Abstract
Electrosurgery (ES) offers a promising alternative to conventional steel scalpel surgery (SSS), providing superior hemorrhage control and efficient tissue dissection with minimal invasiveness. Given the limited literature, this study aims to compare the clinical efficacy of ES with that of SSS in bovine umbilical herniorrhaphy. Fourteen crossbred male calves with reducible umbilical hernias, aged less than one month and weighing 25–47 kg, were randomly assigned to two experimental groups: group A (ES) and group B (SSS), each containing seven calves. Clinical parameters, such as temperature, heart rate, respiratory rate, and SpO2, were recorded 10 min preoperatively, intraoperatively at 20 and 40 min, and 10 min postoperatively. Hematobiochemical parameters, including total erythrocyte count, hemoglobin, packed cell volume, total serum protein, blood urea nitrogen, and creatinine, were also assessed at these intervals. Skin incision length, volume of blood loss, surgical duration, healing time, postoperative complications, and postoperative pain response were documented. Furthermore, a sonographic evaluation of postoperative wound healing was conducted. The clinical and hematobiochemical parameters did not vary significantly between the groups. However, significant variations (p<0.05) were noted in skin incision length, volume of blood loss, surgical duration, and healing time between the groups. Group A calves experienced smaller incisions, reduced blood loss, shorter surgical duration, quicker recovery, and fewer postoperative complications and pain. The ultrasonographic evaluation revealed superior postoperative wound healing in group A compared to group B. These findings suggest that ES is a more effective clinical approach than traditional SSS for umbilical herniorrhaphy in bovine calves.
Keywords: Umbilical hernia, Scalpel versus electrosurgery, Incision and hemorrhage, Postoperative complications, Wound healing
1. Introduction
A hernia is the protrusion or displacement of an organ, segment of an organ, or tissue outside the body cavity through an abnormal opening in the cavity wall [1,2]. The external hernias are visible outside an animal's body and detectable with clinical examination. In small and large animals, various types of hernias (i.e., umbilical, inguinal, scrotal, femoral, perineal, ventral, and abdominal hernias) are observed [3]. The umbilical hernia is the most common congenital hernia in neonatal calves [4]. Umbilical hernias can be symptomatic or asymptomatic. If not promptly treated, most umbilical hernias progressively enlarge, potentially leading to pain, anorexia, and weight loss [1,5]. Chronic herniation often results in the adhesion of the herniated contents to the hernial sac, with incarceration and strangulation being the most severe and life-threatening complications [2,6].
External hernias (e.g., umbilical hernias) in calves can be treated by various procedures depending on the size of the hernial ring. For small and reducible hernias, conservative treatments, such as the application of bandages, clamps, or ligatures, as well as the injection of irritants around the hernial ring, may be effective in promoting cicatrization [5,7]. However, irreducible hernias necessitate surgical intervention. Herniorrhaphy is a widely used and reliable surgical technique for repairing uncomplicated hernias (both reducible and irreducible) in bovine calves [1,8]. This procedure is typically performed under sedation followed by local anesthesia and involves the use of suture materials [1,[9], [10], [11]]. In cases where the hernia presents with a larger ring diameter, hernioplasty (i.e., the application of mesh) is required for effective repair [12,13]. In both herniorrhaphy and hernioplasty, the standard surgical procedure involves skin incisions, followed by blunt dissection of the subcutaneous tissue and associated muscles to expose the hernia, and finally, reduction of the hernia. This procedure requires instruments to make incisions and devices to control subsequent hemorrhage. While a traditional steel scalpel is limited to cutting only, an electrosurgical unit provides the dual functionality of cutting and coagulation simultaneously [14].
Traditionally, skin incisions are performed using stainless steel scalpels because of their ease of use and widespread availability. The scalpel is widely considered the gold standard for performing surgical incisions, as it allows surgeons to ensure control precisely over the length and depth of the incision [15]. However, such incisions are often associated with significant hemorrhage and pain [16,17]. Moreover, scalpel-related injuries represent the second most common cause of intraoperative injuries among surgical staff, following suture needle injuries [18]. Therefore, scalpels should be used cautiously in surgical procedures, and their use should be restricted whenever feasible. Diathermy and electrosurgery have been introduced as alternatives to overcome the drawbacks of steel scalpels [19,20]. Electrosurgery refers to the deliberate application of high-frequency waveforms or currents through bodily tissues to achieve a controlled surgical effect [21,22]. Unlike electrocautery, which utilizes direct current, electrosurgery is regarded as a superior technique as it employs alternating current to cleave and coagulate tissues [20]. Electrosurgical devices are used to make incisions in the skin, subcutaneous tissue, and muscle [23]. Electrosurgery offers several advantages, including quicker incisions, rapid hemostasis by sealing off blood vessels, and a reduction in overall blood loss [24]. However, electrosurgical techniques may raise concerns about the risks of postoperative scarring, wound infections, and delayed healing [17,24,25], thereby restricting their use for skin and invasive incisions. Subsequently, it is imperative that electrosurgical devices be selected carefully to suit the specific requirements of individual cases.
Comparative studies evaluating the efficacy of steel scalpels and electrosurgical units are well-documented in human subjects [15,23,25,26] and small animals [22,27,28]. However, there is a paucity of similar research on large ruminants. Despite the higher cost and the requirement of expert hands, electrosurgery is a promising technique in veterinary clinical practices [[29], [30], [31]]. Nevertheless, the potential advantages of electrosurgery in ruminant hernia repair remain unexplored. This study intends to evaluate the clinical efficacy of electrosurgery versus conventional steel scalpel surgery for repairing bovine umbilical hernias in a veterinary hospital setting, focusing on their impacts on the animals’ key clinical and hematobiochemical parameters, as well as intraoperative and postoperative outcomes.
2. Materials and methods
2.1. Animals
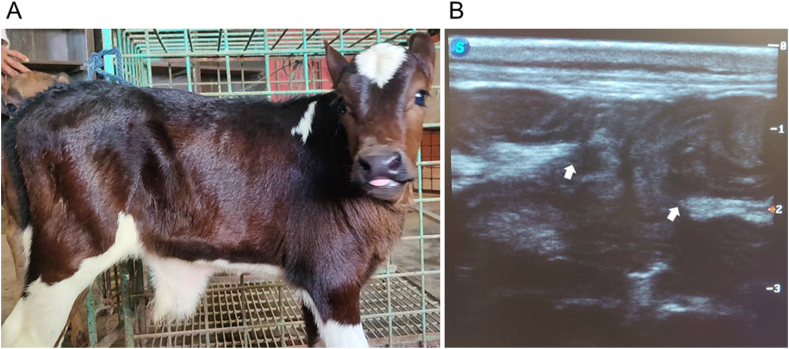
Fourteen crossbred male bovine calves, in apparently good health with a body condition score of 2.5–3 (on a scale of 5), under one month of age, and weighing between 25 and 47 kg, were included in this study. The animals were selected from routine cases admitted to a veterinary teaching hospital, all presenting with uncomplicated reducible umbilical hernias [Fig. 1(A and B)], with hernial ring diameters ranging from 5.08 to 6.35 cm (2–2.5 inches) at the longest axis. The calves were randomly and equally divided into two groups (groups A and B), with seven calves in each group. The sample size per group was determined using the resource equation approach described elsewhere [32]. Group A (n = 7) calves underwent electrosurgery (ES), while the claves in group B (n = 7) were treated with steel scalpel surgery (SSS) for hernia repair.
Fig. 1.
A crossbred bovine calf with an umbilical hernia (irrespective of groups), detected through physical examination and ultrasonography: (A) a reducible umbilical hernia, identified manually by palpation, (B) ultrasonography reveals a notable breach (indicated by white arrows) in the abdominal wall integrity (bright echogenic linear structures) near the umbilicus. The hernial gate (the area between the arrows) demonstrates the projection of hypoechogenic structures, resembling the abdominal contents. Linear probe; orientation: longitudinal, frequency: 7.5 MHz, depth: 3 cm.
