Abstract
Members of the ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family are known to influence development, angiogenesis, coagulation and progression of arthritis. As proteinases their substrates include the von Willebrand factor precursor and extracellular matrix components such as procollagen, hyalectans (hyaluronan-binding proteoglycans including aggrecan), decorin, fibromodulin and cartilage oligomeric matrix protein. ADAMTS levels and activities are regulated at multiple levels through the control of gene expression, mRNA splicing, protein processing and inhibition by TIMP (tissue inhibitor of metalloproteinases). A recent screen of human cartilage has shown that multiple members of the ADAMTS family may be important in connective tissue homeostasis and pathology.
Introduction
ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) proteinases are a group of secreted enzymes; many of them have been found to be expressed in cartilage [1]. Functional investigations of these enzymes have largely been limited to a few specific members, particularly ADAMTS-4, which has been implicated in the progression of arthritis [2,3]. The purpose of this review is to summarise the structure, function and regulation of the entire ADAMTS group of proteinases and to emphasise areas of potential relevance with regard to the homeostasis and pathology of connective tissues.
ADAMTS evolution and structure
ADAMTS proteinases were first described in mice by Kuno and colleagues in 1997 [4] and have subsequently been identified in mammals and Caenorhabditis elegans. They form part of subfamily B (adamalysin subfamily), family M12, in clan MA of the metallopeptidases, as defined in the MEROPS database [5,6] and are structurally and evolutionarily related to the ADAM (a disintegrin and metalloproteinase; also part of the adamalysin subfamily) enzymes and, more distantly, the matrix metalloproteinase (MMP; family M10 in clan MA) enzymes. A comparison of the minimal characteristic domain organisation of these groups of proteinases is shown in Fig. 1.
Figure 1.
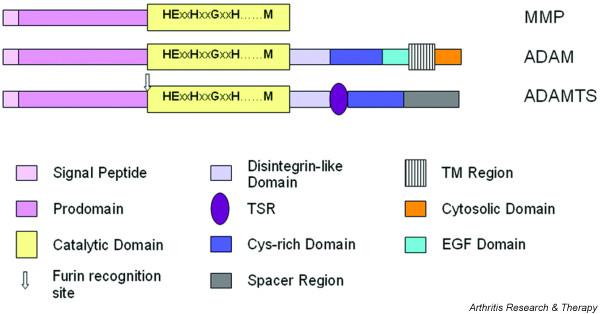
Schematic representation of the minimal domain organisation of matrix metalloproteinase (MMP), ADAM (a disintegrin and metalloproteinase) and ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs; for example ADAMTS-4) proteinases. Note that most MMPs possess additional C-terminal extensions containing domains such as hemopexin-like and fibronectin type II domains. ADAMTS possess from 0 to 14 additional thrombospondin type 1-like repeat (TSR)-like motifs C-terminal to the spacer domain. EGF, epidermal growth factor; TM, transmembrane.
Nineteen distinct human ADAMTS gene products have been identified. A nearest-neighbour dendrogram constructed (using ClustalW 1.7 [7]) from sequence alignments of the entire protein indicates that human ADAMTS proteins can be broadly divided into four subdivisions, which also seem to share structural characteristics and activities (see Fig. 2 and below). A dendrogram constructed from the sequence alignment of the catalytic domains was almost identical, which implies that the catalytic and ancillary domains evolved together (data not shown). The first of the divisions, consisting of ADAMTS-1, -4, -5, -8, -9, -15 and -20, subdivides into two further groups, one composed of ADAMTS-9 and -20 and the other of ADAMTS-1, -4, -5, -8 and -15. A second, well-defined, subgroup contains ADAMTS-2, -3 and -14. ADAMTS-13 stands alone, and the remaining ADAMTS members form a loosely defined subgroup within which members are further divided into four pairs (ADAMTS-19 and -17, ADAMTS-18 and -16, ADAMTS-12 and -7, and ADAMTS-10 and -6) sharing structural features. A detailed study of the phylogenetic relationship of the ADAMTS family members has recently been published [8].
Figure 2.
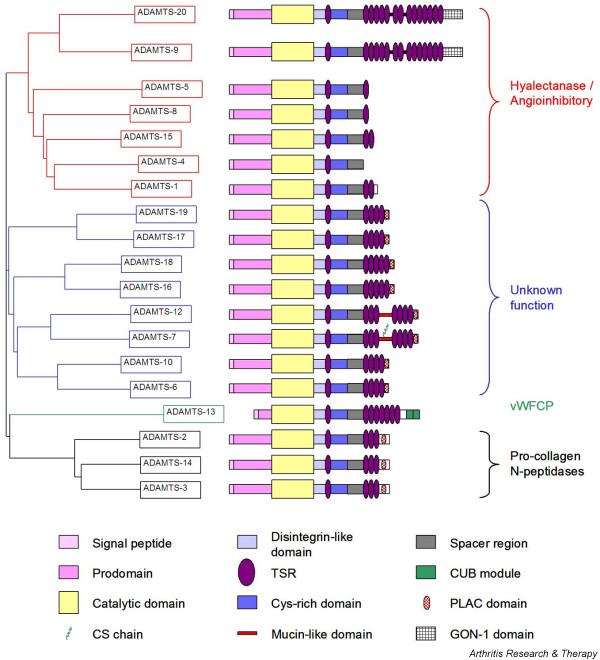
Schematic representation of the structural and evolutionary relationship of the 19 human ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) gene products. The dendrogram was calculated with ClustalW 1.7 [7]. The structural representation of ADAMTS proteins was adapted from [81]. Where applicable the long form of splice variants is shown (see the text). CS, chondroitin sulphate; CUB, complement C1r/C1s, Uegf (epidermal growth factor-related sea urchin protein) and BMP-1 (bone morphogenic protein-1); PLAC, protease and lacunin; TSR, thrombospondin I-like repeat; vWFCP, von Willebrand factor-cleaving protease.
ADAMTS domain structure
The signal sequence of ADAMTS proteins is followed by a pro-region of varying length, but which is unusually short in ADAMTS-13. The pro-domain of all ADAMTS proteinases contains at least one furin cleavage consensus motif; it is therefore generally believed that the zymogen forms of ADAMTS proteinases are cleaved intracellularly and that secreted proteins are in the mature form. This mechanism of maturation is supported by studies of ADAMTS-4, which identify an N terminus of F213ASLS in supernatants conditioned by cells transfected with ADAMTS-4, suggesting that the prodomain is efficiently removed in vivo [9]. The same study also demonstrated that purified proADAMTS-4 could be cleaved by recombinant furin in cell-free experiments. Furin has recently been shown to interact with the pro-form of ADAMTS-4 and to co-localise within the trans-Golgi network [10]. Using furin inhibitors and RNA interference techniques, the removal of the pro-domain was inhibited without affecting secretion, demonstrating an important role for furin in intracellular processing [10]. The same study also revealed the presence of furin-independent pro-domain processing pathways in some cells.
