Abstract
Nephrin is a cell surface receptor of the Ig superfamily that localizes to slit diaphragms, the specialized junctions between the interdigitating foot processes of the glomerular epithelium (podocytes) in the kidney. Mutations in the NPHS1 gene encoding nephrin lead to proteinuria and congenital nephrotic syndrome, indicating that nephrin is essential for normal glomerular development and function. To identify nephrin-binding proteins, we performed mass spectrometry on proteins obtained from pull-down assays with GST-nephrin cytoplasmic domain. Nephrin specifically pulled down six proteins from glomerular lysates, MAGI-2/S-SCAM (membrane-associated guanylate kinase inverted 2/synaptic scaffolding molecule), IQGAP1 (IQ motif-containingGTPase-activatingprotein1),CASK(calcium/calmodulin-dependent serine protein kinase), α-actinin, αII spectrin, and βII spectrin. All of these scaffolding proteins are often associated with cell junctions. By immunofluorescence these proteins are expressed in glomerular epithelial cells, where they colocalize with nephrin in the foot processes. During glomerular development, IQGAP1 is expressed in the junctional complexes between the earliest identifiable podocytes, MAGI-2/S-SCAM is first detected in junctional complexes in podocytes after their migration to the base of the cells. Thus, the nephrin–slit diaphragm protein complex contains a group of scaffolding proteins that function to connect junctional membrane proteins to the actin cytoskeleton and signaling cascades. Despite their special morphology and function, there is considerable compositional similarity between the podocyte slit diaphragm and typical junctional complexes of other epithelial cells.
Keywords: cell adhesion molecules, cell–cell junctions, glomerular epithelial cell, podocyte, slit diaphragm
Slit diaphragms are specialized cell adhesion structures found in the glomerular epithelium (podocytes), where they attach the adjoining foot processes to one another and are essential for glomerular ultrafiltration. Slit diaphragms differentiate from typical junctional complexes during development (1) and share similarities with adherens and tight junctions (1–3). In nephrotic syndrome, which is characterized by proteinuria, the foot process architecture of mature podocytes is lost, and the slit diaphragms are replaced by tight junctions comparable to those found in developing glomeruli (4–6).
Slit diaphragms, like other adhering junctions, express Ig and cadherin superfamily cell adhesion receptors. Nephrin (7) and its homologue Neph1 (8) are members of the Ig superfamily, and nephrin has been suggested to form the framework of the slit diaphragm by means of homophilic interactions between podocytes (9, 10). The classical cadherin P-cadherin (2, 11), another nonclassical cadherin superfamily member FAT (12), and the tight junction protein ZO-1 (6, 13, 14) are also localized at the slit diaphragm.
Nephrin was identified as crucial for glomerular function because it is mutated in the congenital nephrotic syndrome of the Finnish type (7). Subsequently it was shown that mutations in several other slit diaphragm-associated proteins, including Neph1 (8), podocin (15), CD2-associated protein (16), and FAT (17), lead to loss of podocyte foot process architecture and development of nephrotic syndrome. In addition, mutations in α-actinin-4 (18) and α3β1 integrin (19), which attach podocytes to the glomerular basement membrane, and in podocalyxin (20), which is located on the apical domain of podocytes (21), can lead to the same phenotype. The fact that these proteins localize to different subcellular compartments of podocytes and have diverse functions illustrates the complexity of the regulation of podocyte morphology and glomerular function.
We have previously shown that the cytoplasmic domain of nephrin forms a complex with cadherins (22). In this study, we have identified several adherens and tight junction-associated proteins that interact with the intracellular domain of nephrin as components of the nephrin multiprotein complex.
Materials and Methods
Materials and Antibodies. Chemical reagents were from Sigma or Fisher Biotech, and detergents were from CalBiochem. Kodak Biomax MR film was from Fisher Biotech. Rabbit anti-CASK (calcium/calmodulin-dependent serine protein kinase) IgG was from Zymed, and anti-IQGAP1 (IQ motif-containing GTPase-activating protein 1) IgG was obtained from Kozo Kaibuchi (Nagoya University, Nagoya City, Japan) and from Santa Cruz Biotechnology. α-actinin monoclonal antibody (clone AT6.172) was from Chemicon. Rabbit α-actinin-4 IgG was provided by Raghu Kalluri (Harvard Medical School, Cambridge, MA), rabbit anti-MAGI-2/S-SCAM (membrane-associated guanylate kinase inverted 2/synaptic scaffolding molecule) IgG was provided by Yutaka Hata (Tokyo Medical and Dental University, Tokyo), and rabbit anti-MAGI-2 IgG was provided by Charles L. Sawyers and Brian J. Skaggs (University of California, Los Angeles). MAGI-2/S-SCAM is directed against the WW domains of MAGI-2/S-SCAM and recognizes MAGI-2 and its homologue MAGI-1. The MAGI-2 antibody is directed against a unique region between the fourth and fifth PDZ domains of MAGI-2 and is specific for MAGI-2. Anti-MAGI-2 IgG was caprylic acid-purified (23). Rabbit anti-αII spectrin and anti-βII spectrin IgG were obtained from Jon Morrow (Yale University, New Haven, CT), and mouse monoclonal anti-nephrin 5-1-6 IgG was obtained from Fujio Shimizu (Niigata University, Niigata, Japan). Anti-podocalyxin monoclonal IgG (5A) was described (24). Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse IgG were purchased from BioDesign (Saco, ME) and Bio-Rad, respectively. Highly cross-absorbed Alexa Fluor 488 goat anti-mouse and Alexa Fluor 594 goat anti-rabbit F(ab′)2 were from Molecular Probes.
