The devil was always in the details. But as we accumulate more and more quantitative data on living cells, those diabolical details become increasingly finicky and numerical. Small differences that we would have cheerfully disregarded a decade ago as being due to “experimental error” or “differences in technique” suddenly become crucial—the nub of debate if not controversy. Sometimes, even, they reveal cracks in the smooth surface of current dogma and hint at unexplored layers beneath. Take, for example, the question of the “gain” or “amplification” shown by swimming bacteria when they respond to chemical attractants. Arguments about the size of this gain and whether it is consistent with the currently accepted model of signal transduction have been going on for years. Now, an innovative technique applied to the problem measures chemotactic gain with unparalleled accuracy (1). And yes, there really is something strange going on.
The story begins 30 years ago when Howard Berg and Douglas Brown at the University of Colorado used a novel three-dimensional tracking microscope they had developed to follow the swimming of individual Escherichia coli bacteria (2). Swimming was found to consist of smooth “runs” interrupted roughly every second by transient “tumbles” (or “twiddles”). Chemotaxis—the ability of the cells to move toward distant sources of food molecules—was based on the suppression of tumbles in cells that happened by chance to be moving up the gradient. The impressive sensitivity of this process was later measured by applying attractants such as aspartate directly from a pipette onto bacteria tethered onto a coverslip (3). In this tethered-cell method, periodic reversals of rotation of individual flagellar motors are observed and the swimming performance recorded as the rotational bias—the fraction of time spent in counterclockwise rotation. The measurements of Segall et al. (3) revealed that the amplification, or gain, of tethered cells was, to use their adjective, “prodigious.” Bacteria could detect a change in occupancy of the aspartate receptor as little as 0.1–0.2%, corresponding to the binding of one or two molecules per cell. The “gain” of the system—calculated as the change in bias divided by the change in occupancy (both dimensionless quantities)—was about 60 (Fig. 1).
E. coli is able to sense aspartate over a range of at least 5 orders of magnitude in concentration by using just one molecular species of receptor!
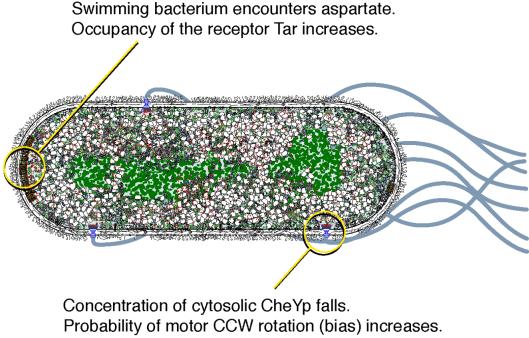
Figure 1.
Chemotactic gain. If an E. coli cell swims into the attractant molecule aspartate, this will lead to an increase in the occupancy of aspartate receptors, typically clustered at one end of the cell, as shown here. A cascade of signal transduction events then leads to a fall in the cytosolic concentration of the protein CheYp, which diffuses to one of the flagellar motors. A fall in CheYp concentration at the motor increases the probability that it will turn in a counterclockwise direction, causing the bacterium to persist in this favorable direction. The probability that a motor turns counterclockwise is expressed as the rotational bias. The chemotactic gain, which is the subject of this commentary, is simply the change in bias divided by the change in occupancy.
Next on the scene was a cohort of talented molecular biologists, biochemists, and geneticists who set about systematically to unravel the molecular hardware of the chemotactic response. The receptors were sequenced and isolated, and parts of them crystallized. The crucial protein kinase CheA, which is regulated by the receptors and transfers a phosphoryl group onto other components of the pathway, was characterized biochemically, and its structure was determined. The small molecule CheY, which picks up a phosphoryl group from CheA and diffuses to the flagellar motors, was crystallized and modified in multiple mutated forms. Progress was exhilaratingly successful and quickly led to the formulation of what has come to be widely accepted as the canonical description of the pathway (Fig. 2). Few if any other signal transduction pathways are known with such completeness and in such detail (the only competitor being the photoresponse of rod outer segments) so that bacterial chemotaxis became a honey pot for modelers. Numerous computer programs were written to represent kinetic and other features of the pathway, with significant success (4, 5). However, one issue remained stubbornly intractable—that of gain. Modelers either introduced arbitrary factors to boost the sensitivity of the system or admitted that their programs were woefully inadequate when compared with a real bacterium.
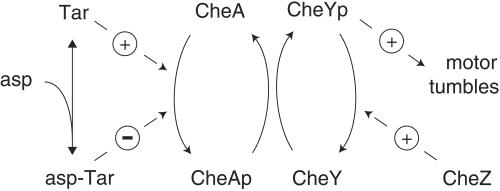
Figure 2.
