CFA and PBS or CFA and BMA. The number of popliteal lymph node cells recovered 10 days after immunization is compiled from three separate experiments. (D) The cellular composition of the recovered popliteal lymph node cells was determined by FACS 10 days after immunization. The proportion of B cells is shown from two separate experiments.
We noticed that ICOS–/– mice immunized with CFA and PBS had much smaller lymph nodes with very significantly decreased cellularity, compared with WT mice (Fig. 4C). Although ICOS–/– mice immunized with BMA and CFA also had significantly reduced cell numbers, compared with BMA-immunized WT mice, this reduction was less pronounced than with CFA alone (Fig. 4C). Lymph nodes from BMA-immunized ICOS–/– mice had significantly more cells than did PBS/CFA-immunized ICOS–/– mice. This observation suggested that BMA could partially rescue the cell-expansion defect observed in immunized ICOS–/– mice. When we examined the cellular compartments in the lymph nodes of immunized mice, we found that the reduced cell numbers were mainly in the B220+ B cell compartment (Fig. 4D). The difference in proportion of B220+ cells was less significant in ICOS–/– mice immunized with BMA and CFA, compared with CFA and PBS (Fig. 4D). These results suggest that BMA has adjuvant properties that can expand the B cell compartment more efficiently than CFA alone in the absence of ICOS. This observation could be due to the stimulation of T-independent antibody production.
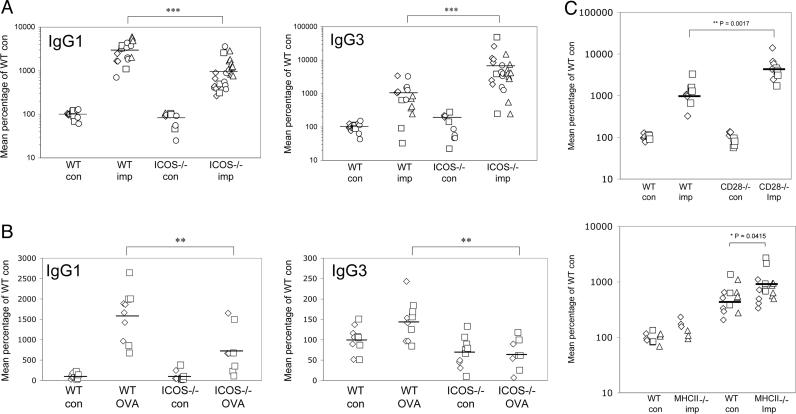
ICOS Deficiency and Antibody Isotype Switching. When we compared the antibody response of ICOS–/– mice with that of WT mice 4–6 weeks after being implanted with the parasites, we found an expected decrease in parasite-specific IgG1 antibodies (Fig. 5A). Interestingly, we observed a very significant (>3-fold) elevation of parasite-specific IgG3 antibodies in implanted ICOS–/– mice (Fig. 5A). Nematode parasites are complex multicellular organisms that are composed of T-dependent protein antigens as well as T-independent antigens such as polysaccharides (33). IgG3 is the predominant IgG subclass in responses against T cell-independent type 2 antigens such as polysaccharides found in bacterial cell walls. To determine whether the enhancement of IgG3 responses could be observed against a protein antigen, we compared the antibody responses of ICOS–/– mice and WT mice to a T-dependent protein antigen ovalbumin immunized with CFA, which drives a strong Th1 response. Consistent with previous studies, we found that both the IgG1 and the IgG3 response to ovalbumin was significantly decreased in the ICOS–/– mice 28–30 days after immunization (Fig. 5B).
Fig. 5.
Reduced IgG1 and enhanced IgG3 in infected ICOS–/– mice. (A) Sera from parasite-implanted mice were assayed for parasite-specific IgG1 and IgG3 isotypes by ELISA. Results were normalized between experiments by conversion to a percentage of the mean of WT control mice (at 100%). Data shown are compiled from four separate experiments. (B) Sera from mice immunized with ovalbumin (OVA) and CFA s.c. were analyzed for OVA-specific IgG1 and IgG3 28–30 days after immunization. The data shown are compiled from two separate experiments. (C) Sera from parasite-implanted CD28–/– and MHC class II–/– mice were assayed for parasite-specific IgG3 by ELISA. The data shown are compiled from two separate experiments.