Preparation of Glomerular Lysates. Glomeruli were isolated from kidney cortices of male Sprague–Dawley rats (150–200 g) by graded sieving as described (25) and lysed by incubation in 1% Nonidet P-40/20 mM Hepes, pH 7.5/150 mM NaCl/100 mM KI with 1× Complete, EDTA-free proteinase inhibitor mixture (Roche)/50 mM NaF/1 mM Na3VO4 at 4°C for 30 min. KI was added to the lysis buffer to increase the solubility of nephrin (25). Detergent-insoluble material was removed by centrifugation (10,000 × g for 2 min).
Pull-Down Assays. GST and GST–nephrin tail fusion proteins were produced as described (22). Glomerular lysates were precleared with glutathione-Sepharose beads (Amersham Biosciences) at 4°C for 1 h, followed by incubation (4°C for 4 h) with GST-nephrin tail or GST alone (20 μg of each) immobilized on beads. Beads were washed extensively with lysis buffer and boiled in Laemmli sample buffer. Proteins were separated by 6% SDS/PAGE for GelCode blue staining (Pierce) or immunoblotting as described (22).
Mass Spectrometry. Pull-down assays were performed on glomerular lysates as above. Bound proteins were separated by 6% SDS/PAGE and stained with GelCode blue. The bands were excised from the gel and digested with trypsin in-gel. Tryptic peptides were analyzed by mass spectrometry as described (26), and the peptides obtained were identified by searching the ensembl database.
Indirect Immunofluorescence. Adult rat kidneys were infiltrated with 2 M glucose or perfusion fixed with paraformaldehyde (PFA) (25). Two-day-old rat kidneys were fixed by immersion in PFA. For preparation of semithin cryosections, PFA-fixed kidneys were cryoprotected and frozen in liquid nitrogen, and semithin cryosections were cut as described (27). For MAGI-2 staining semithin sections prepared from glucose-infiltrated tissue were fixed for 3 min in acetone (–20°C). Sections were incubated with primary antibodies in PBS containing 1% BSA for2hat room temperature. For double labeling, sections were incubated simultaneously with two primary antibodies followed by Alexa Fluor 488 goat antimouse and Alexa Fluor 594 goat anti-rabbit F(ab′)2 in PBS containing 1% BSA (2 h at room temperature) and examined with a Zeiss Axiophot. Images were collected with an ORCA-ER camera (Hamamatsu, Bridgewater, NJ) using image 1.59 (Scion, Frederick, MA) software. Images were processed with photoshop 5.5 (Adobe Systems, Palo Alto, CA).
Results
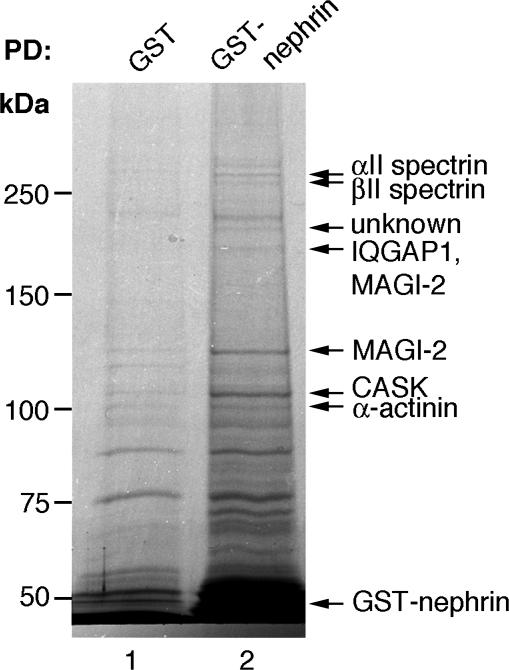
Nephrin Forms a Complex with Cell Junction-Associated Proteins. When pull-down assays were carried out on glomerular lysates with GST-nephrin tail and the proteins were separated by SDS/PAGE and stained with GelCode blue, seven bands were identified that bound specifically to GST–nephrin tail but not to GST alone (Fig. 1). Each band was cut out, digested with trypsin, and identified by mass spectrometry. Only proteins with at least two different peptide matches were considered significant. The >250-kDa bands were identified as αII spectrin and βII spectrin, and the 180-kDa band contained two proteins, IQGAP1 and MAGI-2/S-SCAM. The 130-kDa band also consisted of MAGI-2. The 110-kDa band was identified as CASK, and the 90-kDa band represented peptides present in all four isoforms of α-actinin. The ≈200-kDa band represented a previously uncharacterized protein.
Fig. 1.
Identification of nephrin interaction partners by GST–nephrin tail pull-downs assays and mass spectrometry. GST–nephrin tail (20 μg) or GST (20 μg) were incubated for 4 h with 400 μg of glomerular lysate, and the bound proteins were separated by 6% SDS/PAGE followed by staining with GelCode blue. To upscale the reactions, three parallel pull-downs were combined per lane. The bands observed in GST–nephrin tail pull-downs (lane 2) were identified by mass spectrometry as αII spectrin, βII spectrin, IQGAP1, MAGI-2, CASK, and α-actinin. The ≈200-kDa band represents an unknown protein.
