Abstract
Direct communication between arteries and veins without intervening capillary beds is the primary pathology of arteriovenous malformations (AVMs). Although Notch signaling is implicated in embryonic arteriovenous (AV) differentiation, its function in the adult mammalian vasculature has not been established due to the embryonic lethality that often occurs in both gain- and loss-of-function mutants. We expressed a constitutively active Notch4, int3, in the adult mouse endothelium by using the tetracycline-repressible system to suppress int3 during embryogenesis. int3 caused profound blood vessel enlargement and AV shunting, which are hallmarks of AVM, and led to lethality within weeks of its expression. Vessel enlargement, a manifestation of AVM, occurred in an apparently tissue-specific fashion; the liver, uterus, and skin were affected. int3-mediated vascular defects were accompanied by arterialization, including ectopic venous expression of ephrinB2, increased smooth muscle cells, and up-regulation of endogenous Notch signaling. Remarkably, the defective vessels and illness were reversed upon repression of int3 expression. Finally, endothelial expression of a constitutively active Notch1 induced similar hepatic vascular lesions. Our results provide gain-of-function evidence that Notch signaling in the adult endothelium is sufficient to render arterial characteristics and lead to AVMs.
Keywords: arterialization, Notch, signaling, angiogenesis, vascular remodeling
Ahierarchically branched vascular network of arteries, arterioles, capillaries, postcapillary venules, and veins is essential for adequate support throughout the body. How vascular hierarchy and arteriovenous (AV) communication are established during embryonic development and how such exquisite organization is maintained in adults, however, remains poorly understood. Mechanical forces such as blood flow and shear stress have long been hypothesized to regulate these processes (1). These mechanical cues ultimately trigger biochemical responses in the endothelial cell (EC) lining of blood vessels and its pericyte and smooth muscle cell (SMC) support layers to generate biological effects (2). Less is known about the genetic and biochemical pathways that regulate blood vessel caliber and AV identity. Overexpression of VEGF or angiopoietin-1 can induce blood vessel dilation (3, 4), although these proteins are best known for their ability to promote neovascularization.
Notch, originally discovered in Drosophila, is best known for its function in controlling cell fate decisions and creating boundaries through cell–cell communication (5). The core Notch signaling pathway involves ligand-induced activation of the receptor, proteolytic cleavage, and subsequent translocation of its intracellular domain to the nucleus, where it functions as a transcriptional regulator (5). Notch proteins lacking the extracellular domain are constitutively active (5). Mammalian Notch receptors (Notch 1, 3, and 4) and their cell-surface ligands [Delta like-4 (Dll4), Jagged-1, and Jagged-2] are expressed in blood vessels (6). Manipulation of several genes in the Notch pathway in mice leads to embryonic lethality due to defects in vascular remodeling. Although the phenotypes are complicated, loss of Notch function often leads to reduction of blood vessel diameter (7–13), whereas gain of Notch function leads to dilation (14). In addition, Notch signaling can regulate AV differentiation during embryogenesis (10, 13, 15, 16).
Notch receptor and ligand expression persists in adult arteries but not veins (6, 17). Mutations in Notch3 or Jagged1 lead to the human diseases CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) and Alagille syndrome, respectively, both of which result in vascular pathology (16). Thus, understanding of the function of Notch in adult blood vessels is of significant interest. We have discovered that int3, when expressed in adult endothelium, causes AV shunting and subsequent AV malformation (AVM), which are reversible upon repression of int3. Therefore, Notch signaling may provide cues to further investigate the genetic control of blood vessel hierarchy and AV communication.
Materials and Methods
Mice. The Tie2-tTA construct contains the tetracycline transactivator (tTA) between the 2.1-kb Tie2 promoter and 10-kb enhancer (18). The TRE-int3 transgene (in which TRE designates the tetracycline response element) encodes amino acid 1411–1964 of mouse Notch4, and the TRE-Notch1ICD transgene encodes amino acid 1756–2556 of human Notch1. Mice were derived in the FVB/N background as described in ref. 37. Primer sequences for genotyping are listed in Supporting Text, which is published as supporting information on the PNAS web site.
Vascular Staining. LacZ activity was detected by standard protocols (19) or with an anti-β-galactosidase antibody (MP Biomedicals, Irvine, CA). Lectin staining was performed as described in ref. 20. Immunostaining was performed with anti-CD31 (MEC13.3, Pharmingen) and anti-SMC-α-actin (anti-α-SMA) (1A4-AP, Sigma). Quantitative analysis was performed by measuring the pixel density of lectin and LacZ staining using nih image 1.62 software.
Isolation of Liver ECs and Gene Expression Analysis. Mouse liver ECs were isolated with anti-CD31-coated magnetic beads (Dynal, Great Neck, NY) as described in ref. 21. Total RNA was extracted from whole liver or CD31-positive liver ECs with TRIzol (Invitrogen) and reverse-transcribed. Normalized expression was calculated using hypoxanthine guanine phosphoribosyltransferase (HPRT) as a reference. Primer sequences are listed in Supporting Text.