The increase in B. malayi-specific IgG3 antibodies suggested that there was an increase in T-independent antibody production in the absence of the ICOS–B7h interaction. To investigate this further, we implanted parasites into the peritoneal cavity of CD28–/– mice, which also have a defect in antibody isotype switching (27). We found that CD28–/– mice also make more IgG3 antibodies against the parasite (Fig. 5C). To determine whether this effect is mediated by the absence of MHC class II-restricted CD4+ T cell help, we implanted the parasites into MHC class II–/– mice. The level of IgG3 antibodies against B. malayi also was significantly higher in MHC class II–/– mice, compared with WT mice, although not as markedly as in the CD28- and ICOS-deficient animals. Parasite-specific IgG1 antibody responses were almost completely absent in CD28–/– and MHC class II–/– mice (data not shown).
These results are consistent with the original studies of B cell function in MHC class II–/– mice (34). Immunization of class II-deficient mice with TNP-Ficoll, a type 2 thymic-independent antigen (TI2), induced higher levels of TNP-specific IgG1, IgG2a, IgG2b, and IgG3 antibodies, compared with control (35). Indeed, the immunization of both ICOS- and B7h-deficient mice also have shown signs of elevated IgG3 responses to TNP-Ficoll (7, 16). These results suggest that during an infection with a nematode parasite that is composed of a mixture of T-dependent and T-independent antigens, costimulatory molecules CD28 and ICOS could play a role in shifting the balance of isotypes toward the production of T-dependent antigens, presumably during T cell/B cell interactions in the germinal center (36).
Discussion
By comparing the immune responses of WT and ICOS–/– mice against a chronic tissue-dwelling nematode parasite, B. malayi, in multiple experiments, we have shown that ICOS is not required for the establishment or maintenance of a chronic inflammatory IL-4 response either in the spleen as a secondary lymphoid organ or the tertiary peripheral tissues as in the peritoneal cavity. This surprising observation was in contrast to predictions based on other studies demonstrating an important role for ICOS in Th1 and Th2 responses during infection. The role of ICOS has been examined in infection models of Leishmania mexicana (11), Listeria monocytogenes (37), Toxoplasma gondii (38), Schistosoma mansoni (39), N. brasiliensis (9), Trichinella spiralis (22), and several different viruses (9, 40). In many of these studies, ICOS has appeared as a positive regulator for both Th1 and Th2 responses but not for CD8+ cytotoxic T lymphocytes. Although studies with S. mansoni (39) and Toxoplasma gondii (38) infection have suggested a role of ICOS costimulation during effector T cell activation, we have not observed any differences in effector Th2 responses in the peripheral tissues of 4GET/ICOS–/– mice. There also were no differences in the recruitment of eosinophils, or AAMΦ, both hallmarks of an effector Th2 responses.
We think that the main difference between our studies and previous studies is the observation of an acute phase response vs. a chronic phase response. Previous studies have suggested a synergistic role for ICOS and CD28 (9, 19, 38). A chronic phase response might allow time for CD28 to compensate for the lack of ICOS function. Consistent with previous studies, we observed fewer IL-4-producing cells in the 4GET/ICOS–/– mice induced during the acute phase in the local lymph nodes 10 days after immunization with the Th2-driving soluble BMA. ICOS could be promoting T cell/B cell interactions during the primary expansion of lymphocytes early on in an immune response. This interaction could generate a positive-feedback loop that leads to an increasing number of germinal-center B cells as well as IL-4-producing T cells in the lymph nodes. These observations are in agreement with previous studies showing that ICOS plays an important role in the clonal expansion of T cells during the primary expansion phase of an immune response (13, 41). Our studies are consistent with the hypothesis that ICOS plays a more important role in expanding effector T cells than in enhancing their effector function. It also is possible that the extremely strong Th2 stimuli as exerted by a nematode parasite could be capable of overcoming ICOS deficiency. However, observations that effector Th2 responses are deficient in the absence of ICOS–B7h interactions during acute infection by the gastrointestinal parasites N. brasiliensis (9) and Trichinella spiralis (22) suggest that this is not the case. It should be noted, however, that the reductions in IL-4 responses observed in these studies were quite modest (20–30%) although significant.