The catalytic domains of ADAMTS proteinases share a high degree of similarity and contain the zinc-binding sequence HEXXHXXGXXH, in which the catalytic zinc is coordinated by the three histidine residues. This arrangement is facilitated by the conserved glycine, which permits a tight hairpin loop and enables the third histidine to occupy its correct position [11,12]. As in all MMPs and adamalysins, the zinc-binding sequence is followed at a short distance C-terminally by a conserved methionine residue, an active-site arrangement that has been termed 'metzincin-type'. This methionine constitutes the 'Metturn', a tight turn arranged as a right-handed screw that seems to serve an important function in the structure of the active site [11].
The catalytic domain is followed by a region with 25 to 45% identity to the snake venom disintegrins, although it does not contain the cysteine arrangement of the latter [13]. This domain has therefore been termed disintegrin-like, though there is currently no published evidence that this ADAMTS domain interacts with integrins.
Unlike ADAM proteins, ADAMTS proteinases possess a well-conserved thrombospondin type 1-like repeat (TSR), homologous to the type I repeats of thrombospondins 1 and 2 [14], between the disintegrin-like and cysteine-rich domain (CRD). By analogy to thrombospondins 1 and 2 [15], the central TSR of ADAMTS proteinases is believed to function as a sulphated glycosaminoglycan-binding domain. The independently expressed central TSR of murine ADAMTS-1 required 0.46 to 0.66 M NaCl for elution from a heparin affinity column, indicating that this motif forms a functional heparin-binding unit [16].
The CRD is a well-conserved cysteine-rich sequence containing 10 cysteine residues. In contrast to ADAM proteins, in which the CRD is followed by epidermal growth factor (EGF)-like repeats, a transmembrane domain and C-terminal cytosolic region, all ADAMTS proteinases possess instead a cysteine-free 'spacer' region. This domain varies in length and contains several conserved hydrophobic residues in the N-terminal portion and an extremely variable C-terminal portion. The expression of various domain-deletion constructs of murine ADAMTS-1 revealed the CRD-spacer sequence as a functional extracellular matrix (ECM)-binding domain [16]. This role was supported by investigation of C-terminally processed forms of human ADAMTS-4, which, in combination with a deletion construct lacking the CRD-spacer sequence, indicated that these domains also bind to both heparin and the glycosaminoglycans of aggrecan (predominantly keratan and chondroitin sulphates) [9]. Three putative heparin-binding sequences were identified within the CRD-spacer sequence of ADAMTS-4, one within the CRD and two within the spacer, and peptides corresponding to these sequences were shown to inhibit the binding of ADAMTS-4 to heparin [9].
With the exception of ADAMTS-4, which terminates after the spacer region, all ADAMTS proteinases possess between 1 and 14 TSRs C-terminal to the spacer region (Fig. 2). The sequence of these additional TSRs is more variable between the ADAMTS proteinases than is the central TSR, but the independent expression of the C-terminal TSRs of murine ADAMTS-1 has indicated that these motifs can form functional heparin-binding units [16]. The TSRs of the C-terminal region are arranged in one, two or three tandem arrays. Between arrays is either a short linker sequence (ADAMTS-9 and ADAMTS-20) or a mucin-like domain (ADAMTS-7 and ADAMTS-12) [17].
Four additional types of module have been described in the ADAMTS group and all are present C-terminal to the TSR arrays. ADAMTS-9 and -20 contain a unique module, also found in the C. elegans ADAMTS GON-1, containing 10 conserved cysteine residues [18]. Several ADAMTS proteinases (-6, -7, -10, -12, -16, -17, -18 and -19) possess a PLAC (protease and lacunin) domain containing six conserved cysteine residues, which is found in some pro-protein convertases [19]. A C-terminal extension containing a unique embedded PLAC domain is present in ADAMTS-2, -3 and -14. Finally, CUB (complement C1r/C1s, Uegf (EGF-related sea urchin protein) and BMP-1 (bone morphogenic protein-1)) domains are present at the C terminus of ADAMTS-13 [20]. This domain is also present in spermadhesins, tumour necrosis factor-stimulated gene-6 and the complement proteins C1r, C1s and mannan-binding lectin-associated serine proteinases, among others [21], and there is evidence to suggest that these domains mediate protein-protein interactions with other CUB domain-containing proteins [22,23].
Functions of ADAMTS proteins
Hyalectanases: ADAMTS-1, -4, -5, -8, -9, -15 and -20
ADAMTS-1, -4, -5, -8 -9 and -15 cleave the hyalectan (hyaluronan-binding proteoglycan) aggrecan between a glutamate in the P1 pocket and small aliphatic residues in P1' (using the terminology of Schechter and Berger [24]), an activity that has previously been identified and termed 'aggrecanase' [25]. Several of these ADAMTS proteinases also cleave the related hyalectan versican at analogous sites and ADAMTS-4 has been demonstrated to cleave a further hyalectan, brevican [26]. These ADAMTS proteinases may therefore be termed 'hyalectanases'. ADAMTS-4 has recently been shown to cleave COMP (cartilage oligomeric matrix protein) as well as fibromodulin and decorin, indicating that this subgroup of proteinases is not restricted to the cleavage of proteglycans and might have a wider proteolytic spectrum [27]. There are no published reports of hyalectanase activity for ADAMTS-20, but the catalytic domain of this proteinase has been demonstrated to be proteolytically active [28].
Although aggrecan is also processed by additional proteinases, aggrecanase-mediated cleavage is a characteristic event in the catabolism of cartilage aggrecan in the arthritides [29-31]. The potential importance of aggrecanase-mediated degradation of aggrecan in cartilage, particularly with regard to the development of arthritis, is highlighted by the report that aggrecan protects collagen fibrils from degradation by collagenases [32]. Aggrecanase-mediated cleavages of aggrecan and versican have also been reported to occur in the tensile regions of tendon, suggesting that such activities might be important in the homeostasis of this tissue [33].
The involvement of ADAMTS proteinases in connective tissue turnover has been demonstrated using both cleavage-epitope-specific antibodies and inhibitors that exclude the involvement of other proteinases, particularly the MMPs [3,32]. ADAMTS-4 and -5 have been implicated as the aggrecanases involved in aggrecan degradation in osteoarthritis (OA) on the basis of mRNA and protein expression [3]. Investigations into the relative contributions of ADAMTS-4 and -5 to the aggrecanase-mediated degradation of cartilage aggrecan in arthritis models have recently been conducted in mice with the use of catalytic domain knockouts of each gene [34-36]. Both ADAMTS-4 and -5 knockout mice were phenotypically normal and indistinguishable from wild-type littermates, indicating that each enzyme was dispensable for normal development [34-36]. In a surgically induced OA model there was a significant reduction in the severity of induced OA in ADAMTS-5 knockout mice but no difference in progression or severity in ADAMTS-4 knockout mice [34,35]. Similarly, in a model of inflammatory arthritis, ADAMTS-5, but not ADAMTS-4, knockout mice were protected against aggrecan loss [36]. Furthermore, aggrecanase activity was inducible in articular cartilage explants from wild-type and ADAMTS-4 knockout mice but not from ADAMTS-5 knockout littermates [34-36]. Together these studies suggest that, at least in these mouse models, ADAMTS-5 is primarily responsible for the increased aggrecanase activity.