Vascular Shunting. Fifteen-micrometer fluorescent microspheres (Molecular Probes) were injected into the portal vein of anesthetized mice, and tissues were examined with a fluorescence dissecting microscope. For casting studies, blood was cleared by left ventricular perfusion with 0.85% (wt/vol) saline. Five milliliters of Microfil compound (Flowtech, Carver, MA) (2.5 ml of MV-120 mixed with 2.5 ml of MV diluent) was then injected through the left ventricle. Tissues were fixed in 10% formalin and then cleared with ethanolmethyl salicylate.
Cell Proliferation. Mice were injected with 40 mg/kg BrdUrd and killed after 3 h. Staining was performed with a BrdUrd kit (Zymed).
Echocardiography. Echocardiography was performed as described in ref. 22. Details are provided in Supporting Text.
Results
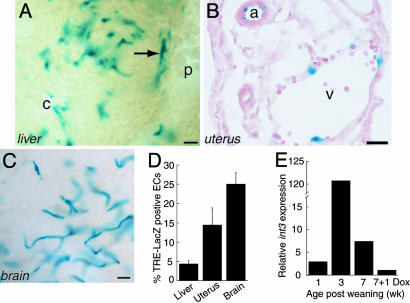
tTA Drives Transgene Expression Specifically in Adult ECs. We first generated Tie2-tTA mice in which the tTA is expressed in ECs by the Tie2 promoter and enhancer (18). To assess transgene expression in adult mice, we performed a reporter assay with mice carrying LacZ under the control of the TRE (23). To achieve adult expression in this experiment and all others, doxycycline (Dox), a more stable derivative of tetracycline, was administered throughout the gestation period until weaning (3 weeks). In the liver, LacZ activity was detected in the hepatic arterial, portal, and central venous, as well as the sinusoidal endothelium (Fig. 1A). EC-specific staining was detected in arteries, veins, and capillaries in all organs examined, including the uterus and brain (Fig. 1 B and C) but not outside of the circulatory system. To assess the proportion of ECs with active tTA, we compared the β-galactosidase staining pattern to that of Lycopersicon esculentum lectin staining, which labels all ECs. The proportion of ECs containing detectable tTA activity varied across organs and was estimated to be 5% in the liver, 15% in the uterus, and 25% in the brain (Fig. 1D; see also Fig. 6, which is published as supporting information on the PNAS web site).
Fig. 1.
Tie2-tTA drives transgene expression specifically in adult ECs. (A–C) β-Galactosidase staining of liver (A, 100 μm), uterus (B, 10 μm), and brain (C, 100 μm) sections. Arrow, hepatic artery; p, portal vein; c, central vein; v, uterine vein; a, uterine artery. (Scale bars, 25μm.) (D) Percentage of tTA-positive ECs in Tie2-tTA/TRE-LacZ organs. (E) int3 expression by quantitative RT-PCR. Each bar represents normalized int3 expression in a Tie2-tTA/TRE-int3 mouse relative to a TRE-int3 mouse.
To express int3 in ECs, we generated TRE-int3 mice and then crossed them with Tie2-tTA mice. As expected, in the Tie2-tTA/TRE-int3 double transgenics (also called mutants), int3 expression during development led to embryonic lethality. However, mutants fed with Dox were born live and healthy. To assess transgene expression, we analyzed ECs isolated from the liver. At 1 week postweaning, mutants expressed slightly higher levels of int3 mRNA than did controls, and int3 mRNA levels peaked at ≈3 weeks postweaning. int3 mRNA levels fell to background within 1 week after readministration of Dox (Fig. 1E). Thus, Tie2-tTA drives transgene expression not only in an EC-specific fashion, but also in a temporally regulatable fashion.
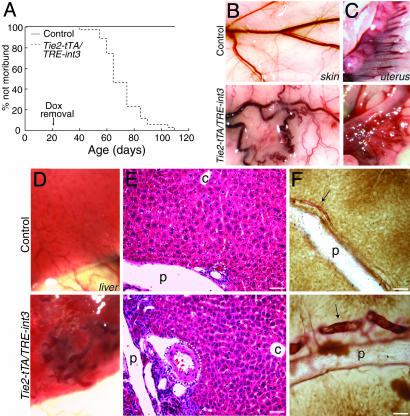
int3 Causes Lethality and Blood Vessel Enlargement. Within 4 to 7 weeks after weaning, >80% of mutants died or were killed due to severe illness (Fig. 2A). Over the same time period, none of the controls (Tie2-tTA, TRE-int3, and nontransgenic) became sick or died. Sick mice exhibited weight loss, respiratory distress, lethargy, and a disheveled coat. To identify the effects of EC int3, we performed gross phenotypic analysis of moribund mutants and detected enlarged and tortuous blood vessels in several organs, most notably the skin, uterus, and liver, at an approximate frequency of 60%, 90%, and 100%, respectively (Fig. 2 B–D). Based on anatomical, histological, and AV marker analyses, both the arteries and veins were enlarged in the skin and uterus (data not shown). Cardiac enlargement also occurred in 100% of moribund mutants. By contrast, we did not detect any cardiovascular defects in the controls. The effect of int3 on blood vessels appeared to be organ-specific, as evidenced by the fact that we did not observe obviously enlarged blood vessels in the brain, despite the presence of tTA activity (and, presumably, int3 expression) in this organ.