2.2. Clinical monitoring
The clinical parameters, including temperature, heart rate (HR), respiration rate (RR), and peripheral oxygen saturation (SpO2) of the calves in both groups, were monitored 10 min preoperatively (without premedication or sedative induction), intraoperatively at 20 and 40 min, and again 10 min postoperatively. Temperature was measured manually from the rectum using a clinical thermometer. HR and RR were recorded with a stethoscope. SpO2 was documented using a digital patient monitor (Oxysmart-M®, Oxycon Co. Ltd.). The same clinical team monitored these parameters in both groups to ensure consistency.
2.3. Blood collection and hematobiochemical evaluation
Blood samples were obtained via jugular venipuncture from both groups 10 min preoperatively (without premedication or sedative induction), intraoperatively at 20 and 40 min, and again 10 min postoperatively. A total of 5 mL of blood was aspirated in each case; 3 mL were transferred to a vacutainer without anticoagulant (clot activator tube), while the remaining 2 mL were transferred to a vacutainer containing ethylenediaminetetraacetic acid (EDTA tube). Samples in clot activator tubes were centrifuged at room temperature for 15 min at 3000 rotations per minute. In each case, the supernatant serum was collected for biochemical analysis. A biochemical analyzer (Microlab 300 Semi-automated Biochemistry Analyzer, ELITECH) was used to evaluate total serum protein (TSP), blood urea nitrogen (BUN), and creatinine. Besides, samples in EDTA tubes were considered for hematological analysis. A hematology analyzer (Z5Vet, Zybio) was used to assess total erythrocyte count (TEC), hemoglobin (Hb), and packed cell volume (PCV).
2.4. Surgical procedures and postoperative management
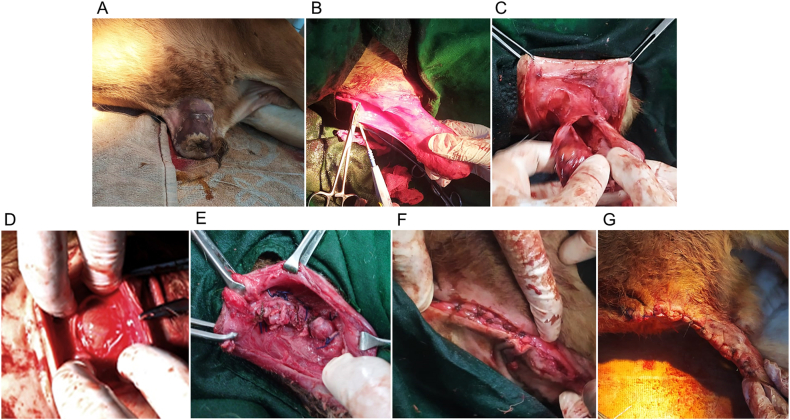
Before surgery, in both groups, the calves were kept off feed and water for 16 h and then stabilized with intravenous 500–940 mL of 5 % dextrose in normal saline (0.9 % NaCl), adjusted according to their body weights (25–47 kg). Each calf was premedicated with atropine sulfate at 0.04 mg/kg body weight (BW) (Atrovet®, Techno Drugs Ltd.) followed by deep sedation with xylazine hydrochloride (HCl) at 0.1 mg/kg BW (Xylaxin®, Indian Immunologicals Ltd.) via intramuscular (IM) injections. Aseptic preparation of the umbilical region (surgical site) was conducted (Fig. 2A). Linear local infiltration of 2 % lidocaine HCl (Jasocaine®, Jayson Pharmaceuticals Ltd.) was performed, encircling the hernial ring. Under deep sedation with appropriate regional desensitization, the calves were positioned in right lateral recumbency. Two elliptical skin incisions, lateral to each other, were made over the surgical site in each case. In group A calves, the incisions were made using an electro-scalpel connected to an electrosurgical unit (Meditom® MT-400, Italy via C. Bonozzi), with power settings at 50 Watts (W) for cutting and 30 W for coagulation (alternating current radio frequency: 400 kHz modulated by 33 kHz, as appropriate). In contrast, incisions in group B calves were made using a steel scalpel (handle number 4 and blade number 20). Then the incised skin edges were gently everted, and the subcutaneous tissue and abdominal muscles were bluntly dissected to expose the hernial sac (Fig. 2B). In group B, hemostasis was achieved with gauze pressures, mosquito artery forceps, or vessel ligation with suture, whereas in group A, electro-coagulation was performed following electro-incision. The tip of the hernial sac was incised carefully to reveal the herniated contents, ensuring no injury. In both groups, no adhesions were detected between the herniated content(s) and the sac, and the content(s) were readily reducible through the hernial ring (Fig. 2C). In each case, the hernial sac was trimmed just at the level of the ring, and the herniated content(s) were returned to the abdominal cavity through the ring (Fig. 2D). Peritoneal lavage was not conducted. Following hernial reduction, herniorrhaphy was performed. This involved the closure of the hernial ring (Fig. 2E) using horizontal mayo-mattress sutures with polyglactin 910 of size 1 (VicrylTM, Ethicon, J & J Medical Devices Companies), following a gentle circumferential scraping of the mucosal layer of the ring to expose a raw surface, thereby promoting healing. Once the ring was closed, the surrounding muscle layers were approximated (Fig. 2F) using simple interrupted sutures with Vicryl™ of size 1. The excess loose skin was surgically excised, and the remaining skin edges were sutured together (Fig. 2G) in a simple interrupted pattern using 2-0 nylon (Ethilon®, Ethicon, J & J Medical Devices Companies). Finally, a protective belly bandage with medicated gauze was applied to the surgical site. All surgical procedures in both groups were conducted by the same surgeon, accompanied by the same surgical team.
Fig. 2.
Intraoperative phases of herniorrhaphy in a calf (irrespective of groups): (A) preparation of the surgical site, (B) exposure of the hernia, (C) absence of adhesions and reduction of herniated contents, (D) repositioning of herniated contents into the abdominal cavity following trimming of the hernial sac at the level of the hernial ring, (E) closure of the hernial ring, (F) muscle layers closure, (G) approximation of the skin edges.
Postoperatively, the calves received a medication regimen that included ceftriaxone IM at 15 mg/kg (Trizon Vet, Acme Laboratories Ltd.) twice a day for seven consecutive days, ketoprofen IM at 3.3 mg/kg once a day for three days, and pheniramine maleate IM at 1 mg/kg once a day for five days. Fly repellent was routinely used in the calf barns, while 5 % povidone-iodine (Viodin® 5 % ointment, Square Pharmaceuticals Ltd.) was topically applied to the wound areas twice a day for five days. Furthermore, it was recommended that all calves be provided with half of their standard diets and subject to movement restrictions for three weeks post-surgery. In most cases, the skin sutures were removed two weeks postoperatively in both groups, revealing healing at the surgical sites. However, in group B, complications such as discharge from the surgical site and dehiscence of skin wounds were observed in two cases within five days post-surgery. Treatment involved the application of an antiseptic dressing with 10 % povidone-iodine (Viodin® 10 % solution, Square Pharmaceuticals Ltd.) twice a day for three days, followed by surgical debridement and suture replacement, along with an additional course of the antibiotic. The complications resolved, and healing was noted within one week after the additional treatment; thereafter, the external sutures were removed. Postoperative monitoring for both groups was conducted by the same observer, a member of the clinical team, ensuring uniformity in data collection.
2.5. Measuring the skin incision length
The skin incision length was measured in inches using a measuring scale for each case in both groups to facilitate comparison.
2.6. Evaluating the volume of blood loss
Before each surgery, the surgical gloves, gauze pieces, and drapes were accurately weighed using a digital weighing balance (SF-400, Precision Digital Scale) under aseptic conditions, and the weights were recorded in grams (g). The gauze pieces were used to soak blood from the surgical site intraoperatively. The blood-moistened gauze pieces were promptly transferred, one after another, into a polybag (following their use) to minimize the evaporative loss of the blood's fluid portion. Immediately after surgery, the surgical drapes were collected. The blood-stained surgical gloves, blood-moistened gauze pieces, and drapes were reweighed using the same scale. The total amount of blood loss was calculated by subtracting the pre-surgery weights from the post-surgery weights of the gloves, gauze pieces, and drapes.