In addition to proteoglycan cleavage, members of this subgroup may also regulate angiogenesis. Vascularisation is a feature of both chronic tendon pathology [37] and arthritis [38], and specific ADAMTS proteins may have a role in its regulation. ADAMTS-1 and -8 possess a potent angioinhibitory activity, suppressing both fibroblast growth factor-2 and vascular endothelial growth factor (VEGF)-mediated angiogenic effects on endothelial cells but not on smooth muscle cells or fibroblasts [39]. An upregulation of ADAMTS-4 mRNA expression has also been reported in an in vitro model of angiogenesis [40]. ADAMTS-1 has been shown to interact with VEGF165 but does not cleave the growth factor, suggesting that the inhibitory effect is brought about by sequestering VEGF from its cell receptor [41]. By analogy to the thrombospondins, the angioinhibitory activity might be assumed to be mediated through the TSR motifs [42], but an ADAMTS-1 C-terminal construct consisting of the central TSR, CRD, spacer region and C-terminal TSRs was an ineffective inhibitor in a functional angiogenesis assay [43]. Furthermore, an ADAMTS-1 active site mutant displayed no angioinhibitory activity [43], therefore suggesting that both the metalloproteinase and ancillary domains are necessary in bringing about the angioinhibitory effects.
There is increasing evidence that members of this ADAMTS subgroup have roles in development. The C. elegans ADAMTS, GON-1, which is most closely related to ADAMTS-9 and -20, is involved in cell migration during the development of the gonad [18,44,45]. Studies in mice indicate that ADAMTS-20 is required for the migration of melanoblasts during embryogenesis, and that mutation of ADAMTS-20 causes a white-spotting mutation, Belted [46]. ADAMTS-1-null mice display several developmental abnormalities, primarily within the urogenital systems, affecting normal growth, organ morphology and function, and female fertility [47,48]. A role for ADAMTS-1 in ovulation has been inferred from studies in rats [49], mice [50] and horses [51], which indicate that upregulation of ADAMTS-1 mRNA correlates temporally with the appearance of ADAMTS-cleaved versican within the ECM of the cumulus oocyte complex [52]. The active form of ADAMTS-4 (but not ADAMTS-5) has been reported to co-localise with ADAMTS-mediated aggrecan cleavage in developing long bones in the rat, implying that ADAMTS-4 mediates the developmental turnover of aggrecan during long bone formation [53]. However, an ADAMTS-4 knockout mouse showed no signs of skeletal abnormalities despite evidence of ADAMTS-4 expression and activity in the growth plates of wild-type mice [34].
Procollagen N-propeptidases: ADAMTS-2, -3 and -14
ADAMTS-2 cleaves the amino peptides of type I, type II and type III procollagens [54,55], and genetic analysis has indicated that mutations in the ADAMTS2 gene correlate with the incidence of both Ehlers–Danlos syndrome type VII C and dermatosparaxis in cattle [56]. Ehlers–Danlos syndrome is a recessively inherited connective-tissue disorder that arises as a result of incorrectly processed procollagen N-telopeptides and is characterised by extreme skin fragility, joint laxity and droopy skin [57]. adamts2 knockout mice seem normal at birth but soon develop fragile skin, and male mice are infertile with decreased testicular sperm, suggesting that ADAMTS-2 has important functions both in regulating the formation and structure of skin and in the maturation of spermatogonia [58]. Despite these abnormalities, a large fraction of both type I N-propeptides in skin and type II N-propeptides in cartilage are cleaved in adamts2 knockout mice, indicating the presence of additional procollagen N-peptidases. ADAMTS-3 has since been identified as a type II procollagen N-propeptidase, whose expression is much lower than ADAMTS-2 in skin but is about 5-fold that of ADAMTS-2 in cartilage [59]. It has been suggested that the relative expression patterns of ADAMTS-2 and -3 explain the relative sparing of tissues such as cartilage in dermatosparaxis [59]. ADAMTS-14 has been identified as a homologue of ADAMTS-2, functioning as the major type I procollagen N-propeptidase activity in tendon [60].
Von Willebrand factor-cleaving protease: ADAMTS-13
ADAMTS-13 cleaves the large multimeric von Willebrand factor (vWF) precursor to generate vWF of optimal size for proper coagulation [20,61,62]. Mutations in ADAMTS-13 correlate with the occurrence of the hereditary form [63], and autoantibodies against ADAMTS-13 with the sporadic form [64], of thrombotic thrombocytopenic purpura, a disease in which the cleavage of vWF is decreased resulting in large vWF multimers.
Other ADAMTS proteins: ADAMTS-6, -7, -10, -12, -16, -17, -18 and -19
Functions have not been assigned to this subgroup of ADAMTS proteins. However, ADAMTS-7, -10 and -12 are known to be proteolytically active, although ADAMTS-7 does not cleave aggrecan or versican at the sites characteristic of the hyalectanase ADAMTS subgroup [17,65,66]. The long form of ADAMTS-7 contains both O-linked glycosylations and an N-linked chondroitin sulphate (CS) chain within its mucin-like domain [17]. The presence of this CS chain is reported to reduce the affinity of the protein for heparin [17]. Null mutations of the ADAMTS10 gene have recently been attributed to a recessive form of Weill-Marchesani syndrome, whose symptoms include short stature, brachydactyly, joint stiffness and eye lens abnormalities [67].
Regulation of ADAMTS expression, structure and activity
Regulation of expression
ADAMTS-1 was initially identified as a novel murine cDNA expressed in a cachexigenic adenocarcinoma cell line that could be upregulated by IL-1 [4]. The intravenous administration of lipopolysaccharide into mice induces expression in the kidney and heart, suggesting that ADAMTS-1 is an inflammation-associated gene product [4]. ADAMTS-1, -6 and -9 mRNA levels are upregulated in response to tumour necrosis factor-α in retinal pigment epithelium-derived cells [68] and ADAMTS-4 mRNA expression is increased by IL-17 in articular chondrocytes [69], indicating that other ADAMTS proteins might also be upregulated by inflammatory cytokines. The induction of ADAMTS-4 mRNA in β-amyloid-treated rat astrocytes supports an inflammatory-associated role for this gene [70].
In articular cartilage an upregulation in mRNA level by transforming growth factor β has been shown for ADAMTS-4 but not ADAMTS-5 [71], indicating that gene expression of these aggrecanases might be differentially regulated. Differential regulation of these genes has also been reported with an immortalised human chondrocyte cell line, in which IL-1α together with oncostatin M, but not either cytokine alone, upregulated ADAMTS-4 mRNA, whereas ADAMTS-5 mRNA was upregulated by IL-1α and there was no effect of oncostatin M [72]. An induction by IL-1α of both ADAMTS-4 and -5 in mouse cartilage explants has been reported [36]. However, studies in human articular cartilage and bovine nasal cartilage and synovium suggest that the expression of ADAMTS-4 and -5 mRNA is relatively insensitive to retinoic acid or IL-1α, despite the marked upregulation of aggrecanase activity [73-75]. A recent study of ADAMTS-4 suggests that the increase in aggrecanase activity observed after treatment with IL-1α is mediated, at least in part, through the upregulation of other enzymes that then process and activate existing ADAMTS enzyme within the tissue (see also below) [76].