Fig. 2.
int3 expression in adult mice leads to lethality and blood vessel enlargement. (A) Survival curve. (B–D) Gross phenotype. (E) Hematoxylin/eosin liver sections. Hepatic arteries are encircled. p, portal veins; c, central veins. (F) Anti-CD31 (brown) and α-SMA (red) double staining of liver sections. Arrows, hepatic arteries; p, portal veins. (Scale bars, 50 μm.)
Expression of int3 affected the liver most severely, and thus we focused our analyses on the hepatic vasculature. Upon dissection, we observed enlarged and tortuous blood vessels on the surface of mutant livers (Fig. 2D). Further histological and vascular casting analyses demonstrated that hepatic arteries were significantly enlarged (Figs. 2 E and F and 3F). Comparatively, the portal and central veins were mildly enlarged (Figs. 2E and 3F). To confirm this finding, we examined 100-μm-thick liver sections that were immunostained for both CD31 and α-SMA and observed enlarged and tortuous hepatic arteries accompanied by increased α-SMA staining (Fig. 2F). The vascular SMC content often varies in dilated blood vessels, and thus we further performed α-SMA immunostaining. Low levels of α-SMA were observed in livers from control mice, and the staining was confined to a subset of hepatic arteries (Fig. 4A). In contrast, strong α-SMA staining was observed in hepatic arteries and portal and central veins of mutant liver (Fig. 4B). Increased α-SMA was also observed in the enlarged uterine vessels (data not shown). Taken together, these findings demonstrate that expression of int3 in ECs results in hepatic arterial enlargement and enhanced SMC content.
Fig. 3.
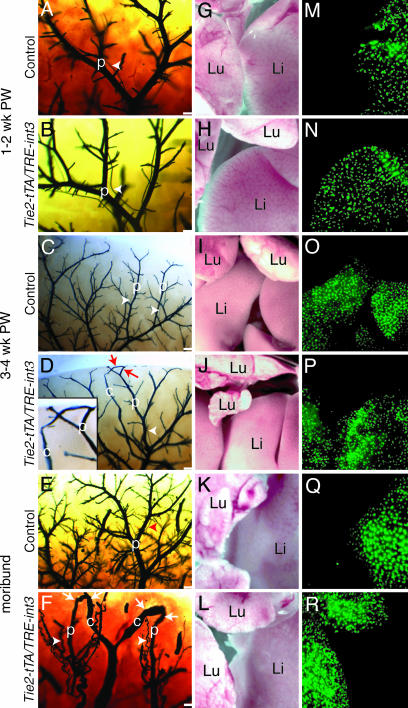
Portosystemic shunting in Tie2-tTA/TRE-int3 mice. (A–F) Liver vascular casts of control (A, C, and E) and Tie2-tTA/TRE-int3 (B, D, and F) mice at the indicated age postweaning (PW). Arrowheads, hepatic arteries; arrows, connections between portal (p) and central (c) veins. Central veins were distinguished from portal veins by the absence of adjacent hepatic arteries. (Scale bars, 0.2 mm.) (D Inset) A higher magnification of the shunt. (G–R) Light and fluorescence photomicrographs after portal vein microsphere injections into control (G, I, K, M, O, and Q) and Tie2-tTA/TRE-int3 (H, J, L, N, P, and R) mice. Li, liver; Lu, lung.
Fig. 4.
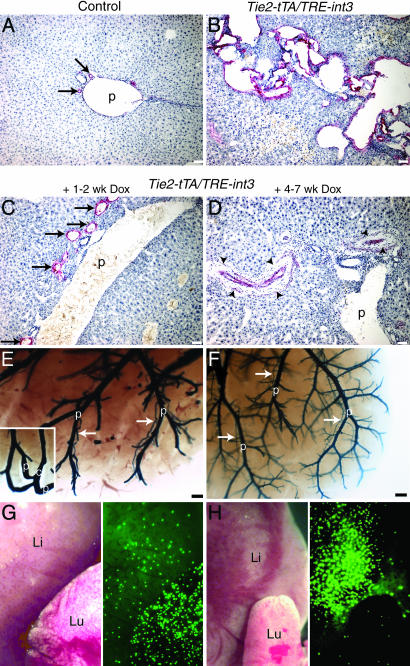
int3-induced hepatic vascular effects are reversible. (A–D) Liver sections stained with anti-α-SMA from control (A), moribund Tie2-tTA/TRE-int3 (B), and moribund Tie2-tTA/TRE-int3 mice fed with Dox (C and D). (E–H) Liver vascular casts (E and F) and microsphere assay (G and H) of moribund Tie2-tTA/TRE-int3 mice fed with Dox. (E Inset) A shunt in another region of the same liver. Arrows, hepatic arteries; p, portal veins; c, central veins; arrow-heads, fibrosis surrounding regressed hepatic arteries; Li, liver; Lu, lung. (Scale bars: A–D, 50 μm; E and F, 0.2 mm.)