Excitatory signal cascade. That portion of the signal cascade that relays a change in aspartate binding to a change in swimming behavior is shown in simplified form. Reactions responsible for adaptation are omitted for clarity. Tar is the receptor that binds aspartate (asp); CheA is the autophosphorylating kinase; CheY is the response regulator. After phosphorylation by CheA, CheYp diffuses to the six or so flagellar motors, increasing the likelihood that they turn clockwise and thereby initiate a tumble. CheZ is a protein that interacts with CheYp and stimulates its dephosphorylation. For further details see ref. 15.
Simply put, the issue is as follows. The slowest reaction in the cascade of reactions is the generation of phosphoryl groups by CheA—appropriately, because this is the step regulated by the receptor. Subsequent reactions are much faster, so CheYp should rise and fall more or less in parallel with changes in receptor occupancy. If a change in concentration of an attractant increases receptor occupancy by, say 0.1%, we expect that there should be a fall of at most 0.1% in the concentration of CheYp (Fig. 2). To translate this fall into a change in rotational bias, we use estimates of the power relationship between the two, sometimes termed the “Hill coefficient.” Early estimates of this value put it at around 4, leading to an expected change in bias of 0.002—which is roughly 50 times too small to be detectable by humans, or be useful to a bacterium. The mystery can be expressed in a different way. Estimates of the binding affinity of aspartate to the membrane receptor of wild-type E. coli typically give a dissociation constant in the range 1–5 μM (6). A bacterium responding to a change in occupancy of 0.1% is therefore sensing concentrations of aspartate of a few nanomolar. And yet we know from decades of observations that the same bacterium is also capable of responding to gradients of aspartate that extend up to 1 mM. Somehow, E. coli is able to sense aspartate over a range of at least 5 orders of magnitude in concentration by using just one molecular species of receptor!
It became apparent to many workers that to understand how this biological sensor works we need to look at its wiring. In other words, we need to know how the concentrations of the various elements in the pathway change when the bacterium encounters an attractant—a difficult task given that the cell can respond in less than a tenth of a second. The most pivotal point of control is arguably the diffusible signaling molecule CheYp, which links signals from the receptors to swimming behavior. Two laboratories used genetic tricks to stabilize CheYp so that its concentration could be estimated. In one study, mutant bacteria were constructed that, because of an absence of CheZ and other lesions, had essentially all of their CheY fully phosphorylated (7). In another study, a mutant CheY that works as though it were CheYp was used (8). In either case, the CheYp, real or ersatz, was produced from a plasmid that was induced at different levels in populations of bacteria, and their swimming behavior was followed. Despite the differences in approach, these two studies gave broadly similar estimates of the Hill coefficient between 3–5, and this value was therefore generally accepted. But not for long—a further genetic manipulation in which CheY was coupled to the fluorescent protein GFP resulted in a relationship between CheYp and bias much steeper than had been appreciated, with an apparent Hill coefficient of 10 ± 1 (9). The truly innovative feature of this latter work was that it estimated concentrations and responses in single cells, rather than the large populations of cells that had always been used before. Why should single cells give a higher Hill coefficient? Well, the possibility favored by the authors is that in large populations the true steepness of the response is masked by stochastic variations in the timing of different cells. A similar conclusion had been reached a few years previously in a very different system—the mitogen-activated protein (MAP) kinase cascade in frog oocytes (10).
Returning to the question of gain, it might appear that, with such a steep relationship between CheYp and bias, it would be easy to generate the necessary sensitivity. Not so, alas, as demonstrated in a series of studies of the swimming response by using a novel photo-activated form of attractant, which could be applied instantly, at the flash of a light. The swimming performance of populations of bacteria was monitored by video analysis, and their rate of change in direction in response to sudden onset of aspartate was analyzed. A series of reports employing this technique showed clearly a discrepancy of perhaps 30-fold between real bacteria and simulated bacteria, even allowing for the large Hill coefficient (11). This large gain was present in mutants lacking CheZ (suggesting that it does not contribute to the amplification), but deficient in mutants lacking CheR and CheB, the two enzymes responsible for adaptation, suggesting that the receptors were involved. These findings were then supported and extended by the new study by Sourjik and Berg (1) in which they measured fluctuations of CheYp directly. These authors used fluorescence energy transfer (FRET) to show that the stage between aspartate binding and CheYp concentration has an amplification 30–40 times greater than expected. When this “front end” amplification is conflated with a Hill coefficient of around 10, it gives an overall chemotactic gain similar in magnitude to that seen by Howard Berg and his colleagues thirty years ago.