Certainly, the strongest effects in the absence of ICOS–B7h interactions have consistently been on class-switched antibody responses. IgG1 responses have always been diminished, and we found that this also was the case for B. malayi implantation. Although we observed that the total serum IgE levels were significantly reduced in ICOS–/– mice, this reduction was not as dramatic as that in the CD28–/– mice and also not as significant as that in IgE observed in infection with Leishmania mexicana (11). Interestingly, we also observed enhanced parasite-specific IgG3 antibody isotypes in the ICOS–/– mice that could be directed at T-independent antigens. IgG3 is the main isotype in antibody responses against polysaccharides and other T cell-independent type 2 antigens in mice, the human equivalent being IgG2 (42). Antibodies of this isotype are considered to be especially important against bacterial infections. Mice that have the γ3 region of the Ig gene knocked out are more susceptible to pneumococcal infection (43), and children deficient for IgG2 are also more susceptible to pneumococcal infection in the sinus and pulmonary system (44). Our results suggest that in the absence of the ICOS–B7h interaction, B cells that are activated are biased toward production of T-independent antibodies of this isotype. The defect in T cell help for B cells during an infection with pathogens that are composed of a mixture of protein and carbohydrate antigens could lead to more B cells producing T cell-independent carbohydrate antigens of the IgG3 isotype instead of isotypes against T cell-dependent protein antigens. Further studies will be needed to test this hypothesis.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute and the Wellcome Trust. P.L. is a recipient of the Wellcome International Research Fellowship. X.Z. is a fellow of the Cancer Research Institute.
Author contributions: P.L. and J.P.A. designed research; P.L., X.Z., L.H., and R.W. performed research; X.Z. and R.M.L. contributed new reagents/analytic tools; P.L. and J.E.A. analyzed data; and P.L. wrote the paper.
Abbreviations: ICOS, inducible costimulator; Th, T helper cell; PEC, peritoneal exudate cells; BMA, B. malayi antigen; CFA, complete Freund's adjuvant; AAMΦ, alternatively activated macrophages.
References
- 1.Carreno, B. M. & Collins, M. (2002) Annu. Rev. Immunol. 20, 29–53. [DOI] [PubMed] [Google Scholar]
- 2.Greenwald, R. J., Freeman, G. J. & Sharpe, A. H. (2004) Annu. Rev. Immunol. 23, 515–548. [DOI] [PubMed] [Google Scholar]
- 3.Hutloff, A., Dittrich, A. M., Beier, K. C., Eljaschewitsch, B., Kraft, R., Anagnostopoulos, I. & Kroczek, R. A. (1999) Nature 397, 263–266. [DOI] [PubMed] [Google Scholar]
- 4.Yoshinaga, S. K., Whoriskey, J. S., Khare, S. D., Sarmiento, U., Guo, J., Horan, T., Shih, G., Zhang, M., Coccia, M. A., Kohno, T., et al. (1999) Nature 402, 827–832. [DOI] [PubMed] [Google Scholar]
- 5.Grimbacher, B., Warnatz, K. & Peter, H. H. (2003) Curr. Opin. Allergy Clin. Immunol. 3, 409–419. [DOI] [PubMed] [Google Scholar]