int3 Triggers Vascular Shunting Before Hepatic Artery Enlargement. To test whether EC expression of int3 results in AV shunting in adults, we performed vascular casting. There are three possible hepatic shunts: portosystemic (portal vein to central vein), AV (hepatic artery to central vein), and arterioportal (hepatic artery to portal vein). The casting reagent filled the liver vasculature in two phases: an early phase entering through the hepatic arteries and a late phase entering through the portal veins. It did not pass through the sinusoids, and thus the central veins, in the absence of shunting, were invisible. Both control and mutants at 1–2 weeks postweaning displayed a hierarchically branched network of hepatic arteries running alongside portal veins (Fig. 3 A and B). Both vessel types were relatively straight, and no shunts were observed. At 3–4 weeks postweaning, after the induction of int3 but before any signs of overt illness, the hierarchical network of portal veins and hepatic arteries appeared similar between the mutants and controls. However, we found several central vein casts in the mutants, which connect with portal veins directly (Fig. 3 C and D). At this stage, we did not detect obvious hepatic artery enlargement. As the health of the mutants deteriorated, we observed more severe portosystemic shunting as well as massively enlarged and tortuous hepatic arteries, often accompanied by AV shunts (Fig. 3 E and F and data not shown).
To determine the physiological impact of shunts, we injected fluorescent microspheres that are too large (15 μm in diameter) to pass through capillaries into the portal vein and then examined their retention in the liver and the lung. As predicted from the casting studies, all microspheres were retained in the livers when these injections were performed on control and mutants at 1–2 weeks postweaning (Fig. 3 G, H, M, and N). Despite the presence of portosystemic shunts by vascular casting in mutant livers at 3–4 weeks postweaning, most beads were retained in the liver (Fig. 3 I, J, O, and P). In sharp contrast, microspheres injected into the portal vein of moribund mutants completely bypassed the liver and lodged in the lungs, indicating the presence of portosystemic shunts (Fig. 3 K, L, Q, and R). These findings indicate that EC expression of int3 leads to shunting, which is the earliest vascular abnormality detected thus far.
int3-Triggered Animal Illness and the Hepatic AVM Are Reversible. To test whether sustained expression of int3 is required for the maintenance of vascular defects, we fed moribund mice with Dox. Remarkably, within a few days of Dox treatment, the mice became more active, gained weight, and ultimately recovered from their illness (Movies 1 and 2, which are published as supporting information on the PNAS web site). The hepatic blood vessels in the mutants treated with Dox for only 1–2 weeks showed evidence of regression of both vessel enlargement and shunting, as shown by histology, vascular casting, and microsphere perfusion (Fig. 4 C, E, and G). Livers from moribund mice treated with Dox for 4–7 weeks displayed a near complete regression of enlarged hepatic arteries as well as a dramatic reduction in α-SMA immunoreactivity (Fig. 4 D and F). At this stage, only occasional shunts were detected, and, accordingly, very few microspheres bypassed the liver (Fig. 4H). These data show that both the animal illness and hepatic vascular defects regress once int3 expression is turned off, demonstrating that int3 is required for the maintenance of these abnormalities.
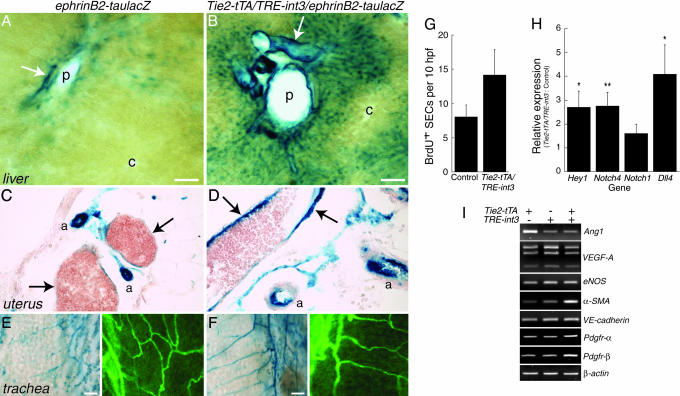
int3 Induces Robust Arterialization in Adult Blood Vessels. Because the Notch pathway is implicated in AV specification during embryogenesis and because ephrinB2 is an arterial EC marker that is genetically downstream of the Notch signaling pathway (16), we next examined ephrinB2 expression. Semiquantitative RT-PCR analysis demonstrated a strong up-regulation of ephrinB2 in the mutant liver (data not shown). To confirm this finding and to determine the localization of the increased ephrinB2 expression, we used reporter mice, ephrinB2-tauLacZ, in which LacZ is present in arteries under the control of the ephrinB2 regulatory sequences (19). Hepatic arteries in control ephrinB2-tauLacZ mice stained strongly for LacZ; the portal veins and periportal sinusoids stained weaker; and the pericentral sinusoids and central veins were negative (Fig. 5A). In contrast, we observed stronger LacZ staining in the enlarged hepatic arteries and portal veins of Tie2-tTA/TRE-int3/ephrinB2-tauLacZ mice (Fig. 5B). In addition, there was an extension of LacZ-positive sinusoidal ECs from the periportal to the pericentral region (Fig. 5B). Furthermore, we observed ectopic or increased LacZ staining in otherwise negative or low LacZ ECs in the uterine veins and in the trachea despite a similar overall vascular density (Fig. 5 C–F). These results suggest that int3 expression leads to more ECs exhibiting arterial identity.
