Abstract
Introduction
Menopausal hormone therapy (HT) is typically withheld from breast cancer survivors because of concerns about risk for recurrence. Our objectives were to estimate the effects of HT on recurrence in breast cancer survivors and to examine the reliability of these estimates.
Methods
In a systematic review of the literature we identified all reports of HT use in breast cancer survivors that included comparison groups. Study design features that might affect selection of participants, detection of recurrence, and manuscript publication were assessed. The relative risks for breast cancer recurrence associated with HT were combined with random effects models.
Results
Two randomized and eight observational studies included 1,316 breast cancer survivors who used HT and 2,839 nonusers. In the observational studies, HT users were younger and more commonly node negative; only two reported balanced restaging for HT and control groups. Randomized trials suggest that HT increased the risk for recurrence (relative risk 3.41, 95% confidence interval 1.59–7.33), whereas observational studies suggest that HT decreased this risk (relative risk 0.64, 95% confidence interval 0.50–0.82).
Conclusion
Results from observational studies of HT conducted in breast cancer survivors are discrepant with results from randomized trials. Observational studies of HT use in breast cancer survivors have design limitations that cannot be controlled for using standard statistical methods. Therefore, the randomized clinical trial data provide the only reliable estimates of the effect of HT use on recurrence risks in breast cancer survivors.
Introduction
Most breast cancer survivors are menopausal either at diagnosis or as a result of premature therapy-induced menopause, and they frequently experience climacteric symptoms [1]. Menopausal hormone therapy (HT), either with estrogen alone or with combined estrogen and progestin, relieves estrogen deficiency symptoms [2] but it is commonly withheld from women with diagnosed breast cancer because of concerns regarding an increased risk for recurrence [3].
The available data from observational studies indicate that use of HT is associated with increased risk for breast cancer [4]. In postmenopausal women, the randomized Women's Health Initiative HT trials found an increased risk for breast cancer with estrogen plus progestin [5] but not with unopposed estrogen [6]. An apparent reduction in risk seen during the first 2 years of combination HT was attributed to a masking of breast cancer detection, with a higher risk for more advanced breast cancers subsequently [5]. In breast cancer survivors, observational studies have consistently reported similar or lower risks for recurrence among women using HT as compared with nonusers [7], albeit with methodological weaknesses [8]; this has been interpreted as evidence of the safety or perhaps benefit of HT in women with breast cancer. However, the first large randomized trial in this population reported that HT significantly increased the risk for recurrence [9].
The objectives of this meta-analysis were to estimate the impact HT has on recurrence risk among observational and randomized studies, and to examine the reliability of these estimates.
Materials and methods
A previous Medline search from 1966 to 1999 [7] was updated to February 2004 using the medical subject headings 'breast neoplasm', 'neoplasm recurrence', 'estrogens', 'estrogen replacement therapy', 'hormone replacement therapy', and 'estradiol', and reference lists of abstracted manuscript and protocols were reviewed. Only studies that included women with invasive breast cancer who received oral HT, that had an explicitly defined comparison group, and that reported breast cancer recurrences were included. Studies that reported overlapping or redundant data were excluded [10-16], as were those that did not adequately describe the selection or composition of control groups [17,18] or that included only topical hormones [19].
Two of the authors (NFC and JAK) independently abstracted data on the following variables: sample size, age at diagnosis and at trial induction, tumor stage, nodal status, estrogen and progesterone receptor status, disease-free interval (DFI) between initial breast cancer diagnosis and initiation of HT, type and duration of HT used, follow up after initiation of HT, and number and timing of breast cancer recurrences.
Each study was systematically reviewed for features that could introduce bias, including procedures for identifying participants, whether institutional review board approval and/or informed consent was obtained, whether risk factors for recurrence were similar at diagnosis, and whether restaging before entry (to exclude metastatic disease) and duration of follow up were similar for HT users and nonusers. Observational studies were classified as 'clinical experiences' if one or more study authors provided health care to the cohort with potential participation in the decision to use HT.