Post-transcriptional regulation
Post-transcriptional regulation through alternative splicing has been identified for several of the ADAMTS proteins, including ADAMTS-6, -7 and -9 [68,77]. Initial reports predicted that ADAMTS-6 and -7 proteins would terminate after a single C-terminal TSR [78] and that ADAMTS-9 would terminate after three C-terminal TSRs [79,80]. However, the full coding sequences of ADAMTS-6, -7 and -9 are now believed to encode four C-terminal TSRs followed by a PLAC domain, seven C-terminal TSRs interrupted by a mucin-like domain and followed by a PLAC domain, and 14 C-terminal TSRs, respectively [81] (Fig. 2). In addition, other splice variants of ADAMTS-6 have been identified that terminate after the pro-domain and immediately after the catalytic domain [77]. The identification of spliced variants of ADAMTS-6, -7 and -9 suggests that splicing might be an important mechanism of regulation of this family of enzymes in which the ancillary domains of the proteins are altered. It is worth noting that the full coding sequences of ADAMTS-16 and -18 are also predicted to be longer than initially reported [80], each encoding five C-terminal TSRs followed by a PLAC domain [81].
Present studies of ADAMTS-6 have identified a potential translational regulatory mechanism for the expression of this protein [77]. The full 5' untranslated region of this mRNA contains multiple upstream ATG initiation codons followed by very short open reading frames that are predicted to recruit ribosomes to non-productive sites, thereby reducing the rate of translation. By switching to a transcript lacking these upstream ATGs, either by mRNA processing or through the use of an alternative promoter, protein production could be increased, a form of regulation that has been implicated in several diseases [82].
Post-translational regulation
ADAMTS enzymes are synthesised as zymogens that undergo the constitutive removal of the pro-domain in the secretory pathway by pro-protein convertases such as furin (see above). Secreted ADAMTS proteinases can undergo additional processing at their C-terminal end. Studies of ADAMTS-1 and -4 have identified cleavages within the respective spacer domains, and a study of ADAMTS-12 revealed a cleavage within the mucin-like domain that released the C-terminal TSR quadruplet [83]. For ADAMTS-4, two such events have been identified so far, one resulting in the removal of most of the spacer region, the other in the removal of the spacer region and most of the CRD [9]. Although C-terminal processing of ADAMTS-4 has been shown to occur through an autocatalytic mechanism [9], cell-based experiments have suggested that such cleavages are mediated by MMP [83-85]. A processing pathway in which the full-length mature enzyme is bound at the cell surface and cleaved to generate both identified truncated forms by membrane-type 4-MMP (MT4-MMP, also termed MMP-17) has been proposed [85]. The processed form retaining the CRD seems to be maintained at the cell surface by syndecan-1 through interactions with both CS and heparan sulphate chains, whereas the shortest form is released into the medium.
The cleavage of ADAMTS-1 and -4 proteins within the CRD-spacer region reduces the affinity of both for heparin, suggesting that the spacer region influences these interactions [83,84]. These processing events also alter the activities of these proteins; for example, they reduce the angioinibitory capacity of ADAMTS-1 and alter the activity of ADAMTS-4 against aggrecan [83,84]. The processed ADAMTS-4 is able to cleave aggrecan within the interglobular domain (IGD) in addition to sites within the CS attachment region that are also cleaved by the full-length proteinase [83,84]. Similarly, investigations of ADAMTS-13 proteins revealed that the major epitopes of inhibitory autoantibodies from patients with thrombotic thrombocytopenic purpura reside within the CRD-spacer region and that removal of the CRD-spacer region results in a marked reduction in vWF cleaving activity [86]. It therefore seems that the CRD-spacer region influences the activities of the catalytic domains of the ADAMTS proteinases.
Studies of ADAMTS-4 have given an insight into the influence of the ancillary domains on the proteinase activities. A recent study of truncated recombinant forms of ADAMTS-4 indicated that the presence of the CRD was required for maximal aggrecanase activity, whereas the inclusion of the spacer region prevented cleavage of aggrecan at the IGD site [27]. In addition, the presence of the spacer region prevented the cleavage of non-proteoglycan substrates such as deglycosylated aggrecan, chemically modified transferrin, and fibromodulin, an activity that required only the catalytic and disintegrin-like domains [27].
These data suggest the ADAMTS-4 catalytic domain possesses a proteolytic activity that is modulated by the spacer region so that the characteristic non-IGD 'aggrecanase' sites of aggrecan are favoured over the IGD 'aggrecanase' site and non-hyalectan substrates. In addition, the requirement of the CRD for optimal cleavage of glycosylated aggrecan compared with its negligible influences on the cleavage of non-proteoglycan substrates suggests that this domain promotes hyalectan cleavage by mediating interactions with sulphated glycosaminoglycan, in a similar manner to that proposed for the activation by heparan sulphate of growth-factor-receptor signalling [87].
The processing of ADAMTS enzymes is likely to be of importance in pathologies such as the arthritides, in which loss of aggrecan seems to be a primary event [88]. The activity of the full-length ADAMTS-4 seems to be restricted to cleavages within the CS region of aggrecan, events that would truncate the proteoglycan but would not be expected to remove aggrecan from cartilage. However, processing events removing the spacer region might generate a more promiscuous and destructive activity capable of disaggregating aggrecan–hyaluronan complexes through IGD proteolysis, resulting in the loss of aggrecan from the tissue. Additional processing, removing the CRD, may reduce activity towards aggrecan in favour of non-proteoglycan substrates. When considering the role of ADAMTS-4 or other ADAMTS proteinases in arthritic pathologies it may therefore be prudent to consider the enzyme form in addition to the absolute levels of the protein.
Regulation by inhibitors
The most significant endogenous ECM inhibitor of ADAMTS proteinases to be identified is tissue inhibitor of metalloproteinases-3 (TIMP-3) [89], although other TIMP members possess a limited inhibitory capacity [90]. TIMP-3 is unique among the TIMP family in that it binds tightly to, and is found exclusively within, the ECM [91]. In addition to the TIMP family, papilin, a protein with homology to the ancillary domains of ADAMTS proteins, is a non-competitive inhibitor of ADAMTS-2 [92]. Recently, the C-terminal portion of fibronectin has also been reported as a potent inhibitor of ADAMTS-4 [93].
Roles of the ADAMTS family in connective tissues
There have been few comprehensive studies of ADAMTS in connective tissues. However, the expression of the entire ADAMTS subfamily was recently investigated in normal and OA cartilage [1]. This study indicated that, with the exception of ADAMTS-7 and -8, normal cartilage expressed the entire ADAMTS subfamily. The most highly expressed of the ADAMTS proteinase mRNAs were those for ADAMTS-1, -5, -6, -15, -18 and -19, and observed differences between normal and late-stage OA cartilage included the lower expression of 'hyalectanase' ADAMTS proteinases (ADAMTS-1, -5, -9 and -15) and a higher expression of procollagen peptidases (ADAMTS-2 and -14). Furthermore, two ADAMTS members of unknown function (ADAMTS-12 and -16) were more highly expressed in OA. Although the levels of hyalectanases were not increased in late-stage OA this result does not preclude a role for this proteinase in the early stages of OA. In a comparison of normal and chronic painful tendon by this group (GC Jones, AN Corps, CJ Pennington, IM Clark, DR Edwards, MM Bradley, BC Hazleman, GP Riley, unpublished work), the expression of all 19 ADAMTS genes was detected. A lower expression level of ADAMTS-5 and higher levels of ADAMTS-2, -12, -14 and -17 were observed in chronic painful tendon. These data suggest that many members of the ADAMTS subfamily might possess important roles in both the homeostasis and pathology of connective tissues and are worthy of further investigation.