Weight calculation formula:
Dry weight of surgical gloves = A g, dry weight of gauze pieces = B g, dry weight of surgical drapes = C g, weight of blood-stained gloves = D g, weight of blood-moistened gauze pieces = E g, weight of drapes after surgery = F g, and weight of polybag = G g
Therefore, the amount of blood loss = [(D + E + F + G) – (A + B + C + G)] g.
According to an earlier study [33], the density of blood is comparable to that of water, allowing 1 g of blood loss to be equivalent to 1 mL. Based on this observation, the blood loss measured in ‘g’ was converted to ‘mL’ and was referred to as the volume of blood loss.
2.7. Determining the duration of surgery and healing time
The total duration of surgery was determined by recording the time in minutes with a stopwatch from the initial skin incision to the final skin closure for each case in both groups. The healing time was assessed through manual inspection of the surgical site postoperatively in both groups, and it was recorded as the number of days required for skin wound recovery. For this purpose, the animals were monitored weekly for three weeks following surgery.
2.8. Evaluating the postoperative complications and pain response
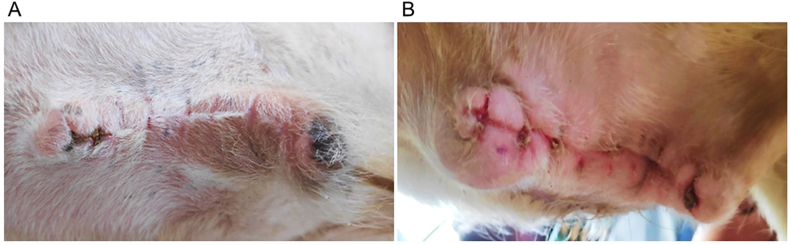
The calves in both groups were carefully monitored twice a day after surgery for any postoperative complications, including surgical site discharge, skin wound dehiscence, hernial recurrence, and deep scar formation. The monitoring commenced 48 h post-surgery and continued for up to three weeks. Apart from scar formation (a minor and transient skin defect) [Fig. 3(A and B)], all other complications observed within this period were effectively treated until recovery, as outlined in the postoperative management section. A six-month postoperative follow-up revealed no complications in either group. The local pain response was assessed in both groups once a day at three-day intervals from the third to the nineteenth postoperative days. This assessment was conducted manually through gentle and compassionate handling of the animals, accompanied by a physical examination that involved light palpation of the surgical site to elicit a withdrawal reflex. The incidence of postoperative complications and pain response were expressed as percentages.
Fig. 3.
Scar formation noted after skin suture removal two weeks postoperatively: (A) negligible stitch scar (fine line) in a calf from group A, (B) deep stitch scar (hypertrophic) in a calf from group B.
2.9. Evaluating the postoperative wound healing through ultrasonography
An ultrasound scanner (SonoScape P15 Ultrasound Machine, SonoScape) was employed for sonographic assessments of the postoperative wound healing, focusing on echogenicity to facilitate the detection of granulation tissue formation (an important indicator of the healing process) and wound contraction at the surgical site. This assessment was conducted 35 days post-surgery for each case in both groups.
2.10. Statistical analysis
The data obtained from this experiment were calculated and presented as ‘mean ± standard error of the mean’ and percentages for both groups. Independent Samples t-tests were performed to compare the means of parametric variables (i.e., clinical and hematobiochemical indices, skin incision length, volume of blood loss, surgical duration, and healing time) between the two groups, using GraphPad Prism (version 9.3.1). A p-value of <0.05 was considered statistically significant. Descriptive statistics (i.e., case frequency and percentage) were presented to report the postoperative complications and pain response.
3. Results
3.1. Variations in the clinical and hematobiochemical parameters
The clinical and hematobiochemical parameters assessed perioperatively for both groups are presented in Table 1, Table 2, respectively. Throughout the experimental period, there were negligible variations in these parameters, and no significant differences (p > 0.05) were observed between the groups.
Table 1.
Clinical parameters of the calves (perioperatively).
| Parameters (Mean ± SEM) | Groups | Preoperative |
Intraoperative |
Postoperative |
|
|---|---|---|---|---|---|
| −10 min | 20 min | 40 min | +10 min | ||
| T (°F) |
A (ES, n = 7) | 102.15 ± 0.25 | 102.01 ± 0.35 | 102.07 ± 0.30 | 102.20 ± 0.33 |
| B (SSS, n = 7) | 101.80 ± 0.12 | 101.73 ± 0.18 | 101.84 ± 0.16 | 101.90 ± 0.14 | |
|
p-value |
0.489 |
0.431 |
0.453 |
0.453 |
|
| HR (beat/min) |
A (ES, n = 7) | 83.71 ± 4.37 | 78.84 ± 5.42 | 79.29 ± 5.34 | 83.11 ± 5.81 |
| B (SSS, n = 7) | 86.85 ± 5.41 | 84.86 ± 8.17 | 84.71 ± 5.86 | 89.00 ± 8.67 | |
|
p-value |
0.554 |
0.541 |
0.557 |
0.854 |
|
| RR (breath/min) |
A (ES, n = 7) | 25.25 ± 4.68 | 22.57 ± 3.96 | 22.86 ± 3.57 | 27.00 ± 4.04 |
| B (SSS, n = 7) | 26.40 ± 3.34 | 25.14 ± 3.78 | 25.43 ± 5.74 | 27.14 ± 3.86 | |
|
p-value |
0.751 |
0.980 |
0.652 |
0.920 |
|
| SpO2 (%) | A (ES, n = 7) | 99.00 ± 2.00 | 94.71 ± 4.00 | 94.43 ± 3.23 | 99.18 ± 3.51 |
| B (SSS, n = 7) | 98.99 ± 5.00 | 92.14 ± 2.55 | 91.95 ± 2.83 | 98.96 ± 3.21 | |
| p-value | 0.740 | 0.632 | 0.575 | 0.901 | |
SEM: standard error of the mean, T: temperature, HR: heart rate, RR: respiratory rate, SpO2: saturation of peripheral oxygen, ES: electrosurgery, SSS: steel scalpel surgery.
Table 2.
Hematobiochemical parameters of the calves (perioperatively).
| Parameters (Mean ± SEM) | Groups | Preoperative |
Intraoperative |
Postoperative |
|
|---|---|---|---|---|---|
| −10 min | 20 min | 40 min | +10 min | ||
| Hematological | |||||
| TEC (1012/L) |
A (ES, n = 7) | 7.68 ± 0.27 | 7.02 ± 0.16 | 6.89 ± 0.21 | 7.07 ± 0.13 |
| B (SSS, n = 7) | 8.41 ± 0.93 | 7.91 ± 0.29 | 7.78 ± 0.35 | 8.12 ± 0.82 | |
|
p-value |
0.530 |
0.369 |
0.481 |
0.595 |
|
| Hb (g/dL) |
A (ES, n = 7) | 9.00 ± 1.90 | 8.92 ± 2.10 | 8.24 ± 1.95 | 8.60 ± 2.00 |
| B (SSS, n = 7) | 10.18 ± 1.95 | 9.80 ± 1.63 | 8.97 ± 1.46 | 9.65 ± 1.75 | |
|
p-value |
0.729 |
0.889 |
0.754 |
0.901 |
|
| PCV (%) | A (ES, n = 7) | 26.71 ± 5.07 | 25.11 ± 6.21 | 23.95 ± 4.93 | 24.19 ± 6.07 |
| B (SSS, n = 7) | 29.15 ± 6.23 | 28.37 ± 6.59 | 27.88 ± 5.80 | 28.03 ± 6.66 | |
| p-value | 0.811 | 0.668 | 0.733 | 0.817 | |
| Biochemical | |||||
| TSP (g/dL) |
A (ES, n = 7) | 5.76 ± 0.64 | 5.15 ± 0.24 | 4.98 ± 0.51 | 5.04 ± 0.67 |
| B (SSS, n = 7) | 6.14 ± 0.22 | 5.86 ± 0.47 | 5.10 ± 0.19 | 5.73 ± 0.23 | |
|
p-value |
0.708 |
0.905 |
0.825 |
0.690 |
|
| BUN (mg/dL) |
A (ES, n = 7) | 18.63 ± 2.52 | 18.90 ± 3.15 | 19.47 ± 2.80 | 20.25 ± 2.76 |
| B (SSS, n = 7) | 21.15 ± 3.09 | 21.66 ± 3.58 | 21.93 ± 3.33 | 22.80 ± 2.94 | |
|
p-value |
0.447 |
0.236 |
0.671 |
0.715 |
|
| Cr (mg/dL) | A (ES, n = 7) | 1.45 ± 0.62 | 1.45 ± 0.38 | 1.47 ± 0.30 | 1.48 ± 0.25 |
| B (SSS, n = 7) | 1.27 ± 0.31 | 1.28 ± 0.22 | 1.31 ± 0.45 | 1.34 ± 0.63 | |
| p-value | 0.623 | 0.971 | 0.890 | 0.558 | |
SEM: standard error of the mean, TEC: total erythrocyte count, Hb: hemoglobin, PCV: packed cell volume, TSP: total serum protein, BUN: blood urea nitrogen, Cr: creatinine, ES: electrosurgery, SSS: steel scalpel surgery.