IQGAP1, αII spectrin, βII spectrin, and α-actinin are all known to be localized at adherens junctions and to associate with actin either directly or indirectly. IQGAP1 binds E-cadherin, β-catenin (28), and actin (29) and is a component of signaling networks that regulate cadherin-mediated cell adhesion (28) and actin cytoskeleton dynamics by means of the small GTPases Cdc42 and Rac1 (30, 31). Spectrins are membrane-associated cytoskeletal proteins that have been shown to coimmunoprecipitate with E-cadherin (32) and to stabilize cell junctions and control cell shape and polarity (33). α-Actinins are actin-binding and bundling proteins. Spectrin (34) and α-actinin (35) are linked to the cadherin/catenin cell–cell adhesion complex by α-catenin. MAGI-2 and CASK are members of the membrane-associated guanylate kinase (MAGUK) family of scaffolding proteins (36). These proteins are found in complexes with members of the Ig superfamily (37–40) and serve to cluster receptors, signaling, and scaffolding proteins at specific membrane regions, including tight and synaptic junctions (38, 41–43).
Thus, the proteins identified in the nephrin multiprotein complex are all found in cell–cell junctions and are known to play important roles in regulating cell adhesion, signaling, and actin cytoskeleton dynamics.
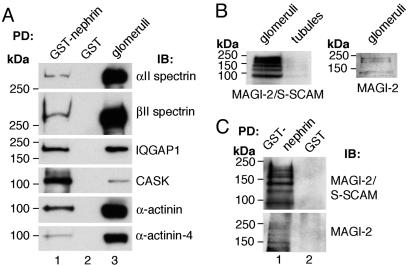
Confirmation of the Presence of Cell Junction-Associated Proteins in the Nephrin Complex. The presence of spectrins, IQGAP1, MAGI-2, CASK, and α-actinin was confirmed by immunoblotting of pull-downs obtained from glomerular lysates with GST-nephrin tail (Fig. 2 A and C). The presence of α-actinin was confirmed by using an antibody that recognizes all four α-actinin isoforms as well as an antibody specific for α-actinin-4. α-actinin-4 has previously been shown to be expressed in podocytes and, when mutated in humans, can lead to familial focal segmental glomerulosclerosis (18).
Fig. 2.
Confirmation of the interactions between nephrin and the proteins identified by mass spectrometry. (A) αII spectrin, βII spectrin, IQGAP1, CASK, and α-actinin bind to GST–nephrin tail (lane 1) but not to GST alone (lane 2). Glomerular lysate (10 μg) is included as a control (lane 3). For α-actinin, antibodies recognizing all four isoforms of the protein as well as those specific for α-actinin were used. (B) Immunoblotting (IB) with two different antibodies indicated that MAGI-2 is expressed in glomeruli but not in tubules. Glomerular or tubular lysates (50 μg) was separated by 7.5% SDS/PAGE and immunoblotted with anti-MAGI-2/S-SCAM IgG, which recognizes MAGI-1 and MAGI-2 (Left), and with anti-MAGI-2 IgG, which is specific for MAGI-2 (Right). (C) Immunoblotting with anti-MAGI-2/S-SCAM IgG and anti-MAGI-2 IgG showed that MAGI-2 binds to GST–nephrin tail (lane 1) but not to GST alone (lane 2). (A and C) GST–nephrin tail (20 μg) or GST alone (20 μg) was incubated with 400 μg of glomerular lysate, as described in Fig. 1, and immunoblotted with antibodies against αII spectrin, βII spectrin, IQGAP1, CASK, and α-actinin (A) or MAGI-2/S-SCAM and MAGI-2 (C).
Because MAGI-2 was not detected previously in kidney lysates with the MAGI-2/S-SCAM antibody, which recognizes MAGI-1 and MAGI-2 (42), we checked the presence of MAGI-2 in glomerular fractions by using this antibody and a MAGI-2-specific antibody (Fig. 2B). MAGI-1 has previously been shown to be expressed in podocytes (44, 45). Multiple bands representing previously characterized isoforms of MAGI-1 (45) and MAGI-2 (46) were observed in glomerular lysates with both antibodies (Fig. 2B). Immunoblotting of nephrin pull-downs with both antibodies confirmed the presence of MAGI-2 in the nephrin complex (Fig. 2C). We conclude that spectrins, IQGAP1, MAGI-2, CASK, and α-actinin-4 are part of the nephrin multiprotein complex.
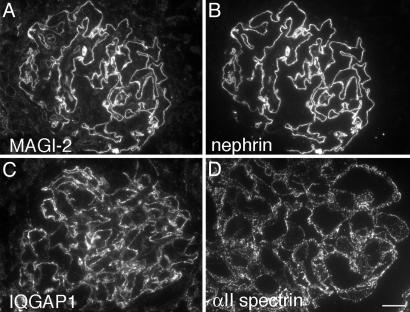
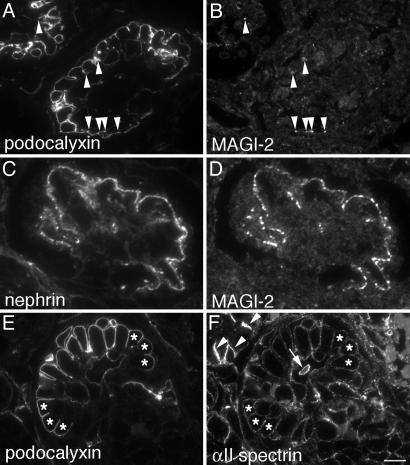
Proteins Identified in the Nephrin Complex Are Localized in the Glomerular Epithelium (Podocytes) in Rat Kidney. Next we performed immunofluorescence on semithin cryosections from rat kidney to determine the localization of the putative nephrin interaction partners identified in glomerular lysates. All these proteins are expressed in glomeruli. MAGI-2 (Fig. 3A), IQGAP1 (Fig. 3C), and α-actinin (data not shown) specifically stained the podocytes. Staining for all these proteins was confined to the foot process layer that lines the capillary loops of the podocytes and codistributes with nephrin (Fig. 3B). αII spectrin (Fig. 3D) and βII spectrin (data not shown) were distributed in a punctate pattern in the foot process layer. CASK was diffusely distributed throughout the cytoplasm in the podocyte foot processes and cell bodies (22). IQGAP1, CASK, and spectrins were detected in kidney tubules as well as in glomeruli, whereas MAGI-2 and α-actinin were restricted to podocytes. Thus, the proteins identified in the nephrin complex were found to be expressed in podocytes and to codistribute with nephrin in the foot processes.