Fig. 5.
Cellular and molecular effects of int3.(A–F) EphrinB2-LacZ reporter assay. (A and B) Liver sections (100 μm). Arrows, hepatic arteries; p, portal veins; c, central veins. (C and D) Uterus sections (10 μm). Arrows, uterine veins; a, arteries. (E and F) LacZ-(Left) and lectin-(Right) stained trachea whole mounts. (Scale bars, 50 μm.) (G) Proliferation analysis. Liver sinusoidal EC (SEC) BrdUrd labeling in 10 high-power fields (hpf) was quantified. The data represent the mean ± SEM of eight control and six mutants at 3–4 weeks postweaning (P = 0.13 by Student's t test). (H) Notch pathway gene expression by quantitative RT-PCR. Data represents the mean ± SEM of HPRT normalized expression in moribund Tie2-tTA/TRE-int3 mice (n = 4 or 6) relative to expression in age-matched controls (n = 4; two Tie2-tTA and two TRE-int3). *, P < 0.05; **, P < 0.01 of expression in Tie2-tTA/TRE-int3 mice relative to controls by Student's t test. The P value of Notch1 expression is 0.057. (I) Gene expression by RT-PCR.
To examine whether int3 induced EC proliferation, we performed in vivo BrdUrd labeling experiments. We observed a slight but statistically insignificant increase in the number of BrdUrd-positive sinusoidal ECs in the mutant livers compared with controls by histological evaluations (Fig. 5G). Because these analyses relied on morphological criteria to identify sinusoidal ECs, we injected BrdUrd into mice and then analyzed the number of CD31+BrdUrd+ nuclei relative to total CD31+ nuclei in isolated ECs. Again, we found a slight but insignificant increase in EC proliferation in mutants relative to controls (0.98% ± 0.22% versus 0.71% ± 0.15%, P = 0.38). Thus, EC expression of int3 does not lead to significant increases in EC proliferation.
Up-Regulation of Notch Pathway Gene Expression in the Mutant Liver. To investigate the potential molecular mechanisms of int3, we performed quantitative RT-PCR on total liver RNA (Fig. 5H). As expected, we observed increased transcription of Hey1, a down-stream Notch target (12). int3 caused the up-regulation of endogenous Notch4 and Dll4, genes that are expressed primarily in arterial ECs (6). Furthermore, the mRNA level of Notch1, which is expressed primarily in ECs in the liver (24), was slightly increased. Our results are consistent with in vitro studies that show that expression of int3 leads to the up-regulation of Dll4 in cultured ECs (25) and demonstrate that int3 can up-regulate the expression of endogenous Notch pathway genes in vivo.
In contrast, we did not detect an increase in the expression of Ang1 (angiopoietin-1), VEGF-A, or eNOS (endothelial nitric oxide synthase), genes that can promote blood vessel enlargement in adult mice (Fig. 5I) (3, 4, 26). We also examined genes involved in epithelial-to-mesenchymal transitions, because constitutively active Notch can induce EC expression of α-SMA while inhibiting vascular endothelial (VE)-cadherin expression (27, 28). Although α-SMA and platelet derived growth factor receptor-α and -β were up-regulated, we did not detect a decrease in VE-cadherin expression in Tie2-tTA/TRE-int3 liver (Fig. 5I). In summary, arterial-specific genes and genes in the Notch signaling pathway are up-regulated in response to int3 expression.
Cardiomegaly Is a Compensatory Response to int3-Induced AV Shunting. Autopsies revealed that the mutants developed enlarged hearts (Fig. 7, which is published as supporting information on the PNAS web site). Therefore, we investigated whether the cardiac enlargement was associated with a primary heart defect or resulted from the pathophysiological effects of AV shunting. Cardiomegaly that occurs after the onset of primary heart failure is a compensatory response to the marked reduction in cardiac output (29). By contrast, cardiomegaly in response to peripheral vascular defects such as AV shunting is a compensatory response to an increase in cardiac output (30). Echocardiography demonstrated that cardiac output and estimated left ventricular mass were ≈25–35% higher in Tie2-tTA/TRE-int3 mice than in controls (Fig. 7). This increase in cardiac output and heart mass correlated with an increase in left ventricular end diastolic and systolic volume (Fig. 7). Considered together, these findings are consistent with the notion that cardiomegaly and, eventually, heart failure are compensatory effects of peripheral AVM. This conclusion is supported by our finding that cardiomegaly and heart failure were reversed when int3 was turned off (data not shown).
Expression of Constitutively Active Notch1 in the ECs Leads to a Similar Vessel Enlargement Phenotype. To investigate whether Notch1 bears similar function, we crossed the Tie2-tTA mice with mice expressing constitutively active Notch1 under the control of a TRE. Tie2-tTA/TRE-Notch1ICD mice survived and appeared phenotypically normal throughout the monitoring period. However, at 1 year of age, three of four Tie2-tTA/TRE-Notch1ICD mice displayed enlarged hepatic blood vessels containing excessive SMCs (Fig. 8, which is published as supporting information on the PNAS web site). Taken together, these data demonstrate that constitutively active Notch1 and Notch4 can elicit vascular enlargement in mice when expressed in adult ECs, suggesting that the effects are specific to the Notch genes.