- 6.Coyle, A. J., Lehar, S., Lloyd, C., Tian, J., Delaney, T., Manning, S., Nguyen, T., Burwell, T., Schneider, H., Gonzalo, J. A., et al. (2000) Immunity 13, 95–105. [DOI] [PubMed] [Google Scholar]
- 7.Tafuri, A., Shahinian, A., Bladt, F., Yoshinaga, S. K., Jordana, M., Wakeham, A., Boucher, L. M., Bouchard, D., Chan, V. S., Duncan, G., et al. (2001) Nature 409, 105–109. [DOI] [PubMed] [Google Scholar]
- 8.Dong, C., Juedes, A. E., Temann, U. A., Shresta, S., Allison, J. P., Ruddle, N. H. & Flavell, R. A. (2001) Nature 409, 97–101. [DOI] [PubMed] [Google Scholar]
- 9.Kopf, M., Coyle, A. J., Schmitz, N., Barner, M., Oxenius, A., Gallimore, A., Gutierrez-Ramos, J. C. & Bachmann, M. F. (2000) J. Exp. Med. 192, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rottman, J. B., Smith, T., Tonra, J. R., Ganley, K., Bloom, T., Silva, R., Pierce, B., Gutierrez-Ramos, J. C., Ozkaynak, E. & Coyle, A. J. (2001) Nat. Immunol. 2, 605–611. [DOI] [PubMed] [Google Scholar]
- 11.Greenwald, R. J., McAdam, A. J., Van der Woude, D., Satoskar, A. R. & Sharpe, A. H. (2002) J. Immunol. 168, 991–995. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalo, J. A., Tian, J., Delaney, T., Corcoran, J., Rottman, J. B., Lora, J., Al-garawi, A., Kroczek, R., Gutierrez-Ramos, J. C. & Coyle, A. J. (2001) Nat. Immunol. 2, 597–604. [DOI] [PubMed] [Google Scholar]
- 13.Mak, T. W., Shahinian, A., Yoshinaga, S. K., Wakeham, A., Boucher, L. M., Pintilie, M., Duncan, G., Gajewska, B. U., Gronski, M., Eriksson, U., et al. (2003) Nat. Immunol. 4, 765–772. [DOI] [PubMed] [Google Scholar]
- 14.Dong, C., Temann, U. A. & Flavell, R. A. (2001) J. Immunol. 166, 3659–3662. [DOI] [PubMed] [Google Scholar]
- 15.McAdam, A. J., Greenwald, R. J., Levin, M. A., Chernova, T., Malenkovich, N., Ling, V., Freeman, G. J. & Sharpe, A. H. (2001) Nature 409, 102–105. [DOI] [PubMed] [Google Scholar]
- 16.Wong, S. C., Oh, E., Ng, C. H. & Lam, K. P. (2003) Blood 102, 1381–1388. [DOI] [PubMed] [Google Scholar]
- 17.Nurieva, R. I., Mai, X. M., Forbush, K., Bevan, M. J. & Dong, C. (2003) Proc. Natl. Acad. Sci. USA 100, 14163–14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimbacher, B., Hutloff, A., Schlesier, M., Glocker, E., Warnatz, K., Drager, R., Eibel, H., Fischer, B., Schaffer, A. A., Mages, H. W., et al. (2003) Nat. Immunol. 4, 261–268. [DOI] [PubMed] [Google Scholar]
- 19.Suh, W. K., Tafuri, A., Berg-Brown, N. N., Shahinian, A., Plyte, S., Duncan, G. S., Okada, H., Wakeham, A., Odermatt, B., Ohashi, P. S. & Mak, T. W. (2004) J. Immunol. 172, 5917–5923. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence, R. A. & Devaney, E. (2001) Parasite Immunol. (Oxf.) 23, 353–361. [DOI] [PubMed] [Google Scholar]
- 21.Maizels, R. M. & Yazdanbakhsh, M. (2003) Nat. Rev. Immunol. 3, 733–744. [DOI] [PubMed] [Google Scholar]
- 22.Scales, H. E., Ierna, M. X., Gutierrez-Ramos, J. C., Coyle, A. J., Garside, P. & Lawrence, C. E. (2004) Eur. J. Immunol. 34, 2854–2862. [DOI] [PubMed] [Google Scholar]
- 23.Maizels, R. M., Sartono, E., Kurniawan, A., Partono, F., Selkirk, M. E. & Yazdanbakhsh, M. (1995) Parasitol. Today 11, 50–56. [DOI] [PubMed] [Google Scholar]
- 24.Falcone, F. H., Loke, P., Zang, X., MacDonald, A. S., Maizels, R. M. & Allen, J. E. (2001) J. Immunol. 167, 5348–5354. [DOI] [PubMed] [Google Scholar]