Fig. 3.
Localization of MAGI-2, nephrin, IQGAP1, and αII spectrin in adult rat glomeruli. (A, C, and D) MAGI-2 (A), IQGAP1 (C), and αII spectrin (D) are distributed in a linear pattern along the glomerular capillaries. (B) Double labeling with nephrin shows that MAGI-2 and nephrin colocalize in the foot process layer of podocytes. Rat kidneys were cryoprotected, infiltrated with glucose, and processed for semithin cryosectioning. (A and B) Sections were fixed with acetone (3 min) before labeling for MAGI-2 and nephrin. (C and D) Rat kidneys were perfusion-fixed with PFA before processing for semithin cryosectioning. Sections were labeled with anti-IQGAP1 and anti-αII spectrin IgG and examined by immunofluorescence. (Bars: A–C, 15 μm; D, 7.5 μm.)
Differentiation of Slit Diaphragms from Junctional Complexes During Glomerular Development. Glomeruli differentiate from metanephric mesenchymal cells in a stepwise process in which several stages (renal vesicle, S-shaped body, capillary loop, and maturing glomerulus) are recognized (1). The presumptive podocytes can first be identified at the S-shaped body stage by expression of the sialoglycoprotein podocalyxin (Fig. 4A) on their apical surface (1). At the beginning of this stage, the foot processes and slit diaphragms have not yet formed and the cells destined to be podocytes are typical polarized epithelial cells connected at their apical surfaces by tight and adherens junctions (Fig. 4A, arrowheads). As this stage progresses, the junctional complexes migrate from the apical surface toward the base of the cells and podocalyxin marks the apical domain of the podocytes above the junctional complex (3) (Fig. 4C). Subsequently capillaries “invade” the epithelium, foot process differentiation and interdigitation begins, and the slit diaphragms start to differentiate (capillary loop stage). Junctional migration and foot process interdigitation can be followed by staining for the tight junction protein ZO-1, which localizes to the migrating junctional complex (6). Nephrin is first detected at the approximate time that junctional migration begins, when it colocalizes with ZO-1 in the junctional complex (11). P-cadherin is expressed in the renal vesicle before expression of podocalyxin and nephrin (11).
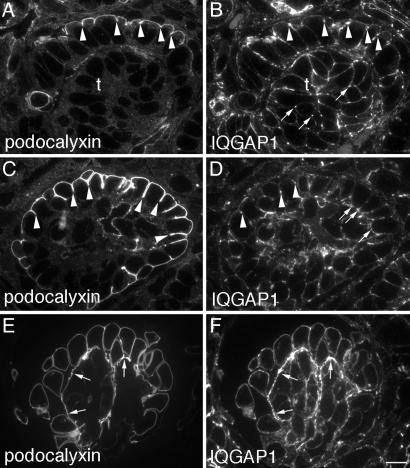
Fig. 4.
Expression of IQGAP1 and podocalyxin during glomerular development. (A) In the S-shaped vesicle, podocalyxin labels the apical domain of differentiating podocytes. (B) IQGAP1 is observed on all domains of future podocytes and tubule cells but is concentrated at the migrating junctional complexes (arrowheads) in the podocytes and at cell–cell junctions (arrows) in presumptive proximal tubule cells. (C) In the early capillary loop stage, the junctions migrate to the base of the podocytes, and podocalyxin labels the lateral cell surface of podocytes down to the junctional complexes (arrowheads). (D) IQGAP1 is found along the cell junctions (arrowheads) and at the base of the cells (arrows). (E and F) In the late capillary loop stage, podocalyxin (E) and IQGAP1 (F) strongly label the foot processes at the base of the cells lining the capillaries (arrows). Kidneys of 2-day-old rats were immersion-fixed with PFA, processed for semithin cryosectioning, double labeled for IQGAP1 and podocalyxin, and examined by immunofluorescence. (Bar, 7.5 μm.)
Expression of IQGAP1 During Kidney Development Reflects Foot Process Formation. When IQGAP1 was localized during glomerulogenesis in semithin cryosections of newborn rat kidneys, it was detected at cell–cell junctions between the earliest forming podocytes (renal vesicle stage) when the cells destined to become glomerular epithelium are not yet distinguishable from those destined to become proximal tubule cells (data not shown). After podocalyxin appears on the apical domain (Fig. 4A), IQGAP1 is distributed on all cell domains but is most concentrated in the migrating junctional complexes of the differentiating podocytes (Fig. 4B, arrowheads) and at cell–cell junctions between presumptive proximal tubule cells (Fig. 4B, arrows). After migration of podocalyxin (Fig. 4C) and the junctional complexes to the base of the immature podocytes, IQGAP1 begins to be concentrated at their base (Fig. 4D). Upon interdigitation of the foot processes IQGAP1 (Fig. 4F) becomes concentrated in the foot process layer, which is heavily stained for podocalyxin (Fig. 4E).