Discussion
int3 Leads to Reversible AV Shunting in the Established Adult Vasculature. The objective of this study was to ascertain the function of int3 in the adult endothelium. The mammalian vasculature is a complex system that exhibits diverse anatomical and physiological properties in different organs. By focusing on the liver, our data provide crucial insights into the mechanism of int3 action. The first detectable vascular abnormality is portosystemic shunting, formed de novo days after maximal int3 expression. An obvious concern is whether int3 exacerbates developmental defects in Tie2-tTA/TRE-int3 mice due to leaky transgene expression. We have not detected any abnormal vascular structures in mice 2 weeks after weaning when int3 expression is low by histology, vascular casting, microsphere perfusion, or overall health monitoring. Therefore, our data suggest that the AV shunts develop as a direct consequence of int3 expression in adulthood. Our finding that repression of int3 expression rapidly improves the animals' health and induces regression of the mutant phenotype further supports this notion.
The involvement of Notch signaling in AV communication has been envisaged based on embryonic studies of both fish and mouse Notch mutants. For example, in zebrafish, the loss-of-function Notch mutants mibta52b and EGFPSuDN cause AV shunting (31). Recent studies of mice demonstrate that the loss-of-function Notch mutants Dll4+/– (11) and EC deletion of Rbpsuh (10) exhibit embryonic AV shunting. To our knowledge, our data provide the first demonstration that activation of Notch signaling can induce AV shunts, suggesting that proper levels of Notch signaling are critical for the maintenance of functional AV interfaces.
Related to the shunting effect is the histological manifestation of AVMs, which occur in 100% of moribund Tie2-tTA/TRE-int3 mice. AVMs are devastating vascular anomalies comprising dysplastic arteries intermingled with arterialized veins (32). TGF-β signaling has been implicated in one form of AVM, hereditary hemorrhagic telangiectasia (HHT), because mutations in members of TGF-β pathway are responsible for HHT (33). The involvement of Notch signaling in AVMs has been suspected based on the findings that Notch4 is down-regulated in a form of human AVM, cerebral cavernous malformation (CCM), and because it appears to be genetically downstream of the Ccm1 gene in mice (34). Our data demonstrate that activation of Notch signaling is sufficient to induce AVM-like abnormalities in mice.
AVMs have been presumed to be congenital abnormalities that fail to regress, and surgical resection is the only “cure” for the disease (32). In contrast to this prevailing view, we show in our Tie2-tTA/TRE-int3 model that the defective vasculature regresses upon transgene repression, proposing the tantalizing possibility that correcting the underlying genetic lesions can cure AVMs. One caveat of our regression analysis is that we cannot assess the degree of shunting in individual moribund animals before int3 repression. However, the overt signs of illness correlate 100% with profound AV shunting, and, because both the animals' health and AVMs are cured by Dox treatment, we are reasonably certain that we are observing true regression.
int3 and Blood Vessel Size. Genetic regulation of mammalian blood vessel size is an intriguing but largely unknown subject. The dramatic enlargement of hepatic arteries in the Tie2-tTA/TRE-int3 mice raises the possibility that int3 may affect vessel size directly. This possibility is consistent with findings in embryonic studies (7–14). However, it is well known that AV shunts can alter hemodynamics and lead to arterial enlargement and tortuosity (35). Because hepatic artery enlargement occurs subsequently to portosystemic shunting, it is likely that this vessel enlarges secondarily to shunting. Our studies do not exclude, however, the possibility that int3 expression may regulate vessel caliber directly, but this model is not ideal to define a direct effect, given the concurrent shunting.
int3 Induces Arterialization of Adult Vasculature. Studies of the Notch pathway during zebrafish development first demonstrated its involvement in EC AV differentiation. These studies revealed that loss-of-function mutants exhibit decreased arterial ephrinB2 expression and ectopic venous marker flt4 expression (31). Loss-of-function mutants in the Notch pathway, such as Hey1–/–/Hey2–/– or Notch1–/– (12), Dll4–/– (13), and Rbpsuh–/– (10) also exhibit a loss of ephrinB2 expression in the embryonic dorsal aortae. Here, we demonstrate that EC int3 expression is sufficient to induce ectopic ephrinB2 expression in adult mice. It has been reported that arterial-like flow increases ephrinB2 expression (36), and thus the effects of int3 on ephrinB2 expression in the enlarged vessels is confounded by flow. Our finding that vascular ephrinB2 is up-regulated in several organs, including tracheas that lack other detectable blood vessel abnormalities, suggests that int3 can directly promote ephrinB2 expression and that adult EC can acquire arterial features.
Another measure of arterialization is vascular SMC content, and we have observed enhanced α-SMA expression in dilated hepatic and uterine blood vessel walls. This phenotype is similar to human AVM histology and is likely due to AV shunting (35). It is also possible that int3 up-regulates paracrine factors or acts through cell–cell interfaces to promote SMC proliferation or migration. Because of the AVMs, however, this system is not ideal to delineate the int3-specific effects on SMC.