- 25.Loke, P., MacDonald, A. S., Robb, A., Maizels, R. M. & Allen, J. E. (2000) Eur. J. Immunol. 30, 2669–2678. [DOI] [PubMed] [Google Scholar]
- 26.Loke, P., Nair, M. G., Parkinson, J., Guiliano, D., Blaxter, M. & Allen, J. E. (2002) BMC Immunol. 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahinian, A., Pfeffer, K., Lee, K. P., Kundig, T. M., Kishihara, K., Wakeham, A., Kawai, K., Ohashi, P. S., Thompson, C. B. & Mak, T. W. (1993) Science 261, 609–612. [DOI] [PubMed] [Google Scholar]
- 28.Mohrs, M., Shinkai, K., Mohrs, K. & Locksley, R. M. (2001) Immunity 15, 303–311. [DOI] [PubMed] [Google Scholar]
- 29.Le Goff, L., Loke, P., Ali, H. F., Taylor, D. W. & Allen, J. E. (2000) Infect. Immun. 68, 2513–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDonald, A. S., Loke, P., Martynoga, R., Dransfield, I. & Allen, J. E. (2003) Med. Microbiol. Immunol. 192, 33–40. [DOI] [PubMed] [Google Scholar]
- 31.Raes, G., De Baetselier, P., Noel, W., Beschin, A., Brombacher, F. & Hassanzadeh Gh, G. (2002) J. Leukocyte Biol. 71, 597–602. [PubMed] [Google Scholar]
- 32.Khayyamian, S., Hutloff, A., Buchner, K., Grafe, M., Henn, V., Kroczek, R. A. & Mages, H. W. (2002) Proc. Natl. Acad. Sci. USA 99, 6198–6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tawill, S., Le Goff, L., Ali, F., Blaxter, M. & Allen, J. E. (2004) Infect. Immun. 72, 398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grusby, M. J. & Glimcher, L. H. (1995) Annu. Rev. Immunol. 13, 417–435. [DOI] [PubMed] [Google Scholar]
- 35.Markowitz, J. S., Rogers, P. R., Grusby, M. J., Parker, D. C. & Glimcher, L. H. (1993) J. Immunol. 150, 1223–1233. [PubMed] [Google Scholar]
- 36.Liang, L., Porter, E. M. & Sha, W. C. (2002) J. Exp. Med. 196, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittrucker, H. W., Kursar, M., Kohler, A., Yanagihara, D., Yoshinaga, S. K. & Kaufmann, S. H. (2002) J. Immunol. 169, 5813–5817. [DOI] [PubMed] [Google Scholar]
- 38.Villegas, E. N., Lieberman, L. A., Mason, N., Blass, S. L., Zediak, V. P., Peach, R., Horan, T., Yoshinaga, S. & Hunter, C. A. (2002) J. Immunol. 169, 937–943. [DOI] [PubMed] [Google Scholar]
- 39.Rutitzky, L. I., Ozkaynak, E., Rottman, J. B. & Stadecker, M. J. (2003) Infect. Immun. 71, 4040–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertram, E. M., Tafuri, A., Shahinian, A., Chan, V. S., Hunziker, L., Recher, M., Ohashi, P. S., Mak, T. W. & Watts, T. H. (2002) Eur. J. Immunol. 32, 3376–3385. [DOI] [PubMed] [Google Scholar]
- 41.Smith, K. M., Brewer, J. M., Webb, P., Coyle, A. J., Gutierrez-Ramos, C. & Garside, P. (2003) J. Immunol. 170, 2310–2315. [DOI] [PubMed] [Google Scholar]
- 42.Mond, J. J., Lees, A. & Snapper, C. M. (1995) Annu. Rev. Immunol. 13, 655–692. [DOI] [PubMed] [Google Scholar]
- 43.McLay, J., Leonard, E., Petersen, S., Shapiro, D., Greenspan, N. S. & Schreiber, J. R. (2002) J. Immunol. 168, 3437–3443. [DOI] [PubMed] [Google Scholar]
- 44.Umetsu, D. T., Ambrosino, D. M., Quinti, I., Siber, G. R. & Geha, R. S. (1985) N. Engl. J. Med. 313, 1247–1251. [DOI] [PubMed] [Google Scholar]