MAGI-2 Is Up-Regulated upon Foot Process Differentiation. In contrast to IQGAP1, MAGI-2 (Fig. 5B) is expressed much later during glomerulogenesis (late capillary loop stage). The protein is first detected after migration of podocalyxin (Fig. 5A) and the junctions to the base of the cells. After the foot processes begin to differentiate MAGI-2 colocalizes with nephrin in the foot processes (Fig. 5 C and D).
Fig. 5.
Expression of MAGI-2, αII spectrin, nephrin, and podocalyxin during glomerular development. (A) Podocalyxin stains the lateral domain of the podocyte cell membrane down to the migrating junctional complexes (arrowheads). (B) The first spot-like concentrations of MAGI-2 are observed during junctional migration (arrowheads) (early capillary loop stage). (C and D) Later in the capillary loop stage, nephrin (C) and MAGI-2 (D) partially colocalize in the interdigitating foot processes lining the capillary loops. (E and F) αII spectrin (F) is concentrated along all cell membrane domains in differentiating podocytes (asterisks), whereas podocalyxin (E) stains only the apical and lateral cell domains of the podocytes (asterisks). In tubule cells, αII spectrin is concentrated along the lateral cell membranes (arrowheads) down to the junctional complexes. (F) The arrow indicates a blood cell present in the lumen of the glomerular capillary. Kidneys of 2-day-old rats were processed as described in Fig. 4 and double labeled for podocalyxin (A) and MAGI-2 (B), nephrin (C) and MAGI-2 (D), or podocalyxin (E) and αII spectrin (F). (Bar, 7.5 μm.)
Spectrin Is Present Throughout Glomerulogenesis. αII spectrin is expressed along the cell membrane in both the podocytes and presumptive tubule cells throughout glomerulogenesis (Fig. 5 E and F). In tubule cells αII spectrin is concentrated along lateral cell margins (Fig. 5F, arrowheads) (47), but in glomeruli no significant concentration at junctional complexes is observed (Fig. 5F, asterisks).
Discussion
The podocyte slit diaphragm is a specialized cell junction adapted for facilitating glomerular filtration. It is derived from typical junctional complexes of polarized epithelial cells that are composed of a group of transmembrane cell adhesion molecules and a cytoplasmic plaque of regulatory and scaffolding proteins modulating cell adhesion, signaling, and cytoskeletal connections. Although there are morphological differences between the slit diaphragms and epithelial cell junctions, they share several key structural proteins of both adherens junctions and tight junctions including the presence of cadherins and catenins (2, 22) and ZO-1 (2, 6).
We have previously shown that nephrin, a cell adhesion receptor of the Ig superfamily, and P-cadherin, a classical cadherin, are components of a multiprotein complex (22). Here we report the identification by mass spectrometry of several additional cell junction-associated proteins in nephrin complexes. Four of the proteins identified, IQGAP1, αII spectrin, βII spectrin, and α-actinin, represent adherens junction-associated proteins (28, 34, 35), and two, MAGI-2/S-SCAM and CASK, represent MAGUK family scaffolding proteins that associate with Ig superfamily proteins (37–40) and are found in tight junctions and synapses (38, 41–43). All these proteins have previously been shown to be involved in the regulation of cell adhesion. We have localized these proteins to the foot processes of podocytes in the adult rat kidney, confirming that they are properly distributed to interact with nephrin and regulate cell adhesion. The presence of these proteins in slit diaphragms and their association with nephrin suggests that they may form a scaffolding protein complex in the podocyte slit diaphragm and thus contribute to the regulation of ultrafiltration by binding slit membrane proteins and establishing their cytosolic connections.
The presence of IQGAP1 in slit diaphragms is particularly intriguing because it binds E-cadherin (28) and actin (29) and controls cadherin-mediated cell adhesion (28, 48). IQGAP1 contains multiple protein–protein interaction domains, including calponin homology, WW, IQ, and RasGAP-related domains (31), and has been shown to be a component of signaling networks that regulate actin cytoskeleton dynamics and cell motility (30, 49). IQGAP1 binds actin directly (29), and it regulates the actin cytoskeleton indirectly via the small GTPases Cdc42 and Rac1 (30, 31). IQGAP1 also associates with calmodulin (31), the major mediator of Ca2+ signaling in cells, which regulates the actin-binding activity of IQGAP1 (29). Overexpression of IQGAP1 (28) or translocation of endogenous IQGAP1 to cell–cell junctions (48) coincides with a decrease in E-cadherin-mediated homophilic adhesion. Binding of IQGAP1 to E-cadherin displaces α-catenin from the cadherin/catenin complex, thus reducing the interaction of E-cadherin with the cytoskeleton and decreasing cell adhesion (48). Ca2+ signaling also plays an important role in the regulation of cell adhesion via IQGAP1, because Ca2+/calmodulin competes with E-cadherin for binding to IQGAP1 (48).
Thus, identification of IQGAP1 in nephrin pull-downs suggests that it may play an important role in controlling slit diaphragm function by modulating cadherin adhesion and actin cytoskeleton dynamics. In keeping with our findings in glomeruli, while this paper was in preparation, another study reported identification of IQGAP1 by nephrin tail pull-down assay on cell lysates obtained from podocytes in culture (50).