In summary, we conclude that the primary effect of int3 is to render arterial characteristics to nonarterial ECs, which triggers the initial AV shunting that ultimately leads to the progression of AVM in mice. We believe that our Tie2-tTA/TRE-int3 mouse model is a powerful tool for dissecting the mechanisms of AVM pathogenesis, which may ultimately offer prospects for therapeutic interventions.
Supplementary Material
Acknowledgments
We thank Dr. J. Michael Bishop, in whose laboratory the Tie2-tTA mice were generated; Dr. Shuji Joho for performing echocardiograms; Drs. Bruce Conklin (University of California, San Francisco) and David Anderson (California Institute of Technology, Pasadena, CA) for providing the TRE-LacZ and ephrinB2-tauLacZ mice, respectively; and Drs. Henry Bourne and Douglas Hanahan for critical reading of the manuscript. We also thank Dr. Yung Hae Kim and other members of our laboratory for helpful discussions and the UCSF Liver Center, which is supported by National Institutes of Health (NIH) Grant P30-DK26743, for histology. This work was primarily supported by startup funds from the Pacific Vascular Research Institute and the Howard Hughes Medical Institute University of California, San Francisco, Biomedical Research Support Program (to R.W.). Additional funding was provided by the NIH; The G. W. Hooper Foundation (J.M.B.); Department of Defense Breast Cancer Program DAMD17-9818193 (Y.Y.); National Cancer Institute Grants CA72006 and AI053194 (to Z.W.); and Grants NIH R01 CA 83736, ACS RPG LBC99465, and LLS 1298-02 (to A.J.C.).
Author contributions: T.R.C., Y.Y., X.W., M.T.L., G.L.T., and R.W. designed research; T.R.C., Y.Y., X.W., M.T.L., G.L.T., and R.W. performed research; Y.Y., L.J.B., A.J.C., Z.W., and R.W. contributed new reagents/analytic tools; T.R.C., X.W., M.T.L., G.L.T., L.M.M., Z.W., and R.W. analyzed data; and T.R.C. and R.W. wrote the paper.
Abbreviations: AV, arteriovenous; AVM, arteriovenous malformation; EC, endothelial cell; SMC, smooth muscle cell; Dox, doxycycline; tTA, tetracycline transactivator; TRE, tetracycline response element; α-SMA, SMC-α-actin.
References
- 1.Murray, C. D. (1926) Proc. Natl. Acad. Sci. USA 12, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehoux, S. & Tedgui, A. (2003) J. Biomech. 36, 631–643. [DOI] [PubMed] [Google Scholar]
- 3.Suri, C., McClain, J., Thurston, G., McDonald, D. M., Zhou, H., Oldmixon, E. H., Sato, T. N. & Yancopoulos, G. D. (1998) Science 282, 468–471. [DOI] [PubMed] [Google Scholar]
- 4.Dor, Y., Djonov, V., Abramovitch, R., Itin, A., Fishman, G. I., Carmeliet, P., Goelman, G. & Keshet, E. (2002) EMBO J. 21, 1939–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai, E. C. (2004) Development (Cambridge, U.K.) 131, 965–973. [DOI] [PubMed] [Google Scholar]
- 6.Villa, N., Walker, L., Lindsell, C. E., Gasson, J., Iruela-Arispe, M. L. & Weinmaster, G. (2001) Mech. Dev. 108, 161–164. [DOI] [PubMed] [Google Scholar]
- 7.Xue, Y., Gao, X., Lindsell, C. E., Norton, C. R., Chang, B., Hicks, C., Gendron-Maguire, M., Rand, E. B., Weinmaster, G. & Gridley, T. (1999) Hum. Mol. Genet. 8, 723–730. [DOI] [PubMed] [Google Scholar]
- 8.Swiatek, P. J., Lindsell, C. E., del Amo, F. F., Weinmaster, G. & Gridley, T. (1994) Genes Dev. 8, 707–719. [DOI] [PubMed] [Google Scholar]
- 9.Krebs, L. T., Xue, Y., Norton, C. R., Shutter, J. R., Maguire, M., Sundberg, J. P., Gallahan, D., Closson, V., Kitajewski, J., Callahan, R., et al. (2000) Genes Dev. 14, 1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 10.Krebs, L. T., Shutter, J. R., Tanigaki, K., Honjo, T., Stark, K. L. & Gridley, T. (2004) Genes Dev. 18, 2469–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gale, N. W., Dominguez, M. G., Noguera, I., Pan, L., Hughes, V., Valenzuela, D. M., Murphy, A. J., Adams, N. C., Lin, H. C., Holash, J., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 15949–15954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer, A., Schumacher, N., Maier, M., Sendtner, M. & Gessler, M. (2004) Genes Dev. 18, 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duarte, A., Hirashima, M., Benedito, R., Trindade, A., Diniz, P., Bekman, E., Costa, L., Henrique, D. & Rossant, J. (2004) Genes Dev. 18, 2474–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uyttendaele, H., Ho, J., Rossant, J. & Kitajewski, J. (2001) Proc. Natl. Acad. Sci. USA 98, 5643–5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawson, N. D. & Weinstein, B. M. (2002) Nat. Rev. Genet. 3, 674–682. [DOI] [PubMed] [Google Scholar]