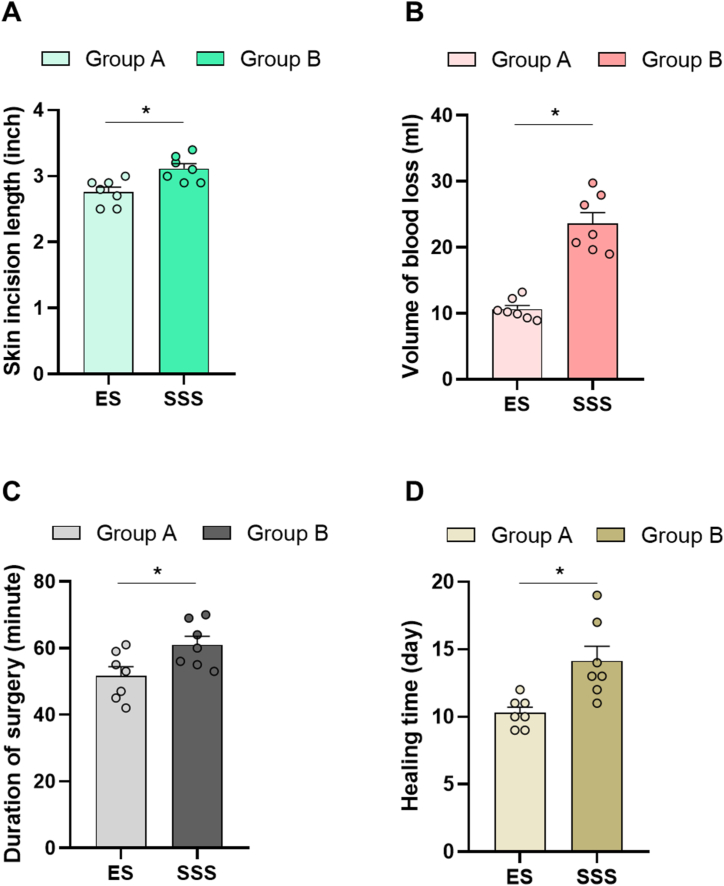
3.2. Variations in the skin incision length, blood loss, surgical duration, and healing time
The skin incision length, volume of blood loss, duration of surgery, and healing time for both groups are illustrated in Fig. 4(A–D). Significant differences (p < 0.05) were observed in all these parameters between the two groups. The calves in group A, which underwent ES, demonstrated significantly shorter skin incision lengths, reduced blood loss, shorter surgical duration, and faster recovery than those in group B with SSS. Notably, the mean volume of blood loss in group A was less than 50 % of that observed in group B.
Fig. 4.
Intraoperative and postoperative parameters evaluating the efficacy of ES and SSS: bar diagrams showing the intergroup variations in (A) skin incision length, (B) volume of blood loss, (C) duration of surgery, and (D) healing time (∗ = p < 0.05).
3.3. Variations in the postoperative complications and pain response
The postoperative complications and pain response of the calves for both groups are demonstrated in Table 3. No complications were recorded postoperatively in group A calves, however, postoperative complications, such as surgical site discharge and skin wound dehiscence (28.57 %) as well as deep scar formation (42.86 %), were observed in group B calves. No instances of hernial recurrence were identified in either group.
Table 3.
Postoperative complications and pain response of the calves.
| Postoperative complications | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Groups | Surgical site discharge and skin wound dehiscence |
Hernial recurrencea |
Deep scar formationb |
|||||||
| F | % | F | % | F | % | |||||
| A (ES, n = 7) | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | ||||
| B (SSS, n = 7) | 2 | 28.57 | 0 | 0.00 | 3 | 42.86 | ||||
| Days postoperative pain responsec | ||||||||||
| Groups |
Day 3 | Day 7 | Day 11 | Day 15 | Day 19 | |||||
| F |
% |
F |
% |
F |
% |
F |
% |
F |
% |
|
| A (ES, n = 7) | 7 | 100 | 7 | 100 | 4 | 57.14 | 0 | 0.00 | 0 | 0.00 |
| B (SSS, n = 7) | 7 | 100 | 7 | 100 | 6 | 87.71 | 2 | 28.57 | 0 | 0.00 |
ES: electrosurgery, SSS: steel scalpel surgery, F: frequency, %: percentage.
Data recorded until six months post-surgery.
Data recorded through visual inspection of the skin at the surgical site until three weeks post-surgery.
Based on gentle palpation of the surgical site and concurrent withdrawal reflex of the animals.
3.4. Variations in the postoperative wound healing
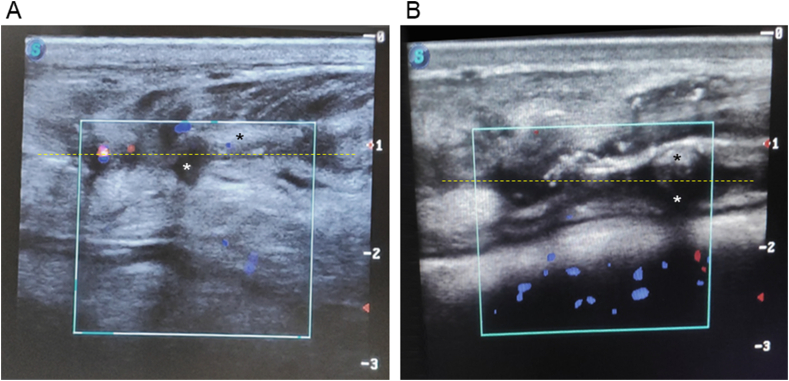
The postoperative ultrasonographic evaluation of wound healing is illustrated in Fig. 5(A and B), featuring a representative case from each group. The areas of granulation tissue and wound contraction were more pronounced in group A than in group B. Conversely, the areas indicating the wound gaps were comparatively smaller in group A than in group B.
Fig. 5.
Ultrasonographic evaluation of wound healing 35 days postoperatively: (A) a calf from group A demonstrating superior outcomes compared to (B) a calf from group B, as assessed by the observation of granulation tissue formation, wound contraction, and wound gap. The areas of granulation tissue (heteroechoic transverse zones) are highlighted with yellow dotted lines, while black stars denote the areas of wound contraction (hyperechoic zones), and white stars indicate the wound gaps (anechoic zones). The assessment was performed using a linear probe with a longitudinal orientation, a frequency of 7.5 MHz, and a depth setting of 3 cm. The blue spots indicate blood flow away from the probe, while the red spots indicate blood flow toward the probe.