In addition to IQGAP1, three other proteins often associated with adherens junctions, α-actinin-4, αII spectrin, and βII spectrin, were identified in the nephrin multiprotein complex. In podocytes, α-actinin-4 is part of the microfilament network connecting podocytes to the glomerular basement membrane (51). In other cell types, α-actinin-4 has also been shown to be in complex with cadherins and catenins (35) and with the Ig superfamily member ICAM-1 (intercellular adhesion molecule 1) (52). α-Actinin-4 is essential for maintaining podocyte function, because mutations in α-actinin-4 lead to focal segmental glomerulosclerosis (18), which is associated with disorganizaton of the foot processes and filtration slits. Mutated α-actinin-4 shows enhanced binding of the mutated protein to actin and disregulation of the actin cytoskeleton. Interestingly, a mouse model mimicking the human disease exhibited reduced levels of nephrin expression (53). Our finding of spectrins in nephrin/P-cadherin protein complexes is in keeping with previous findings that spectrin can associate with the Ig superfamily and the cadherin-mediated cell adhesion receptors (54). Spectrin also associates with α-catenin at sites of E-cadherin-mediated cell–cell contact (34). αII and βII spectrins form a tetramer, and the complex has been suggested to provide anchoring points for transmembrane proteins at regions specified by cell adhesion proteins and, thus, strengthen cell junctions (55). Similarly, in podocytes spectrin may be involved in the regulation of cell junctions.
The MAGUK family proteins ZO-1 and MAGI-1 have previously been localized in podocytes (6, 14, 44, 45) and shown to associate with the Ig superfamily cell adhesion receptor Neph1 (56, 57) and JAM4 (junctional adhesion molecule 4) (44), respectively. Here we report identification of two new members of the MAGUK family of proteins, MAGI-2/S-SCAM and CASK, as components of the nephrin multiprotein complex. MAGUK proteins contain multiple protein–protein interaction domains, including guanylate kinase, WW, and PDZ domains, and they homodimerize and heterodimerize to form scaffolding complexes (36).
MAGI-2/S-SCAM has previously been shown to localize in junctions in certain synapses in brain called puncta adherentia (40). Puncta adherentia resemble adherens junctions morphologically, and they are composed of cadherins, catenins (58), and nectin (40) (an Ig superfamily member cell adhesion receptor), which are all components of adherens junctions. MAGI-2/S-SCAM has been suggested to link cadherins and nectin in puncta adherentia, thus connecting these cadherin and Ig family cell adhesion receptors (40). Furthermore, MAGI-2/S-SCAM directly interacts with the adherens junction protein β-catenin (59).
CASK has also been found in cell–cell adhesion sites, including synapses (37, 41) and tight junctions, and it has been shown to associate with Ig domain protein JAM (38) and with cadherins, catenins, and kainate receptors (60). CASK has been postulated to link membrane proteins to the cytoskeleton and regulate epithelial cell polarity (36).
The MAGUK family scaffolding proteins MAGI-1, MAGI-2, CASK, and ZO-1, which localize to podocyte foot processes, most likely function as scaffolds to link Ig family receptors, including nephrin, and cadherins in podocytes.
Collectively, the adherens junction-associated proteins and MAGUK family scaffolding proteins found in the nephrin multiprotein complex may be expected to play a fundamental role in regulating slit diaphragm assembly during development and its function in mature glomeruli. Further studies are needed to analyze the functional consequences of these interactions in glomeruli in vivo under normal and proteinuric conditions.
Acknowledgments
This work was supported by National Institutes of Health Grant DK17724 (to M.G.F.), fellowships from the Academy of Finland and Sigrid Jusélius Foundation (to S.L.), and by Faculty Transition Award NHGRI K22 and the Ludwig Institute for Cancer Research (to J.J.R. and H.Z.).
Author contributions: S.L., H.Z., and M.G.F. designed research; S.L., J.J.R., K.K., and N.I. performed research; J.J.R. and H.Z. contributed new reagents/analytic tools; S.L., H.Z., and M.G.F. analyzed data; and S.L. and M.G.F. wrote the paper.
Abbreviations: PFA, paraformaldehyde; MAGUK, membrane-associated guanylate kinase.