- 16.Shawber, C. J. & Kitajewski, J. (2004) BioEssays 26, 225–234. [DOI] [PubMed] [Google Scholar]
- 17.Uyttendaele, H., Marazzi, G., Wu, G., Yan, Q., Sassoon, D. & Kitajewski, J. (1996) Development (Cambridge, U.K.) 122, 2251–2259. [DOI] [PubMed] [Google Scholar]
- 18.Schlaeger, T. M., Bartunkova, S., Lawitts, J. A., Teichmann, G., Risau, W., Deutsch, U. & Sato, T. N. (1997) Proc. Natl. Acad. Sci. USA 94, 3058–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, H. U., Chen, Z. F. & Anderson, D. J. (1998) Cell 93, 741–753. [DOI] [PubMed] [Google Scholar]
- 20.Ezaki, T., Baluk, P., Thurston, G., La Barbara, A., Woo, C. & McDonald, D. M. (2001) Am. J. Pathol. 158, 2043–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeCouter, J., Moritz, D. R., Li, B., Phillips, G. L., Liang, X. H., Gerber, H. P., Hillan, K. J. & Ferrara, N. (2003) Science 299, 890–893. [DOI] [PubMed] [Google Scholar]
- 22.Ishizaka, S., Sievers, R. E., Zhu, B. Q., Rodrigo, M. C., Joho, S., Foster, E., Simpson, P. C. & Grossman, W. (2004) Am. J. Physiol. Heart Circ. Physiol. 286, H1208–H1215. [DOI] [PubMed] [Google Scholar]
- 23.Redfern, C. H., Coward, P., Degtyarev, M. Y., Lee, E. K., Kwa, A. T., Hennighausen, L., Bujard, H., Fishman, G. I. & Conklin, B. R. (1999) Nat. Biotechnol. 17, 165–169. [DOI] [PubMed] [Google Scholar]
- 24.Nijjar, S. S., Crosby, H. A., Wallace, L., Hubscher, S. G. & Strain, A. J. (2001) Hepatology 34, 1184–1192. [DOI] [PubMed] [Google Scholar]
- 25.Shawber, C. J., Das, I., Francisco, E. & Kitajewski, J. (2003) Ann. N.Y. Acad. Sci. 995, 162–170. [DOI] [PubMed] [Google Scholar]
- 26.Boo, Y. C. & Jo, H. (2003) Am. J. Physiol. Cell Physiol. 285, C499–C508. [DOI] [PubMed] [Google Scholar]
- 27.Noseda, M., McLean, G., Niessen, K., Chang, L., Pollet, I., Montpetit, R., Shahidi, R., Dorovini-Zis, K., Li, L., Beckstead, B., et al. (2004) Circ. Res. 94, 910–917. [DOI] [PubMed] [Google Scholar]
- 28.Timmerman, L. A., Grego-Bessa, J., Raya, A., Bertran, E., Perez-Pomares, J. M., Diez, J., Aranda, S., Palomo, S., McCormick, F., Izpisua-Belmonte, J. C. & de la Pompa, J. L. (2004) Genes Dev. 18, 99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jessup, M. & Brozena, S. (2003) N. Engl. J. Med. 348, 2007–2018. [DOI] [PubMed] [Google Scholar]
- 30.Naschitz, J. E., Slobodin, G., Lewis, R. J., Zuckerman, E. & Yeshurun, D. (2000) Am. Heart J. 140, 111–120. [DOI] [PubMed] [Google Scholar]
- 31.Lawson, N. D., Scheer, N., Pham, V. N., Kim, C. H., Chitnis, A. B., Campos-Ortega, J. A. & Weinstein, B. M. (2001) Development (Cambridge, U.K.) 128, 3675–3683. [DOI] [PubMed] [Google Scholar]
- 32.Vikkula, M., Boon, L. M., Mulliken, J. B. & Olsen, B. R. (1998) Trends Cardiovasc. Med. 8, 281–292. [DOI] [PubMed] [Google Scholar]
- 33.Larson, A. M. (2003) J. Clin. Gastroenterol. 36, 149–158. [DOI] [PubMed] [Google Scholar]
- 34.Whitehead, K. J., Plummer, N. W., Adams, J. A., Marchuk, D. A. & Li, D. Y. (2004) Development (Cambridge, U.K.) 131, 1437–1448. [DOI] [PubMed] [Google Scholar]
- 35.Sho, E., Nanjo, H., Sho, M., Kobayashi, M., Komatsu, M., Kawamura, K., Xu, C., Zarins, C. K. & Masuda, H. (2004) J. Vasc. Surg. 39, 601–612. [DOI] [PubMed] [Google Scholar]
- 36.le Noble, F., Moyon, D., Pardanaud, L., Yuan, L., Djonov, V., Matthijsen, R., Breant, C., Fleury, V. & Eichmann, A. (2004) Development (Cambridge, U.K.) 131, 361–375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.