4. Discussion
Bovine umbilical hernia is a commonly reported clinical condition that frequently necessitates surgical intervention [9,34,35]. Currently, there is a significant emphasis on the value and efficiency of surgical procedures. Thus, it is worthwhile to investigate the clinical efficacy of scalpels and electrosurgical units in repairing bovine umbilical hernias, focusing on a less invasive approach with minimal biological impacts. The intraoperative and postoperative parameters investigated in the present study, specifically skin incision length, blood loss, surgical duration, postoperative complications, and healing time, have also been documented as instrumental in facilitating comparisons between various surgical techniques in other research [15,22,25,36,37]. The assessment of clinical parameters (temperature, HR, RR, SpO2) and hematobiochemical parameters (TEC, Hb, PCV, TSP, BUN, and creatinine) was considered important; as ES employing alternating current at specific frequencies to body tissues may influence these parameters, indicating potential alterations in the animals’ vital organs functions [38].
This study demonstrated minimal or negligible variations in the clinical and hematobiochemical parameters of the calves in both groups, none of which were statistically significant. These findings suggest that neither ES nor SSS were associated with adverse effects on the animals' vital functions. Additionally, it may be stated that the flow of alternating current during ES did not typically affect the animals’ physiology. In the present study, the cutting and coagulation electrical energies were set at 50 W and 30 W, respectively, which are in alignment with the findings from previous studies [2,39,40]. Therefore, electrosurgery, at these applied levels of cutting and coagulation current, may be safely employed for hernia repair in bovine calves. However, a comparison of intraoperative blood pressures between the ES and SSS groups would have been valuable. This was not possible in the present study, as this data had not been recorded properly. ES may influence both systolic and diastolic blood pressures, a point to be considered [41]. Although the thermal injury to surrounding tissues associated with ES is an important parameter [42,43], it was not evaluated in this study, underscoring the necessity for further research. In this context, plasma scalpels have been demonstrated to be superior to both electrosurgical scalpels and steel scalpels [44].
In this study, the electrosurgical skin incisions were observed to be shorter, with a mean length of 7.003 cm (2.757 inches), in comparison to traditional scalpel incisions, which exhibited a mean length of 7.910 cm (3.114 inches). It is important to note that these measurements correspond exclusively to the initial skin incisions made for hernia exposure, and do not include those performed for the final trimming of excess loose skin after hernia repair, prior to skin closure. Although these incisions were elliptical, their lengths were measured in a linear orientation after the skin edges had been manually stretched. The shorter incisions achieved with electro-scalpels may be attributed to their capacity to provide finer thermal cuts, reducing the extent of adjacent tissue damage. In contrast, traditional steel scalpels require additional physical force and control to create mechanical cuts, increasing the chances of adjacent tissue damage and potentially leading to relatively greater incisions. The shorter incisions sped up wound closure following hernia repair, contributing to a reduction in overall surgical time. Apart from initial skin incisions, all other incisions made in the fascia, subcutaneous tissues, and muscles either with electro-scalpels or steel scalpels were not measured but the associated hemorrhages were considered in either group.
The hemorrhage associated with the use of electro-scalpels during herniorrhaphy was significantly reduced, measuring less than half of that observed with steel scalpels. This reduction may be ascribed to the simultaneous functionality of the handpiece electro-scalpel, which features integrated cut and coagulation buttons within a probe-like design. This technique allowed for alternate point applications of electro-coagulation following electro-incision to achieve hemostasis. The finding that the electrosurgical technique facilitated a reduction in blood loss is consistent with the observations in related studies [25,45]. Given that ES demonstrated a reduction in intraoperative hemorrhage during umbilical herniorrhaphy, it might be a superior choice for animals presenting with anemia, thrombocytopenia, or coagulation disorders. Intraoperative hemorrhage can obscure the surgical field, compromising visibility. Diffuse or focal abdominal hemorrhage during herniorrhaphy (if any), particularly in the absence of electrosurgical devices, may necessitate the increased use of abdominal swabs, retractors, and hemostats, potentially leading to additional blood loss. In the present study, however, no abdominal swabs were used, given the absence of notable abdominal hemorrhage. Although not investigated in the present study, it is suggested that surgeons may prefer the use of a combination of steel scalpels and electrosurgical devices in clinical practices to enhance the efficiency and convenience of surgical procedures as deemed appropriate.
In the present study, the overall surgical duration was notably shorter with the use of electrosurgical devices, primarily due to shorter incision time and the subsequent decrease in hemorrhage requiring hemostasis. Hemostasis, achieved with gauze pressures, artery forceps, or vessel ligation with suture, was observed to extend the intraoperative time in cases of scalpel surgery, thereby increasing overall surgical duration. This observation is consistent with the findings reported in previous research [26,41,46,47]. The shorter incision time observed with ES during umbilical herniorrhaphy might be attributed to a few factors. Since ES employs a high-frequency current to generate heat for tissue dissection, it allows for precise incisions with minimal handling pressure. This alleviates hand fatigue typically associated with steel scalpels, thereby improving the speed and accuracy of incisions. However, in certain cases, there may be no clinically significant differences in intraoperative duration, particularly concerning incision times between ES and SSS, as documented elsewhere [23].
In addition to shorter incision time, ES offered several benefits during herniorrhaphy in the calves. Electrosurgical scalpels provided uniform cutting across various tissue types, minimizing variability in incision quality. In contrast, the inconsistencies in making incisions with steel scalpels, particularly when addressing tougher or highly vascularized tissues, reasonably prolonged the surgical process. Allowing simultaneous cutting and coagulation, ES reduced the need for frequent instrument-switching intraoperatively, leading to a decrease in total operation theater time. Although not investigated in the present study, this finding suggests that a shorter surgical duration associated with ES may require reduced dosages and duration of anesthesia, facilitating quick anesthetic recovery.
The incidence of postoperative complications, including discharge, wound dehiscence, and deep scarring, was notably higher in the calves treated with steel scalpels. These findings contrast with those of other researchers [45,48,49], who reported an increase in postoperative complications associated with the use of electro-scalpels, especially with the ‘coagulation’ mode or with lower power setting in ‘cut’ mode (≤30 W) for incisions. However, these complications were not observed when the actual ‘cut’ mode of electro-scalpels was used for making incisions, as observed previously [22] and corroborated by the results of the present study. Besides, several studies have suggested no remarkable variations in postoperative complications between ES and SSS groups [15,37,41,50]. In the present study, electro-scalpels provided immediate vessel sealing and tissue coagulation, minimizing postoperative hemorrhage and oozing within the wounds, which likely contributed to the absence of visible discharge and wound dehiscence. The complications documented for manual scalpel surgery might be because of more invasive tissue handling, tissue damage, increased intraoperative time, and hemorrhage, which led the animals to be more stressed than those treated with electro-scalpels. This subsequently might have contributed to increased inflammatory responses during wound healing, leading to surgical site discharge and wound dehiscence, with higher risks of postoperative infections. Greater tissue damage may have induced healing through fibrous connective tissue proliferation, resulting in more prominent scarring in the scalpel group observed until three weeks post-surgery. Conversely, a previous investigation has documented scarring to be more pronounced in the ES group compared to the SSS group seven days post-surgery [45]. This may be because the heat generated by the alternating current caused excessive damage to surrounding tissues. Therefore, the use of electrosurgical devices should be tailored to specific applications, with accurate current settings for both coagulation and cut modes according to tissue types, requiring skilled hands. In addition, the selection of electrosurgical electrode tips, incision speed and pattern, and suturing techniques might be important variables. Besides, several other studies have demonstrated no significant differences in scar tissue formation between ES and SSS groups three months postoperatively [37,51]. These findings suggest that the presence and characteristics of scar tissue may vary depending on the timing of observation of wound healing postoperatively, as scar tissue undergoes continuous remodeling over time [52].