References
- 1.Reeves, W., Caulfield, J. P. & Farquhar, M. G. (1978) Lab. Invest. 39, 90–100. [PubMed] [Google Scholar]
- 2.Reiser, J., Kriz, W., Kretzler, M. & Mundel, P. (2000) J. Am. Soc. Nephrol. 11, 1–8. [DOI] [PubMed] [Google Scholar]
- 3.Schnabel, E., Dekan, G., Miettinen, A. & Farquhar, M. G. (1989) Eur. J. Cell Biol. 48, 313–326. [PubMed] [Google Scholar]
- 4.Farquhar, M. G. & Palade, G. E. (1961) J. Exp. Med. 114, 699–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caulfield, J. P., Reid, J. J. & Farquhar, M. G. (1976) Lab. Invest. 34, 43–59. [PubMed] [Google Scholar]
- 6.Schnabel, E., Anderson, J. M. & Farquhar, M. G. (1990) J. Cell Biol. 111, 1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kestilä, M., Lenkkeri, U., Männikkö, M., Lamerdin, J., McCready, P., Putaala, H., Ruotsalainen, V., Morita, T., Nissinen, M., Herva, R., et al. (1998) Mol. Cell 1, 575–582. [DOI] [PubMed] [Google Scholar]
- 8.Donoviel, D. B., Freed, D. D., Vogel, H., Potter, D. G., Hawkins, E., Barrish, J. P., Mathur, B. N., Turner, C. A., Geske, R., Montgomery, C. A., et al. (2001) Mol. Cell. Biol. 21, 4829–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoshnoodi, J., Sigmundsson, K., Öfverstedt, L.-G., Skoglund, U., Öbrink, B., Wartiovaara, J. & Tryggvason, K. (2003) Am. J. Pathol. 163, 2337–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wartiovaara, J., Öfverstedt, L.-G., Khoshnoodi, J., Zhang, J., Mäkelä, E., Sandin, S., Ruotsalainen, V., Cheng, R. H., Jalanko, H., Skoglund, U. & Tryggvason, K. (2004) J. Clin. Invest. 114, 1475–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruotsalainen, V., Patrakka, J., Tissari, P., Reponen, P., Hess, M., Kestilä, M., Holmberg, C., Salonen, R., Heikinheimo, M., Wartiovaara, J., et al. (2000) Am. J. Pathol. 157, 1905–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue, T., Yaoita, E., Kurihara, H., Shimizu, F., Sakai, T., Kobayashi, T., Ohshiro, K., Kawachi, H., Okada, H., Suzuki, H., et al. (2001) Kidney Int. 59, 1003–1012. [DOI] [PubMed] [Google Scholar]
- 13.Kurihara, H., Anderson, J. M., Kerjaschki, D. & Farquhar, M. G. (1992) Am. J. Pathol. 141, 805–816. [PMC free article] [PubMed] [Google Scholar]
- 14.Kurihara, H., Anderson, J. M. & Farquhar, M. G. (1992) Proc. Natl. Acad. Sci. USA 89, 7075–7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boute, N., Gribouval, O., Roselli, S., Benessy, F., Lee, H., Fuchshuber, A., Dahan, K., Gubler, M.-C., Niaudet, P. & Antignac, C. (2000) Nat. Genet. 24, 349–354. [DOI] [PubMed] [Google Scholar]
- 16.Shih, N.-Y., Li, J., Karpitskii, V., Nguyen, A., Dustin, M. L., Kanagawa, O., Miner, J. H. & Shaw, A. S. (1999) Science 286, 312–315. [DOI] [PubMed] [Google Scholar]
- 17.Ciani, L., Patel, A., Allen, N. D. & ffrench-Constant, C. (2003) Mol. Cell. Biol. 23, 3575–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan, J. M., Kim, S. H., North, K. N., Rennke, H., Correia, L. A., Tong, H.-Q., Mathis, B. J., Rodríguez-Pérez, J.-C., Allen, P. G., Beggs, A. H. & Pollak, M. R. (2000) Nat. Genet. 24, 251–256. [DOI] [PubMed] [Google Scholar]
- 19.Kreidberg, J. A. (1996) Development (Cambridge, U.K.) 122, 3537–3547. [DOI] [PubMed] [Google Scholar]
- 20.Doyonnas, R., Kershaw, D. B., Duhme, C., Merkens, H., Chelliah, S., Graf, T. & McNagny, K. M. (2001) J. Exp. Med. 194, 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerjaschki, D., Sharkey, D. J. & Farquhar, M. G. (1984) J. Cell Biol. 98, 1591–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehtonen, S., Lehtonen, E., Kudlicka, K., Holthöfer, H. & Farquhar, M. G. (2004) Am. J. Pathol. 165, 923–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinney, M. M. & Parkinson, A. (1987) J. Immunol. Methods 96, 271–278. [DOI] [PubMed] [Google Scholar]
- 24.Miettinen, A., Dekan, G. & Farquhar, M. G. (1990) Am. J. Pathol. 137, 929–944. [PMC free article] [PubMed] [Google Scholar]
- 25.Orlando, R. A., Takeda, T., Zak, B., Schmieder, S., Benoit, V. M., McQuistan, T., Furthmayr, H. & Farquhar, M. G. (2001) J. Am. Soc. Nephrol. 12, 1589–1598. [DOI] [PubMed] [Google Scholar]
- 26.Zhou, W., Ryan, J. J. & Zhou, H. (2004) J. Biol. Chem. 279, 32262–32268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCaffery, J. M. & Farquhar, M. G. (1995) Methods Enzymol. 257, 259–279. [DOI] [PubMed] [Google Scholar]
- 28.Kuroda, S., Fukata, M., Nakagawa, M., Fujii, K., Nakamura, T., Ookubo, T., Izawa, I., Nagase, T., Nomura, N., Tani, H., et al. (1998) Science 281, 832–835. [DOI] [PubMed] [Google Scholar]