When not reported, the follow up after HT initiation was assumed to equal the duration of HT use. Any second breast cancer event (local, regional, or distant recurrence or invasive cancer in either breast) was treated as a recurrence because studies did not consistently make these distinctions.
Relative risk (RR) and 95% confidence interval (CI) were calculated for each study for the recurrence rate and mortality rate among HT users and nonusers. A random effects model was used to estimate the combined RR for randomized and observational studies using Meta-Analyst [13].
Results
Ten studies were identified, including a total of 1,316 breast cancer survivors who used HT and 2,839 who did not. Of these 10 studies, two were unblinded randomized controlled trials without placebo arms [9,20], one began as a randomized trial but was reported as an observational study and is considered as such here, and seven were observational studies.
Summary of randomized trials
Both randomized trials were conducted in Europe (one in England and one in Sweden). They involved a total of 445 patients with a mean age of 55.5 years, a mean DFI of 33.2 months, a duration of HT use of 19.9 months, and a mean follow-up period after HT initiation of 25.2 months (Table 1). A total of 36 recurrences and nine deaths occurred during this time in these trials; the pooled RR for the two randomized trials was 3.41 (95% CI 1.59–7.33).
Table 1.
Characteristics of 1316 users and 2839 nonusers of hormone therapy
| Study | Treatment | n | Mean age (years) | Stage | Nodal status | ER status | PgR status | Mean DFI before HT (months) | Estrogen alone (%) | Mean duration of HT (months) | Mean follow-up after HT (months) | Recurrences (n) | Deaths, all cause (n) | Deaths, primary tumor (n) |
| Randomized trials | ||||||||||||||
| Marsden et al. (2000; n = 100) [20] | HT | 51a | 58b | NR | NR | NR | NR | 40b | NR | 6 | NR | 2 | NR | NR |
| No HT | 49a | 55b | NR | NR | NR | NR | 36b | NR | 1 | NR | NR | |||
| Holmberg et al. (2004; n = 345) [9] | HT | 174 | 55.5 | NR | 25.9% (38) positive | 86 positivec | NR | 31.2b | NR | 24 | 25.2b | 26 | 5 | 3 |
| No HT | 171 | 55.0 | NR | 21.4% (31) positive | 73 positivec | NR | 32.4b | 25.2b | 7 | 4 | 4 | |||
| Observational studies | ||||||||||||||
| Ursic-Vrscaj and Bebar (1999; n = 63) [27] | HT | 21d | 47b | 1 G1 10 G2 7 G3 |
14 negative, 7 positive | 5 positive, 16 negative | 8 positive, 13 negative | 62 | 4.8 | 28 | 38g | 4 | 0g | 0 |
| No HT | 42d | 48.2 | 7 G1 17 G2 11 G3 |
28 negative 14 positive | 18 positive, 22 negative | 22 positive, 18 negative | NR | 38g | 5 | 1g | 1 | |||
| DiSaia et al. (2000; n = 487) [22] | HT | 125 | 55.7 | 17 DCIS 52 stage I 27 stage II 10 stage III 1 stage IV |
NR | NR | NR | 46b | 28 | 22b | 92.1g | NR | 4g | NR |
| No HT | 362 | 55.9 | NR | NR | NR | NR | NR | 90.6g | NR | 57g | NR | |||
| O'Meara et al. (2001; n = 869) [36] | HT | 174d | 63.6e | 91 stage I 51 stage II 20 stage I/II 10 stage III 2 stage II/III |