The present study documented relatively short-term postoperative pain in the calves treated with ES, which is correlated with previous findings [25,50,53]. The greater intensity and durability of postoperative pain in the scalpel group might be attributed to increased intraoperative tissue damage, which likely triggered more acute inflammatory responses affecting local nerves and vessels during wound healing. This might have resulted in a comparatively prolonged painful condition postoperatively. Nevertheless, different studies have demonstrated no significant variations in postoperative pain between ES and SSS groups [15,22,47]. In the present study, postoperative pain was assessed by observing the withdrawal reflexes of the animals following manual palpation. This pain assessment procedure was implemented based on individual experience and was consistently carried out by the same observer in both groups to facilitate a reliable comparison. Accurate pain assessment might be difficult in animals, and variations may arise with different observers or the use of various assessment methods, such as pain indexes, scales, or scoring systems described in other studies [54,55]. While the pain assessment in the present study was somewhat superficial depending on manual palpation, the UNESP–Botucatu unidimensional composite pain scale appears to be more reliable for evaluating postoperative pain in calves [56].
The calves treated with ES demonstrated comparatively faster skin wound healing. This might be ascribed to the minimally invasive nature of ES, characterized by shorter incision lengths and reduced tissue trauma, culminating in very minor wound gaps following precise approximation of wound edges; this, in turn, resulted in the absence of the postoperative complications observed with SSS. This finding contrasts the previous report [45], where delayed healing was associated with ES. Additionally, several studies have indicated no significant differences in wound healing between ES and SSS groups [22,37]. Thus, it is worth mentioning that postoperative healing in animals is dependent not only on the surgical devices used (steel scalpel and electro-scalpel) but also on the types of surgical cases, patients, and associated anatomical structures and tissues. Ultrasonography provided valuable insights into postoperative wound healing by detecting the extent of granulation tissue, wound gap, and wound contraction, with superior outcomes observed in the ES group. The feasibility of sonographic techniques in this context is substantiated by relevant studies [57,58].
The procedures used in premedication, sedation, surgical intervention, and suturing, are concordant with those documented in other studies [1,2,59]. No recurrence of hernia was noted in either group, which might be attributed to the effectiveness of the surgical procedures, postoperative medications, and management. This study is limited by its sample size and reliance on manual observations and assessments. Although a postoperative histological evaluation of wound healing was deemed important, it could not be conducted due to the owners’ constraints, as all animals in this study were hospital patients rather than experimental subjects.
The use of electrosurgical devices presents certain limitations compared to steel scalpels. While no additional skills are required to operate surgical scalpels, the safe and effective use of electrosurgical devices necessitates a comprehensive understanding of electrosurgical biophysics, equipment principles, tissue effects, spatial orientation, and hand-eye coordination [60]. Improper or inexperienced application of electrosurgical devices may result in electrothermal injuries, electric shock, unintended burns, accidental tearing of adjacent blood vessels, charring, and production of surgical smoke [61]. Hence, it is imperative to possess appropriate knowledge of the technique and adhere to safety protocols [61] while operating electrosurgical devices for bovine herniorrhaphy. Electrosurgical devices, although commonly used in well-equipped operating theaters, are also available as portable versions [62]. The portable versions, powered by batteries, can be easily transported for field use. Moreover, mobile veterinary clinics and services can deploy these devices in field operations, enhancing accessibility in remote and resource-limited areas [63].
5. Conclusions
The use of ES, employing both cutting and coagulation modes for dissection and hemostasis during umbilical herniorrhaphy in bovine calves, was determined to be superior to the traditional SSS in terms of incision quality, hemorrhage control, postoperative complications, and wound healing. Neither ES nor SSS demonstrated any significant adverse effects on the primary clinical or hematobiochemical parameters of the animals. Hence, ES with an accurate current setting based on tissue types, incorporating skilled hands, should be considered a feasible approach for umbilical herniorrhaphy in calves. However, further research is warranted to explore the thermal damage to surrounding tissues associated with ES, as well as the histological characteristics of wound healing following its application in bovine umbilical herniorrhaphy.
CRediT authorship contribution statement
Mohammad Raguib Munif: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Md Ariful Islam: Writing – original draft, Visualization, Methodology, Investigation, Formal analysis. Md Sabuj Rahman: Visualization, Methodology, Investigation, Data curation. Md Mizanur Rahman: Writing – review & editing, Supervision, Resources. Md Rafiqul Alam: Writing – review & editing, Validation, Supervision, Conceptualization.
Ethics approval and consent to participate
This study involved animals from the clinical workload at the Veterinary Teaching Hospital (VTH) of Bangladesh Agricultural University (BAU) and adhered to the standard protocols in compliance with animal care and welfare guidelines. The methodology was approved by the Animal Welfare and Experimentation Ethics Committee of BAU [Approval No. AWEEC/BAU/2023(5)]. The director of VTH, BAU, approved the publication, and consent for participation was obtained.
Consent for publication
Informed consent for publication was obtained from the animal owners.
Data availability statement
All the relevant data are presented within the article.
Funding
Research conducted by Mohammad Raguib Munif was, in part, funded by the Bangladesh Agricultural University Research System [Grant No. BAURES/387(53)/2022].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The study was conducted in collaboration between the Veterinary Teaching Hospital (VTH) at Bangladesh Agricultural University (BAU) and the Department of Surgery and Obstetrics, BAU.
Contributor Information
Mohammad Raguib Munif, Email: md.raguibmunif@gmail.com.
Md Rafiqul Alam, Email: alammr@bau.edu.bd.
References
- 1.Munif M.R., Masud R.I., Tasnim S. Surgical treatment of right lateral abdominal hernia in a heifer, Iran. J. Vet. Sci. Tech. 2022;14(3):53–57. doi: 10.22067/ijvst.2022.76524.1145. [DOI] [Google Scholar]
- 2.Munif M.R., Masud R.I., Tasnim S. Surgical repair of incarcerated umbilical hernia in a white German Spitz. Bull. Natl. Res. Cent. 2024;48:48. doi: 10.1186/s42269-024-01202-5. [DOI] [Google Scholar]
- 3.Farman R., Al-Husseiny S., Al-Ameer A. Surgical treatment of hernia in cattle: a review. Al-Qadisiyah J. Vet. Med. Sci. 2018;17(2):61–68. https://www.iasj.net/iasj/download/732952d602d81170 Available: [Google Scholar]