Conclusion
The ADAMTS enzymes are a group of secreted proteinases possessing a conserved N-terminal domain architecture followed by a variable C terminus. The phylogenetic relationship of these proteinases broadly defines four subdivisions, which also seem to discriminate protein activities. Evidence suggests that the synthesis of these proteinases is regulated at the level of both expression and translation and that their structures and activities are regulated through mRNA splicing and post-translationally through protein processing and endogenous inhibitors. The cleavage state of individual ADAMTS proteinases seems to be of importance, because ancillary domains (particularly the CRD and spacer region) seem to modulate the specificity of their activities, an event that might be of significance in arthritis [75]. The complex nature of the regulation of these proteinases implies that a precise control of their activities is required for the maintenance of homeostasis. A broad spectrum of ADAMTS proteinases are expressed in both cartilage and tendon, which suggests that multiple ADAMTS proteinases are important in the homeostasis of these tissues.
In conclusion, the ADAMTS proteins are complex, multi-domain, proteinases whose synthesis and activity are subjected to multiple levels of regulation and which are widely expressed in connective tissues, in both health and pathology.
Abbreviations
ADAM = a disintegrin and metalloproteinase; ADAMTS = a disintegrin and metalloproteinase with thrombospondin motifs; CRD = cysteine-rich domain; CS = chondroitin sulphate; CUB = complement C1r/C1s, Uegf (EGF-related sea urchin protein) and BMP-1 (bone morphogenic protein-1); ECM = extracellular matrix; EGF = epidermal growth factor; IGD = interglobular domain; IL = interleukin; MMP = matrix metalloproteinase; OA = osteoarthritis; PLAC = protease and lacunin; TIMP = tissue inhibitor of metalloproteinases; TSR = thrombospondin type 1-like repeat; VEGF = vascular endothelial growth factor; vWF = von Willebrand factor.
Competing interests
The author(s) declare that they have no competing interests.
Acknowledgments
Acknowledgements
The authors wish to thank Tony Corps and Norman McKie for helpful discussion of this manuscript. GCJ is supported by The Isaac Newton Trust, The Rosetrees Trust and Cambridge Arthritis Research Endeavour.
References
- Kevorkian L, Young DA, Darrah C, Donell ST, Shepstone L, Porter S, Brockbank SM, Edwards DR, Parker AE, Clark IM. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50:131–141. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Burn TC, Pratta MA, Abbaszade I, Hollis JM, Liu R, Rosenfeld SA, Copeland RA, Decicco CP, Wynn R, et al. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science. 1999;284:1664–1666. doi: 10.1126/science.284.5420.1664. [DOI] [PubMed] [Google Scholar]
- Malfait AM, Liu RQ, Ijiri K, Komiya S, Tortorella MD. Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J Biol Chem. 2002;277:22201–22208. doi: 10.1074/jbc.M200431200. [DOI] [PubMed] [Google Scholar]
- Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem. 1997;272:556–562. doi: 10.1074/jbc.272.1.556. [DOI] [PubMed] [Google Scholar]
- MEROPS: the Peptidase Database http://merops.sanger.ac.uk
- Barrett AJ, Rawlings ND, O'Brien EA. The MEROPS database as a protease information system. J Struct Biol. 2001;134:95–102. doi: 10.1006/jsbi.2000.4332. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AC, Malik SB, Logsdon JM, Jr, Van Meir EG. Functional evolution of ADAMTS genes: evidence from analyses of phylogeny and gene organization. BMC Evol Biol. 2005;5:11. doi: 10.1186/1471-2148-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery CR, Zeng W, Corcoran C, Collins-Racie LA, Chockalingam PS, Hebert T, Mackie SA, McDonagh T, Crawford TK, Tomkinson KN, et al. Autocatalytic cleavage of ADAMTS-4 (aggrecanase-1) reveals multiple glycosaminoglycan-binding sites. J Biol Chem. 2002;277:42775–42780. doi: 10.1074/jbc.M205309200. [DOI] [PubMed] [Google Scholar]
- Wang P, Tortorella M, England K, Malfait AM, Thomas G, Arner EC, Pei D. Proprotein convertase furin interacts with and cleaves pro-ADAMTS4 (aggrecanase-1) in the trans-Golgi network. J Biol Chem. 2004;279:15434–15440. doi: 10.1074/jbc.M312797200. [DOI] [PubMed] [Google Scholar]
- Bode W, Gomis-Ruth FX, Stockler W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Metturn) and topologies and should be grouped into a common family, the 'metzincins'. FEBS Lett. 1993;331:134–140. doi: 10.1016/0014-5793(93)80312-I. [DOI] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ. Evolutionary families of metallopeptidases. Methods Enzymol. 1995;248:183–228. doi: 10.1016/0076-6879(95)48015-3. [DOI] [PubMed] [Google Scholar]
- Huang TF. What have snakes taught us about integrins? Cell Mol Life Sci. 1998;54:527–540. doi: 10.1007/s000180050181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. Thrombospondins: structure and regulation of expression. FASEB J. 1992;6:3290–3299. doi: 10.1096/fasebj.6.14.1426766. [DOI] [PubMed] [Google Scholar]
- Guo NH, Krutzsch HC, Negre E, Zabrenetzky VS, Roberts DD. Heparin-binding peptides from the type I repeats of thrombospondin. Structural requirements for heparin binding and promotion of melanoma cell adhesion and chemotaxis. J Biol Chem. 1992;267:19349–19355. [PubMed] [Google Scholar]
- Kuno K, Matsushima K. ADAMTS-1 protein anchors at the extracellular matrix through the thrombospondin type I motifs and its spacing region. J Biol Chem. 1998;273:13912–13917. doi: 10.1074/jbc.273.22.13912. [DOI] [PubMed] [Google Scholar]
- Somerville RP, Longpre JM, Apel ED, Lewis RM, Wang LW, Sanes JR, Leduc R, Apte SS. ADAMTS7B, the full-length product of the ADAMTS7 gene, is a chondroitin sulfate proteoglycan containing a mucin domain. J Biol Chem. 2004;279:35159–35175. doi: 10.1074/jbc.M402380200. [DOI] [PubMed] [Google Scholar]
- Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S, Wight TN, Leduc R, Apte SS. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem. 2003;278:9503–9513. doi: 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- Nardi JB, Martos R, Walden KK, Lampe DJ, Robertson HM. Expression of lacunin, a large multidomain extracellular matrix protein, accompanies morphogenesis of epithelial monolayers in Manduca sexta. Insect Biochem Mol Biol. 1999;29:883–897. doi: 10.1016/S0965-1748(99)00064-8. [DOI] [PubMed] [Google Scholar]
- Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059–41063. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- Bork P, Beckmann G. The CUB domain. A widespread module in developmentally regulated proteins. J Mol Biol. 1993;231:539–545. doi: 10.1006/jmbi.1993.1305. [DOI] [PubMed] [Google Scholar]
- Romero A, Romao MJ, Varela PF, Kolln I, Dias JM, Carvalho AL, Sanz L, Topfer-Petersen E, Calvete JJ. The crystal structures of two spermadhesins reveal the CUB domain fold. Nat Struct Biol. 1997;4:783–788. doi: 10.1038/nsb1097-783. [DOI] [PubMed] [Google Scholar]
- Gregory LA, Thielens NM, Arlaud GJ, Fontecilla-Camps JC, Gaboriaud C. X-ray structure of the Ca2+-binding interaction domain of C1s. Insights into the assembly of the C1 complex of complement. J Biol Chem. 2003;278:32157–32164. doi: 10.1074/jbc.M305175200. [DOI] [PubMed] [Google Scholar]
- Schechter I, Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967;27:157–162. doi: 10.1016/S0006-291X(67)80055-X. [DOI] [PubMed] [Google Scholar]
- Sandy JD, Neame PJ, Boynton RE, Flannery CR. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991;266:8683–8685. [PubMed] [Google Scholar]
- Nakamura H, Fujii Y, Inoki I, Sugimoto K, Tanzawa K, Matsuki H, Miura R, Yamaguchi Y, Okada Y. Brevican is degraded by matrix metalloproteinases and aggrecanase-1 (ADAMTS4) at different sites. J Biol Chem. 2000;275:38885–38890. doi: 10.1074/jbc.M003875200. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Enghild JJ, Gendron C, Hughes C, Caterson B, Itoh Y, Nagase H. Altered proteolytic activities of ADAMTS-4 expressed by C-terminal processing. J Biol Chem. 2004;279:10109–10119. doi: 10.1074/jbc.M312123200. [DOI] [PubMed] [Google Scholar]
- Llamazares M, Cal S, Quesada V, Lopez-Otin C. Identification and characterization of ADAMTS-20 defines a novel subfamily of metalloproteinases-disintegrins with multiple thrombospondin-1 repeats and a unique GON domain. J Biol Chem. 2003;278:13382–13389. doi: 10.1074/jbc.M211900200. [DOI] [PubMed] [Google Scholar]
- Sandy JD, Flannery CR, Neame PJ, Lohmander LS. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J Clin Invest. 1992;89:1512–1516. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmander LS, Neame PJ, Sandy JD. The structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis Rheum. 1993;36:1214–1222. doi: 10.1002/art.1780360906. [DOI] [PubMed] [Google Scholar]
- Sandy JD, Verscharen C. Analysis of aggrecan in human knee cartilage and synovial fluid indicates that aggrecanase (ADAMTS) activity is responsible for the catabolic turnover and loss of whole aggrecan whereas other protease activity is required for C-terminal processing in vivo. Biochem J. 2001;358:615–626. doi: 10.1042/0264-6021:3580615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratta MA, Yao W, Decicco C, Tortorella MD, Liu RQ, Copeland RA, Magolda R, Newton RC, Trzaskos JM, Arner EC. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003;278:45539–45545. doi: 10.1074/jbc.M303737200. [DOI] [PubMed] [Google Scholar]
- Samiric T, Ilic MZ, Handley CJ. Characterisation of proteoglycans and their catabolic products in tendon and explant cultures of tendon. Matrix Biol. 2004;23:127–140. doi: 10.1016/j.matbio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Glasson SS, Askew R, Sheppard B, Carito BA, Blanchet T, Ma HL, Flannery CR, Kanki K, Wang E, Peluso D, et al. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum. 2004;50:2547–2558. doi: 10.1002/art.20558. [DOI] [PubMed] [Google Scholar]
- Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- Astrom M, Rausing A. Chronic Achilles tendinopathy. A survey of surgical and histopathologic findings. Clin Orthop. 1995;316:151–164. [PubMed] [Google Scholar]
- Walsh DA. Angiogenesis and arthritis. Rheumatology (Oxford) 1999;38:103–112. doi: 10.1093/rheumatology/38.2.103. [DOI] [PubMed] [Google Scholar]
- Vazquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M, Iruela-Arispe ML. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angioinhibitory activity. J Biol Chem. 1999;274:23349–23357. doi: 10.1074/jbc.274.33.23349. [DOI] [PubMed] [Google Scholar]
- Kahn J, Mehraban F, Ingle G, Xin X, Bryant JE, Vehar G, Schoen-feld J, Grimaldi CJ, Peale F, Draksharapu A, et al. Gene expression profiling in an in vitro model of angiogenesis. Am J Pathol. 2000;156:1887–1900. doi: 10.1016/S0002-9440(10)65062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque A, Carpizo DR, Iruela-Arispe ML. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J Biol Chem. 2003;278:23656–23665. doi: 10.1074/jbc.M212964200. [DOI] [PubMed] [Google Scholar]
- Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993;122:497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Carpizo D, Luque A. ADAMTS1: a matrix metalloprotease with angioinhibitory properties. Ann N Y Acad Sci. 2003;995:183–190. doi: 10.1111/j.1749-6632.2003.tb03221.x. [DOI] [PubMed] [Google Scholar]
- Blelloch R, Anna-Arriola SS, Gao D, Li Y, Hodgkin J, Kimble J. The gon-1 gene is required for gonadal morphogenesis in Caenorhabditis elegans. Dev Biol. 1999;216:382–393. doi: 10.1006/dbio.1999.9491. [DOI] [PubMed] [Google Scholar]
- Blelloch R, Kimble J. Control of organ shape by a secreted metalloprotease in the nematode Caenorhabditis elegans. Nature. 1999;399:586–590. doi: 10.1038/21196. [DOI] [PubMed] [Google Scholar]
- Rao C, Foernzler D, Loftus SK, Liu S, McPherson JD, Jungers KA, Apte SS, Pavan WJ, Beier DR. A defect in a novel ADAMTS family member is the cause of the belted white-spotting mutation. Development. 2003;130:4665–4672. doi: 10.1242/dev.00668. [DOI] [PubMed] [Google Scholar]
- Shindo T, Kurihara H, Kuno K, Yokoyama H, Wada T, Kurihara Y, Imai T, Wang Y, Ogata M, Nishimatsu H, et al. ADAMTS-1: a metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J Clin Invest. 2000;105:1345–1352. doi: 10.1172/JCI8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama H, Wada T, Kobayashi K, Kuno K, Kurihara H, Shindo T, Matsushima K. A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-1 null mutant mice develop renal lesions mimicking obstructive nephropathy. Nephrol Dial Transplant. 2002;17(Suppl 9):39–41. doi: 10.1093/ndt/17.suppl_9.39. [DOI] [PubMed] [Google Scholar]