Sourjik and Berg used genetic tricks to link fluorescent tags of two colors, yellow and cyan, onto CheY and CheZ, the protein that stimulates CheYp dephosphorylation (see Fig. 2). Cells attached to a polylysine-coated coverslip were placed in a flow cell and exposed to rapid changes in attractant (methylaspartate was used because it is not degraded by the cell). Changes of emitted light intensity in the two channels, yellow and cyan, then revealed changes in the number CheYp/CheZ pairs in the bacteria, leading to an estimate of the changes in CheYp concentration. Rapid changes in response to aspartate were interpreted as being due to fluctuation in the level of CheYp generated by the complex between receptors and CheA. In addition to showing a front-end amplification of about 35, which remained roughly constant over a wide range of ambient concentrations of aspartate, this study also uncovered intriguing effects of receptor methylation. Adaptation in this system is accomplished by two enzymes, CheR and CheB, which methylate and demethylate, respectively, the receptors. Sourjik and Berg found dramatic differences in the response of methylated and unmethylated receptors, qualitatively similar to those previously seen in cell-free preparations of receptors (12). There was also a puzzling loss of gain in mutants lacking CheR and CheB that could not easily be attributed to defects in methylation.
Probably the most important question arising from the Sourjik-Berg study is—what is the origin of the front-end amplification? The authors consider two possibilities. The first is that some form of cross-talk occurs between adjacent receptors that allows the effect of one ligand-binding event to spread to neighboring receptors (13). The second is that an inhibitory interaction between CheB and receptors increases amplification by reducing the number of receptors that actually signal (14). It is also conceivable, as suggested by Li and Weiss (12), that the Kd for aspartate is very much lower than everyone supposes. Conventional measurements have always put this value firmly in the 1–10 μM range, and all estimates of receptor occupancy are based on this value. But, if the Tar receptor had a Kd in the nanomolar range, if only transiently and in one conformation, then this could solve the gain problem in one blow.
A reader who has survived to this point might well ask, “Why do we care? What does it matter that the response of a tiny microbe has this or that numerical value?” The answer, surely, is that the chemotaxis pathway of coliform bacteria is so well documented it has become a benchmark for the responses of cells in general to chemical stimuli. It is, in a sense, the bellwether of signal transduction. If we are unable to understand how this simple network of proteins functions as an integrated system, then what hope have we of understanding the complex pathways in eukaryotic cells? In the long run, issues such as the precise value of chemotactic gain may indeed prove to be trivial, reflecting the biological vagaries of one particular organism. But it is also possible that they will lead us to novel principles that apply to many signaling cascades, in cells of all kinds. Which will it be? There is only one way to find out.
Acknowledgments
I am indebted to Matthew Levin and Shahid Khan for their helpful comments.
Footnotes
See companion article on page 123.
References
- 1.Sourjik V, Berg H C. Proc Natl Acad Sci USA. 2002;99:123–127. doi: 10.1073/pnas.011589998. . (First Published December 11, 2001; 10.1073/pnas.011589998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg H C, Brown D A. Nature (London) 1972;239:500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- 3.Segall J E, Block S M, Berg H C. Proc Natl Acad Sci USA. 1986;83:8987–8991. doi: 10.1073/pnas.83.23.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiro P A, Parkinson J S, Othmer H G. Proc Natl Acad Sci USA. 1996;94:7263–7268. doi: 10.1073/pnas.94.14.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray D, Bourret R B, Simon M I. Mol Biol Cell. 1993;4:469–482. doi: 10.1091/mbc.4.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunten P, Koshland D E., Jr J Biol Chem. 1991;266:1491–1496. [PubMed] [Google Scholar]
- 7.Alon L C, Camarena L, Surette M G, Aguera y Arcas B A, Liu Y, Leibler S, Stock J B. EMBO J. 1998;17:4238–4248. doi: 10.1093/emboj/17.15.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scharf B E, Fahrner K A, Turner L, Berg H C. Proc Natl Acad Sci USA. 1998;95:201–206. doi: 10.1073/pnas.95.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cluzel P, Surette M, Leibler S. Science. 2000;287:1652–1655. doi: 10.1126/science.287.5458.1652. [DOI] [PubMed] [Google Scholar]
- 10.Ferrell J E, Machleder E M. Science. 1998;280:895–898. doi: 10.1126/science.280.5365.895. [DOI] [PubMed] [Google Scholar]
- 11.Kim C, Jackson M, Lux R, Khan S. J Mol Biol. 2001;307:119–135. doi: 10.1006/jmbi.2000.4389. [DOI] [PubMed] [Google Scholar]
- 12.Li G, Weis R M. Cell. 2000;100:357–365. doi: 10.1016/s0092-8674(00)80671-6. [DOI] [PubMed] [Google Scholar]
- 13.Duke T A J, Bray D. Proc Natl Acad Sci USA. 1999;96:10104–10108. doi: 10.1073/pnas.96.18.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barkai N, Alon U, Leibler S. Compt Rend Sér IV. 2001;2:1–7. [Google Scholar]
- 15.Bren A, Eisenbach M. J Bacteriol. 2000;182:6865–6873. doi: 10.1128/jb.182.24.6865-6873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]