- 4.Spadola F., Neve V.C., Costa G.L., Musicò M., Spadaro A., Antoci F., Cavallo O., Cascone G. Surgical approach and etiopathogenetic considerations to the umbilical tumefactions in cattle: case review in twenty years (2000/2020) Vet. Anim. Sci. 2022;17 doi: 10.1016/j.vas.2022.100258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortved K. In: Farm Animal Surgery. second ed. Fubini S.L., Ducharme N.G., editors. WB Saunders, Elsevier; Philadelphia, United States: 2017. Miscellaneous abnormalities of the calf; pp. 540–550. [DOI] [Google Scholar]
- 6.Şimşek A., Kocaaslan H., Dirican A., Ateş M. Factors affecting strangulation and necrosis in incarcerated abdominal wall hernias. Cyprus J. Med. Sci. 2020;5(4):279–283. doi: 10.5152/cjms.2020.1075. [DOI] [Google Scholar]
- 7.Doijode V. Umbilical hernia in ruminant calves: a review. Pharma Innov. J. 2019;8(4):164–167. https://www.thepharmajournal.com/archives/2019/vol8issue4/PartC/8-3-30-374.pdf Available: [Google Scholar]
- 8.Yepez P.J., Klabnik J.L., Lozier J.W., Niehaus A.J., Miesner M.D., Prado T.M., Anderson D.E., Mulon P.Y. Surgical management and outcome of acquired inguinal hernias in mature bulls: 13 cases (2005-2017) J. Am. Vet. Med. Assoc. 2021;259(8):909–913. doi: 10.2460/javma.259.8.909. [DOI] [PubMed] [Google Scholar]
- 9.Haile Y., Velappa R., Asrat M. A study on the prevalence of umbilical hernia in calves in and around Gondar Town, North Gondar, North West Ethiopia. Int. J. Vet. Sci. Anim. Husb. 2017;2(2):11–15. https://www.veterinarypaper.com/pdf/2017/vol2issue2/PartA/1-3-27-987.pdf [Google Scholar]
- 10.Costa G.L., Leonardi F., Interlandi C., Licata P., Lizarraga I., Macrì F., Macrì D., Ferrantelli V., Spadola F. Tramadol administered intravenously either as a bolus or a slow injection in pain management of romifidine-sedated calves undergoing umbilical hernia repair. Animals. 2023;13(7):1145. doi: 10.3390/ani13071145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Interlandi C., Spadola F., Neve V.C., Tabbì M., Di Pietro S., Giudice E., Macrì D., Costa G.L. Use of butorphanol as a local anaesthetic for pain management in calves undergoing umbilical hernia repair. Front. Vet. Sci. 2024;11 doi: 10.3389/fvets.2024.1470957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tóth F., Schumacher J. Prosthetic mesh repair of abdominal wall hernias in horses. Vet. Surg. 2018;47(4):536–542. doi: 10.1111/vsu.12773. [DOI] [PubMed] [Google Scholar]
- 13.Spadola F., Costa G.L., Morici M., Interlandi C., Nastasi B., Musicò S.M. Autologous prosthesis for the surgery of two simultaneous hernias in a calf. Large Anim. Rev. 2017;23:195–197. [Google Scholar]
- 14.Munro M.G. In: The SAGES Manual on the Fundamental Use of Surgical Energy (FUSE) Feldman L., Fuchshuber P., Jones D., editors. Springer; New York: 2012. Fundamentals of electrosurgery part I: principles of radiofrequency energy for surgery; pp. 15–59. [DOI] [Google Scholar]
- 15.Zarei F., Shahmorad M.K. Scalpel versus electrocautery for herniorrhaphy incision: a randomized controlled trail. Int. J. Surg. Open. 2021;28:33–36. doi: 10.1016/j.ijso.2020.12.005. 2021. [DOI] [Google Scholar]
- 16.Liboon J., Funkhouser W., Terris D.J. A comparison of mucosal incisions made by scalpel, CO2 laser, electrocautery, and constant-voltage electrocautery. Otolaryngol. Head Neck Surg. 1997;116:379–385. doi: 10.1016/S0194-59989770277-8. [DOI] [PubMed] [Google Scholar]
- 17.Chalya P.L., Mchembe M.D., Mabula J.B., Gilyoma J.M. Diathermy versus scalpel incision in elective midline laparotomy: a prospective randomized controlled clinical study. East Cent. Afr. J. Surg. 2013;18:71–77. https://www.ajol.info/index.php/ecajs/article/view/89927 Available: [Google Scholar]
- 18.Mansour J., Asarkar A., Pang J., Nathan C.O. Can electrocautery replace the scalpel for surgical skin incision? Laryngoscope. 2022;132(12):2299–2300. doi: 10.1002/lary.30114. [DOI] [PubMed] [Google Scholar]
- 19.Boyd D.E., Palmer J.H.M. Surgical diathermy, anaesth. Intensive Care Med. 2013;14(10):431–433. doi: 10.1016/j.mpaic.2013.07.003. [DOI] [Google Scholar]
- 20.Baigrie D., Qafiti F.N., Buicko Lopez J.L. StatPearls Publishing; Treasure Island (FL): 2023. Electrosurgery, StatPearls [Internet]https://www.ncbi.nlm.nih.gov/books/NBK482380 Available: [PubMed] [Google Scholar]
- 21.Wang K., Advincula A.P. “Current thoughts” in electrosurgery. Int. J. Gynaecol. Obstet. 2007;97(3):245–250. doi: 10.1016/j.ijgo.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Meakin L.B., Murrell J.C., Doran I.C.P., Knowles T.G., Tivers M.S., Chanoit G.P.A. Electrosurgery reduces blood loss and immediate postoperative inflammation compared to cold instruments for midline celiotomy in dogs: a randomized controlled trial. Vet. Surg. 2017;46(4):515–519. doi: 10.1111/vsu.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charoenkwan K., Iheozor-Ejiofor Z., Rerkasem K., Matovinovic E. Scalpel versus electrosurgery for major abdominal incisions. Cochrane Database Syst. Rev. 2017;6(6) doi: 10.1002/14651858.CD005987.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar V., Tewari M., Shukla H.S. A comparative study of scalpel and surgical diathermy incision in elective operations of head and neck cancer. Indian J. Cancer. 2011;48:216–219. doi: 10.4103/0019-509X.82904. [DOI] [PubMed] [Google Scholar]
- 25.Patil S.A., Devani R., Radhakrishna V., Patil M. Electrosurgery versus steel scalpel for elective surgery: a prospective study. Saudi Surg. J. 2018;6(2):55–59. doi: 10.4103/ssj.ssj_76_17. [DOI] [Google Scholar]
- 26.Ismail A., Abushouk A.I., Elmaraezy A., Menshawy A., Menshawy E., Ismail M., Samir E., Khaled A., Zakarya H., El-Tonoby A., Ghanem E. Cutting electrocautery versus scalpel for surgical incisions: a systematic review and meta-analysis. J. Surg. Res. 2017;220:147–163. doi: 10.1016/j.jss.2017.06.093. [DOI] [PubMed] [Google Scholar]
- 27.Tzimtzimis E. Outcome of electrosurgery versus scalpel blade for intestinal incisions in dogs. Vet. Evid. 2019;4(3):1–11. doi: 10.18849/ve.v4i3.243. [DOI] [Google Scholar]
- 28.Bamaniya H.V., Vadalia J.V., Talekar S.H., Bhatt R.H., Vagh A.A., Fefar D.T., Kumar N., Rokad H.A. Clinical evaluation of scalpel blade and electrocautery surgical incision techniques for ovariohysterectomy in dogs. Pharma Innov. J. 2022;SP-11(12):727–733. https://www.thepharmajournal.com/archives/2022/vol11issue12S/PartJ/S-11-11-248-139.pdf Available: [Google Scholar]
- 29.Watts J. The use of bipolar electrosurgical forceps for haemostasis in open surgical ovariectomy of bitches and queens and castration of dogs. J. Small Anim. Pract. 2018;59:465–473. doi: 10.1111/jsap.12838. [DOI] [PubMed] [Google Scholar]
- 30.Caspers M.K., Bell C.D., Tatarniuk D.M. Transendoscopic ventriculocordectomy using monopolar electrosurgical instrumentation for conjunctive treatment of laryngeal hemiplegia in horses: 24 cases (2017-2019) Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.628410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith J., Salazar G., Peace C., Olivarez J., Vermeulen K., Black K., Guss C., Tatarniuk D., Dohlman T., Wiley C. Electrosurgical bipolar vessel sealing for a standing flank ovariectomy in beef heifers. Clin. Theriol. 2021;13(1):60–67. doi: 10.58292/ct.v13.9364. [DOI] [Google Scholar]
- 32.Arifin W.N., Zahiruddin W.M. Sample size calculation in animal studies using resource equation approach. Malays. J. Med. Sci. 2017;24(5):101–105. doi: 10.21315/mjms2017.24.5.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vitello D.J., Ripper R.M., Fettiplace M.R., Weinberg G.L., Vitello J.M. Blood density is nearly equal to water density: a validation study of the gravimetric method of measuring intraoperative blood loss. J. Vet. Med. 2015;2015 doi: 10.1155/2015/152730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baird A.N. Umbilical surgery in calves. Vet. Clin. North Am. Food Anim. Pract. 2008;24(3):467–477. doi: 10.1016/j.cvfa.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Salim M., Hashim M.A., Juyena N., Arafat T.A., Dey R.K., Bag M.A.S., Islam M.S. Prevalence of hernia and evaluation of herniorrhaphy in calves. Int. J. Nat. Soc. Sci. 2015;2:35–43. https://ijnss.org/wp-content/uploads/2015/05/IJNSS-V2I4-06-pp-35-43.pdf Available: [Google Scholar]