- Espey LL, Yoshioka S, Russell DL, Robker RL, Fujii S, Richards JS. Ovarian expression of a disintegrin and metalloproteinase with thrombospondin motifs during ovulation in the gonadotropin-primed immature rat. Biol Reprod. 2000;62:1090–1095. doi: 10.1095/biolreprod62.4.1090. [DOI] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA. 2000;97:4689–4694. doi: 10.1073/pnas.080073497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerboom D, Russell DL, Richards JS, Sirois J. Regulation of transcripts encoding ADAMTS-1 (a disintegrin and metalloproteinase with thrombospondin-like motifs-1) and proges-terone receptor by human chorionic gonadotropin in equine preovulatory follicles. J Mol Endocrinol. 2003;31:473–485. doi: 10.1677/jme.0.0310473. [DOI] [PubMed] [Google Scholar]
- Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem. 2003;278:42330–42339. doi: 10.1074/jbc.M300519200. [DOI] [PubMed] [Google Scholar]
- Mort JS, Flannery CR, Makkerh J, Krupa JC, Lee ER. Use of anti-neoepitope antibodies for the analysis of degradative events in cartilage and the molecular basis for neoepitope specificity. Biochem Soc Symp. 2003;70:107–114. doi: 10.1042/bss0700107. [DOI] [PubMed] [Google Scholar]
- Colige A, Li SW, Sieron AL, Nusgens BV, Prockop DJ, Lapière CM. cDNA cloning and expression of bovine procollagen I N-proteinase: a new member of the superfamily of zinc-metalloproteinases with binding sites for cells and other matrix components. Proc Natl Acad Sci USA. 1997;94:2374–2379. doi: 10.1073/pnas.94.6.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WM, Lee S, Steiglitz BM, Scott IC, Lebares CC, Allen ML, Brenner MC, Takahara K, Greenspan DS. Transforming growth factor-beta induces secretion of activated ADAMTS-2. A procollagen III N-proteinase. J Biol Chem. 2003;278:19549–19557. doi: 10.1074/jbc.M300767200. [DOI] [PubMed] [Google Scholar]
- Colige A, Sieron AL, Li SW, Schwarze U, Petty E, Wertelecki W, Wilcox W, Krakow D, Cohn DH, Reardon W, et al. Human Ehlers–Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am J Hum Genet. 1999;65:308–317. doi: 10.1086/302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusgens BV, Verellen-Dumoulin C, Hermanns-Lê T, De Paepe A, Nuytinck L, Piérard GE, Lapière CM. Evidence for a relationship between Ehlers-Danlos type VII C in humans and bovine dermatosparaxis. Nat Genet. 1992;1:214–217. doi: 10.1038/ng0692-214. [DOI] [PubMed] [Google Scholar]
- Li SW, Arita M, Fertala A, Bao Y, Kopen GC, Langsjo TK, Hyttinen MM, Helminen HJ, Prockop DJ. Transgenic mice with inactive alleles for procollagen N-proteinase (ADAMTS-2) develop fragile skin and male sterility. Biochem J. 2001;355:271–278. doi: 10.1042/0264-6021:3550271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes RJ, Hirohata S, Engle JM, Colige A, Cohn DH, Eyre DR, Apte SS. Procollagen II amino propeptide processing by ADAMTS-3. Insights on dermatosparaxis. J Biol Chem. 2001;276:31502–31509. doi: 10.1074/jbc.M103466200. [DOI] [PubMed] [Google Scholar]
- Colige A, Vandenberghe I, Thiry M, Lambert CA, Van Beeumen J, Li SW, Prockop DJ, Lapière CM, Nusgens BV. Cloning and characterization of ADAMTS-14, a novel ADAMTS displaying high homology with ADAMTS-2 and ADAMTS-3. J Biol Chem. 2002;277:5756–5766. doi: 10.1074/jbc.M105601200. [DOI] [PubMed] [Google Scholar]
- Fujikawa K, Suzuki H, McMullen B, Chung D. Purification of human von Willebrand factor-cleaving protease and its identification as a new member of the metalloproteinase family. Blood. 2001;98:1662–1666. doi: 10.1182/blood.V98.6.1662. [DOI] [PubMed] [Google Scholar]
- Soejima K, Mimura N, Hirashima M, Maeda H, Hamamoto T, Nakagaki T, Nozaki C. A novel human metalloprotease synthesized in the liver and secreted into the blood: possibly, the von Willebrand factor-cleaving protease? J Biochem (Tokyo) 2001;130:475–480. doi: 10.1093/oxfordjournals.jbchem.a003009. [DOI] [PubMed] [Google Scholar]
- Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- Scheiflinger F, Knobl P, Trattner B, Plaimauer B, Mohr G, Dockal M, Dorner F, Rieger M. Nonneutralizing IgM and IgG antibodies to von Willebrand factor-cleaving protease (ADAMTS-13) in a patient with thrombotic thrombocytopenic purpura. Blood. 2003;102:3241–3243. doi: 10.1182/blood-2003-05-1616. [DOI] [PubMed] [Google Scholar]
- Cal S, Arguelles JM, Fernandez PL, Lopez-Otin C. Identification, characterization, and intracellular processing of ADAM-TS12, a novel human disintegrin with a complex structural organization involving multiple thrombospondin-1 repeats. J Biol Chem. 2001;276:17932–17940. doi: 10.1074/jbc.M100534200. [DOI] [PubMed] [Google Scholar]
- Somerville RP, Jungers KA, Apte SS. ADAMTS10: discovery and characterization of a novel, widely expressed metalloprotease and its proteolytic activation. J Biol Chem. 2004;279:51208–51217. doi: 10.1074/jbc.M409036200. [DOI] [PubMed] [Google Scholar]
- Dagoneau N, Benoist-Lasselin C, Huber C, Faivre L, Megarbane A, Alswaid A, Dollfus H, Alembik Y, Munnich A, Legeai-Mallet L, et al. ADAMTS10 mutations in autosomal recessive Weill–Marchesani syndrome. Am J Hum Genet. 2004;75:801–806. doi: 10.1086/425231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevitt DJ, Mohamed J, Catterall JB, Li Z, Arris CE, Hiscott P, Sheridan C, Langton KP, Barker MD, Clarke MP, et al. Expression of ADAMTS metalloproteinases in the retinal pigment epithelium derived cell line ARPE-19: transcriptional regulation by TNFalpha. Biochim Biophys Acta. 2003;1626:83–91. doi: 10.1016/s0167-4781(03)00047-2. [DOI] [PubMed] [Google Scholar]
- Sylvester J, Liacini A, Li WQ, Zafarullah M. Interleukin-17 signal transduction pathways implicated in inducing matrix metalloproteinase-3, -13 and aggrecanase-1 genes in articular chon-drocytes. Cell Signal. 2004;16:469–476. doi: 10.1016/j.cellsig.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Satoh K, Suzuki N, Yokota H. ADAMTS-4 (a disintegrin and metalloproteinase with thrombospondin motifs) is transcriptionally induced in beta-amyloid treated rat astrocytes. Neurosci Lett. 2000;289:177–180. doi: 10.1016/S0304-3940(00)01285-4. [DOI] [PubMed] [Google Scholar]