- 29.Bashour, A.-M., Fullerton, A. T., Hart, M. J. & Bloom, G. S. (1997) J. Cell Biol. 137, 1555–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swart-Mataraza, J. M., Li, Z. & Sacks, D. B. (2002) J. Biol. Chem. 277, 24753–24763. [DOI] [PubMed] [Google Scholar]
- 31.Hart, M. J., Callow, M. G., Souza, B. & Polakis, P. (1996) EMBO J. 15, 2997–3005. [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson, W. J., Shore, E. M., Wang, A. Z. & Hammerton, R. W. (1990) J. Cell Biol. 110, 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knust, E. (2000) Curr. Opin. Genet. Dev. 10, 471–475. [DOI] [PubMed] [Google Scholar]
- 34.Pradhan, D., Lombardo, C. R., Roe, S., Rimm, D. L. & Morrow, J. S. (2001) J. Biol. Chem. 276, 4175–4181. [DOI] [PubMed] [Google Scholar]
- 35.Knudsen, K. A., Soler, A. P., Johnson, K. R. & Wheelock, M. J. (1995) J. Cell Biol. 130, 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caruana, G. (2002) Int. J. Dev. Biol. 46, 511–518. [PubMed] [Google Scholar]
- 37.Biederer, T., Sara, Y., Mozhayeva, M., Atasoy, D., Liu, X., Kavalali, E. T. & Südhof, T. C. (2002) Science 297, 1525–1531. [DOI] [PubMed] [Google Scholar]
- 38.Martínez-Estrada, O. M., Villa, A., Breviario, F., Orsenigo, F., Dejana, E. & Bazzoni, G. (2001) J. Biol. Chem. 276, 9291–9296. [DOI] [PubMed] [Google Scholar]
- 39.Shi, S.-H., Cheng, T., Jan, L. Y. & Jan, Y.-N. (2004) Proc. Natl. Acad. Sci. USA 101, 13346–13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamada, A., Irie, K., Deguchi-Tawarada, M., Ohtsuka, T. & Takai, Y. (2003) Genes Cells 8, 985–994. [DOI] [PubMed] [Google Scholar]
- 41.Hata, Y., Butz, S. & Südhof, T. C. (1996) J. Neurosci. 16, 2488–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirao, K., Hata, Y., Ide, N., Takeuchi, M., Irie, M., Yao, I., Deguchi, M., Toyoda, A., Sudhof, T. C. & Takai, Y. (1998) J. Biol. Chem. 273, 21105–21110. [DOI] [PubMed] [Google Scholar]
- 43.Xu, J., Paquet, M., Lau, A. G., Wood, J. D., Ross, C. A. & Hall, R. A. (2001) J. Biol. Chem. 276, 41310–41317. [DOI] [PubMed] [Google Scholar]
- 44.Hirabayashi, S., Tajima, M., Yao, I., Nishimura, W., Mori, H. & Hata, Y. (2003) Mol. Cell. Biol. 23, 4267–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patrie, K. M., Drescher, A. J., Goyal, M., Wiggins, R. C. & Margolis, B. (2001) J. Am. Soc. Nephrol. 12, 667–677. [DOI] [PubMed] [Google Scholar]
- 46.Hirao, K., Hata, Y., Yao, I., Deguchi, M., Kawabe, H., Mizoguchi, A. & Takai, Y. (2000) J. Biol. Chem. 275, 2966–2972. [DOI] [PubMed] [Google Scholar]
- 47.Piepenhagen, P. A. & Nelson, W. J. (1998) Mol. Biol. Cell 11, 3161–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li, Z., Kim, S. H., Higgins, J. M. G., Brenner, M. B. & Sacks, D. B. (1999) J. Biol. Chem. 274, 37885–37892. [DOI] [PubMed] [Google Scholar]
- 49.Mataraza, J. M., Briggs, M. W., Li, Z., Entwistle, A., Ridley, A. J. & Sacks, D. B. (2003) J. Biol. Chem. 278, 41237–41245. [DOI] [PubMed] [Google Scholar]
- 50.Liu, X. L., Kilpeläinen, P., Hellman, U., Sun, Y., Wartiovaara, J., Morgunova, E., Pikkarainen, T., Yan, K., Jonsson, A. P. & Tryggvason, K. (2005) FEBS J. 272, 228–243. [DOI] [PubMed] [Google Scholar]
- 51.Drenckhahn, D. & Franke, R. P. (1988) Lab. Invest. 59, 673–682. [PubMed] [Google Scholar]
- 52.Carpén, O., Pallai, P., Staunton, D. E. & Springer, T. A. (1992) J. Cell Biol. 118, 1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michaud, J.-L., Lemieux, L. I., Dubé, M., Vanderhyden, B. C., Robertson, S. J. & Kennedy, C. R. J. (2003) J. Am. Soc. Nephrol. 14, 1200–1211. [DOI] [PubMed] [Google Scholar]
- 54.Davis, J. Q. & Bennett, V. (1994) J. Biol. Chem. 269, 27163–27166. [PubMed] [Google Scholar]
- 55.Bignone, P. A. & Baines, A. J. (2003) Biochem. J. 374, 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu, G., Kaw, B., Kurfis, J., Rahmanuddin, S., Kanwar, Y. S. & Chugh, S. S. (2003) J. Clin. Invest. 112, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huber, T. B., Schmidts, M., Gerke, P., Schermer, B., Zahn, A., Hartleben, B., Sellin, L., Walz, G. & Benzing, T. (2003) J. Biol. Chem. 278, 13417–13421. [DOI] [PubMed] [Google Scholar]
- 58.Uchida, N., Honjo, Y., Johnson, K. R., Wheelock, M. J. & Takeichi, M. (1996) J. Cell Biol. 135, 767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishimura, W., Yao, I., Iida, J., Tanaka, N. & Hata, Y. (2002) J. Neurosci. 22, 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coussen, F., Normand, E., Marchal, C., Costet, P., Choquet, D., Lambert, M., Mège, R.-M. & Mulle, C. (2002) J. Neurosci. 22, 6426–6436. [DOI] [PMC free article] [PubMed] [Google Scholar]