- 36.Chrysos E., Athanasakis E., Antonakakis S., Xynos E., Zoras O. A prospective study comparing diathermy and scalpel incisions in tension-free inguinal hernioplasty. Am. Surg. 2005;71(4):326–329. doi: 10.1177/000313480507100410. [DOI] [PubMed] [Google Scholar]
- 37.Thakare G.A., Bhola N., Agarwal A., Ghavat C. A comparative analysis of cutting electrocautery and scalpel for performing cutaneous incisions over the neck- A prospective, randomized, single blind study. Acta Sci. Dent. Sci. 2022;6(3):28–35. doi: 10.31080/ASDS.2022.06.1317. [DOI] [Google Scholar]
- 38.Interlandi C., Nastasi B., Morici M., Calabrò P., Costa G.L. Effects of the combination romifidine/tramadol drug administration on several physiological and behavioral variables in calves, Large Anim. Rev. 2017;23:51–54. [Google Scholar]
- 39.Alkatout I., Schollmeyer T., Hawaldar N.A., Sharma N., Mettler L. Principles and safety measures of electrosurgery in laparoscopy. J. Soc. Laparosc. Robot. Surg. 2012;16(1):130–139. doi: 10.4293/108680812X13291597716348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munif M.R., Safawat M.S., Hannan A. Surgical correction of polymelia in the perineal region of a 2-day-old indigenous bovine calf: a case report from Bangladesh. Bull. Natl. Res. Cent. 2023;47:9. doi: 10.1186/s42269-023-00988-0. [DOI] [Google Scholar]
- 41.Akbari H., Mahmoudian A., Mousavi Nasab S., NasiriFormi E., Pouladkhay F. The outcomes of using scalpel and electrosurgery methods for anterior abdominal wall incision during cesarean section. Ann. Mil. Health Sci. Res. 2023;21(2) doi: 10.5812/amh-139181. [DOI] [Google Scholar]
- 42.Zhong Y., Wei Y., Min N., Guan Q., Zhao J., Zhu J., Hu H., Geng R., Hong C., Ji Y., Li J., Zheng Y., Zhang Y., Li X. Comparative healing of swine skin following incisions with different surgical devices, Ann. Transl. Med. 2021;9(20):1514. doi: 10.21037/atm-21-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X.R., Trinh T.T., Chien P.N., Giang N.N., Zhou S.Y., Nam S.Y., Heo C.Y. Safety assessment of electrosurgical electrodes by using mini pig tissue. Heliyon. 2024;10(15) doi: 10.1016/j.heliyon.2024.e35266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lacitignola L., Desantis S., Izzo G., Staffieri F., Rossi R., Resta L., Crovace A. Comparative morphological effects of cold-blade, electrosurgical, and plasma scalpels on dog skin. Vet. Sci. 2020;7(1):8. doi: 10.3390/vetsci7010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott J.E., Swanson E.A., Cooley J., Wills R.W., Pearce E.C. Healing of canine skin incisions made with monopolar electrosurgery versus scalpel blade. Vet. Surg. 2017;46(4):520–529. doi: 10.1111/vsu.12650. [DOI] [PubMed] [Google Scholar]
- 46.Kearns S.R., Connolly E.M., McNally S., McNamara D.A., Deasy J. Randomized clinical trial of diathermy versus scalpel incision in elective midline laparotomy. Br. J. Surg. 2001;88(1):41–44. doi: 10.1046/j.1365-2168.2001.01625.x. [DOI] [PubMed] [Google Scholar]
- 47.Aird L.N., Brown C.J. Systematic review and meta-analysis of electrocautery versus scalpel for surgical skin incisions. Am. J. Surg. 2012;204(2):216–221. doi: 10.1016/j.amjsurg.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 48.Chang E.I., Carlson G.A., Vose J.G., Huang E.J., Yang G.P. Comparative healing of rat fascia following incision with three surgical instruments. J. Surg. Res. 2011;167(1):e47–e54. doi: 10.1016/j.jss.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 49.Bowers C.A., Burns G., Salzman K.L., McGill L.D., MacDonald J.D. Comparison of tissue effects in rabbit muscle of surgical dissection devices. Int. J. Surg. 2014;12(3):219–223. doi: 10.1016/j.ijsu.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 50.Hajibandeh S., Hajibandeh S., Maw A. Diathermy versus scalpel for skin incision in patients undergoing open inguinal hernia repair: a systematic review and meta-analysis. Int. J. Surg. 2020;75:35–43. doi: 10.1016/j.ijsu.2020.01.020. [DOI] [PubMed] [Google Scholar]
- 51.Oley M.H., Oley M.C., Kepel B.J., Manginstar C., Rawung R., Langi F.L.F.G., Barends D., Aling D.M.R., Wagiu A.M.J., Faruk M M. Post-skin incision scar tissue assessment using patient and observer scar assessment scales: a randomised controlled trial. Ann. Med. Surg. 2021;71 doi: 10.1016/j.amsu.2021.103006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzalez A.C., Costa T.F., Andrade Z.A., Medrado A.R. Wound healing - a literature review. An. Bras. Dermatol. 2016;91(5):614–620. doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talpur A.A., Khaskheli A.B., Kella N., Jamal A. Randomized, clinical trial on diathermy and scalpel incisions in elective general surgery. Iran. Red. Crescent Med. J. 2015;17(2) doi: 10.5812/ircmj.14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernandez-Avalos I., Mota-Rojas D., Mora-Medina P., Martínez-Burnes J., Casas Alvarado A., Verduzco-Mendoza A., Lezama-García K., Olmos-Hernandez A. Review of different methods used for clinical recognition and assessment of pain in dogs and cats. Int. J. Vet. Sci. Med. 2019;7(1):43–54. doi: 10.1080/23144599.2019.1680044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tschoner T., Mueller K.R., Zablotski Y., Feist M. Pain assessment in cattle by use of numerical rating and visual analogue scales- A systematic review and meta-analysis. Animals. 2024;14(2):351. doi: 10.3390/ani14020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Della Rocca G., Brondani J.T., de Oliveira F.A., Crociati M., Sylla L., Elad Ngonput A., Di Salvo A., Luna S.P.L. Validation of the Italian version of the UNESP-Botucatu unidimensional composite pain scale for the assessment of postoperative pain in cattle. Vet. Anaesth. Analg. 2017;44(5):1253–1261. doi: 10.1016/j.vaa.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 57.Dyson M., Moodley S., Verjee L., Verling W., Weinman J., Wilson P. Wound healing assessment using 20 MHz ultrasound and photography, Skin Res. Technol. 2003;9(2):116–121. doi: 10.1034/j.1600-0846.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- 58.Matsumoto M., Nakagami G., Kitamura A., Kurita M., Suga H., Miyake T., Kawamoto A., Sanada H. Ultrasound assessment of deep tissue on the wound bed and periwound skin: a classification system using ultrasound images. J. Tissue Viability. 2021;30(1):28–35. doi: 10.1016/j.jtv.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 59.Munif M.R., Safawat M.S., Hannan A. Tube cystostomy for management of uroperitoneum (water belly) in a weaned Brahma bull-calf. Vet. Res. Notes. 2023;3(7):58–62. doi: 10.5455/vrn.2023.c29. [DOI] [Google Scholar]
- 60.Wu M.P., Ou C.S., Chen S.L., Yen E.Y., Rowbotham R. Complications and recommended practices for electrosurgery in laparoscopy. Am. J. Surg. 2000;179(1):67–73. doi: 10.1016/s0002-9610(99)00267-6. [DOI] [PubMed] [Google Scholar]
- 61.El-Sayed M.M., Saridogan E. Principles and safe use of electrosurgery in minimally invasive surgery. Gynecol. Pelvic Med. 2021;4:6. doi: 10.21037/gpm-2020-pfd-10. [DOI] [Google Scholar]
- 62.Hainer B.L. Fundamentals of electrosurgery. J. Am. Board Fam. Pract. 1991;4(6):419–426. https://pubmed.ncbi.nlm.nih.gov/1767694 Available: [PubMed] [Google Scholar]
- 63.Guidelines for Large Animal Mobile Veterinary Clinics in Western Australia. Government of Western Australia Department of Health; 2013. https://www.health.wa.gov.au/PDF/GuidelinesforLarge_Animal_Vet_Clinics_in_WA.ashx [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the relevant data are presented within the article.