- Yamanishi Y, Boyle DL, Clark M, Maki RA, Tortorella MD, Arner EC, Firestein GS. Expression and regulation of aggrecanase in arthritis: the role of TGF-beta. J Immunol. 2002;168:1405–1412. doi: 10.4049/jimmunol.168.3.1405. [DOI] [PubMed] [Google Scholar]
- Koshy PJ, Lundy CJ, Rowan AD, Porter S, Edwards DR, Hogan A, Clark IM, Cawston TE. The modulation of matrix metalloproteinase and ADAM gene expression in human chondrocytes by interleukin-1 and oncostatin M: a time-course study using real-time quantitative reverse transcription-polymerase chain reaction. Arthritis Rheum. 2002;46:961–967. doi: 10.1002/art.10212. [DOI] [PubMed] [Google Scholar]
- Vankemmelbeke MN, Holen I, Wilson AG, Ilic MZ, Handley CJ, Kelner GS, Clark M, Liu C, Maki RA, Burnett D, et al. Expression and activity of ADAMTS-5 in synovium. Eur J Biochem. 2001;268:1259–1268. doi: 10.1046/j.1432-1327.2001.01990.x. [DOI] [PubMed] [Google Scholar]
- Flannery CR, Little CB, Hughes CE, Caterson B. Expression of ADAMTS homologues in articular cartilage. Biochem Biophys Res Commun. 1999;260:318–322. doi: 10.1006/bbrc.1999.0909. [DOI] [PubMed] [Google Scholar]
- Pratta MA, Scherle PA, Yang G, Liu RQ, Newton RC. Induction of aggrecanase 1 (ADAM-TS4) by interleukin-1 occurs through activation of constitutively produced protein. Arthritis Rheum. 2003;48:119–133. doi: 10.1002/art.10726. [DOI] [PubMed] [Google Scholar]
- Patwari P, Gao G, Lee JH, Grodzinsky AJ, Sandy JD. Analysis of ADAMTS4 and MT4-MMP indicates that both are involved in aggrecanolysis in interleukin-1-treated bovine cartilage. Osteoarthritis Cartilage. 2005;13:269–277. doi: 10.1016/j.joca.2004.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevitt DJ, Li Z, Barker MD, Clarke MP, McKie N. Analysis of ADAMTS6 transcripts reveals complex alternative splicing and a potential role for the 5' untranslated region in translational control. Gene. [DOI] [PubMed]
- Hurskainen TL, Hirohata S, Seldin MF, Apte SS. ADAM-TS5, ADAM-TS6, and ADAM-TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM-TS family. J Biol Chem. 1999;274:25555–25563. doi: 10.1074/jbc.274.36.25555. [DOI] [PubMed] [Google Scholar]
- Clark ME, Kelner GS, Turbeville LA, Boyer A, Arden KC, Maki RA. ADAMTS9, a novel member of the ADAM-TS/metallospondin gene family. Genomics. 2000;67:343–350. doi: 10.1006/geno.2000.6246. [DOI] [PubMed] [Google Scholar]
- Cal S, Obaya AJ, Llamazares M, Garabaya C, Quesada V, Lopez-Otin C. Cloning, expression analysis, and structural characterization of seven novel human ADAMTSs, a family of metalloproteinases with disintegrin and thrombospondin-1 domains. Gene. 2002;283:49–62. doi: 10.1016/S0378-1119(01)00861-7. [DOI] [PubMed] [Google Scholar]
- Apte SS. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. Int J Biochem Cell Biol. 2004;36:981–985. doi: 10.1016/j.biocel.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Kozak M. Emerging links between initiation of translation and human diseases. Mamm Genome. 2002;13:401–410. doi: 10.1007/s00335-002-4002-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Milchanowski AB, Dufour EK, Leduc R, Iruela-Arispe ML. Characterization of METH-1/ADAMTS1 processing reveals two distinct active forms. J Biol Chem. 2000;275:33471–33479. doi: 10.1074/jbc.M002599200. [DOI] [PubMed] [Google Scholar]
- Gao G, Westling J, Thompson VP, Howell TD, Gottschall PE, Sandy JD. Activation of the proteolytic activity of ADAMTS4 (aggrecanase-1) by C-terminal truncation. J Biol Chem. 2002;277:11034–11041. doi: 10.1074/jbc.M107443200. [DOI] [PubMed] [Google Scholar]
- Gao G, Plaas A, Thompson VP, Jin S, Zuo F, Sandy JD. ADAMTS4 (aggrecanase-1) activation on the cell surface involves C-terminal cleavage by glycosylphosphatidyl inositol-anchored membrane type 4-matrix metalloproteinase and binding of the activated proteinase to chondroitin sulfate and heparan sulfate on syndecan-1. J Biol Chem. 2004;279:10042–10051. doi: 10.1074/jbc.M312100200. [DOI] [PubMed] [Google Scholar]
- Soejima K, Matsumoto M, Kokame K, Yagi H, Ishizashi H, Maeda H, Nozaki C, Miyata T, Fujimura Y, Nakagaki T. ADAMTS-13 cysteine-rich/spacer domains are functionally essential for von Willebrand factor cleavage. Blood. 2003;102:3232–3237. doi: 10.1182/blood-2003-03-0908. [DOI] [PubMed] [Google Scholar]
- Sasisekharan R, Venkataraman G. Heparin and heparan sulfate: biosynthesis, structure and function. Curr Opin Chem Biol. 2000;4:626–631. doi: 10.1016/S1367-5931(00)00145-9. [DOI] [PubMed] [Google Scholar]
- van Meurs J, van Lent P, Stoop R, Holthuysen A, Singer I, Bayne E, Mudgett J, Poole R, Billinghurst C, van der Kraan P, et al. Cleavage of aggrecan at the Asn341-Phe342 site coincides with the initiation of collagen damage in murine antigen-induced arthritis: a pivotal role for stromelysin 1 in matrix metalloproteinase activity. Arthritis Rheum. 1999;42:2074–2084. doi: 10.1002/1529-0131(199910)42:10<2074::AID-ANR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5) J Biol Chem. 2001;276:12501–12504. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- Hashimoto G, Aoki T, Nakamura H, Tanzawa K, Okada Y. Inhibition of ADAMTS4 (aggrecanase-1) by tissue inhibitors of metalloproteinases (TIMP-1, 2, 3 and 4) FEBS Lett. 2001;494:192–195. doi: 10.1016/S0014-5793(01)02323-7. [DOI] [PubMed] [Google Scholar]
- Yu WH, Yu S, Meng Q, Brew K, Woessner JF., Jr TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem. 2000;275:31226–31232. doi: 10.1074/jbc.M000907200. [DOI] [PubMed] [Google Scholar]
- Kramerova IA, Kawaguchi N, Fessler LI, Nelson RE, Chen Y, Kramerov AA, Kusche-Gullberg M, Kramer JM, Ackley BD, Sieron AL, et al. Papilin in development; a pericellular protein with a homology to the ADAMTS metalloproteinases. Development. 2000;127:5475–5485. doi: 10.1242/dev.127.24.5475. [DOI] [PubMed] [Google Scholar]
- Hashimoto G, Shimoda M, Okada Y. ADAMTS4 (aggrecanase-1) interaction with the C-terminal domain of fibronectin inhibits proteolysis of aggrecan. J Biol Chem. 2004;279:32483–32491. doi: 10.1074/jbc.M314216200. [DOI] [PubMed] [Google Scholar]