128 negative, 31 positive | 84 positive, 39 negative | 71 positive, 45 negative | 47.7e | 79 | 15b | 44.4b,f | 16 | 17 | 5 |
| No HT | 695d | 63.6e | 403 stage I 246 stage II 3 stage I/II 42 stage III 1 stage II/III |
470 negative, 175 positive | 409 positive, 137 negative | 311 positive, 206 negative | 47.7e | 44.4b,f | 101 | 115 | 59 | |||
| Beckmann et al. (2001; n = 185) [24] | HT | 64 | NA | 37 T1 19 T2 8 T3/4 |
44 negative, 20 positive | 31 positive, 33 negative | 34 positive, 30 negative | 0 | NA | 33b | 37b | 6 | 4 | NR |
| No HT | 121 | NA | 62 T1 42 T2 17 T3/4 |
76 negative, 45 positive | 48 positive, 73 negative | 48 positive, 73 negative | 0 | 42b | 17 | 15 | NR | |||
| Marttunnen et al. (2001; n = 131) [26] | HT | 88 | 53.4 | 3 DCIS 67 T1 17 T2 1 T3 |
72 negative, 10 positive | 57 positive, 15 negative | 54 positiveg, 13 negativeg | 50.4 | 38.6 | 30 | 30 | 7 | 2 | 2 |
| No HT | 43 | 52.8 | 1 DCIS 29 T1 11 T2 2 T3 |
30 negative, 13 positive | 29 positive, 9 negative | 30 positiveg, 7 negativeg | 50.4 | 31.2 | 5 | 3 | 3 | |||
| Durna et al. (2002; n = 1122) [23] | HT | 286 | 56.8b | 180 stage I 64 stage II 22 stage III/IV |
NA | NR | NR | 12b | 5.9 | 21b | 69.6b | 44 | 16 | 13 |
| No HT | 836 | 64.7b | 470 stage I 191 stage II 120 stage III/IV |
NA | NR | NR | NR | 61.2b | 247 | 199 | 122 | |||
| Vassilopoulou-Sellin et al. (2002; n = 299) [21] | HT | 56h | 56b | 9 <1 cm 30 1–2.5 cm 15 >2.5 cm |
35 negative, 13 1–3, 6 >3 | 37 negative | NR | 105.6 | 100 | 30 >5 years, 20 2–5 years, 6 2 years | 71 | 2 | 1 | 0 |
| No HT | 243h | 53b | 38 <1 cm 134 1–2.5 cm 67 >2.5 cm |
133 negative, 70 1–3, 33 >3 | 164 negative | NR | 99.6 | NR | 33 | 2 | 1 | |||
| Decker et al. (2003; n = 554) [25] | HT | 277 | 57.4b | 84 DCIS 124 stage I 47 stage IIA 19 stage IIB 3 stage IIIA |
NR | 100 positive, 54 negative | 63 positive, 46 negative | 43.3 | 48.7 | 44.4 | 49.7 | 30 | 7 | 5 |
| No HT | 277 | 59.0b | 84 DCIS 124 stage I 47 stage IIA 19 stage IIB 3 stage IIIA |
NR | 121 positive, 35 negative | 73 positive, 42 negative | NR | 45.6 | 35 | 17 | 9 | |||
| Summary | ||||||||||||||
| Randomized trials | HT | 225 | 56.07 | 38 positive | 86 positive | 33.19 | 19.92 | 25.20 | 28 | 5 | 3 | |||
| No HT | 220 | 55.00 | 31 positive | 73 positive | 33.20 | 25.20 | 8 | 4 | 4 | |||||
| Observational studies | HT | 1091 | 56.98 | 293 negative, 87 positive | 277 positive, 194 negative | 230 positive, 147 negative | 37.70 | 40.4 | 28.02 | 57.46 | 109 | 51 | 25 | |
| No HT | 2619 | 60.87 | 737 negative, 350 positive | 625 positive, 440 negative | 484 positive, 346 negative | 54.01 | 57.02 | 443 | 409 | 195 | ||||
| All combined | HT | 1316 | 56.82 | 293 negative, 125 positive | 363 positive, 194 negative | 230 positive, 147 negative | 36.93 | 40.4 | 26.58 | 53.03 | 137 | 56 | 28 | |
| No HT | 2839 | 60.39 | 737 negative, 381 positive | 698 positive, 440 negative | 484 positive, 346 negative | 50.55 | 54.88 | 451 | 413 | 199 | ||||
aExcluding stage III/IV patients. bMedian value. cRefers to hormone receptor status; specific data concerning estrogen receptor (ER) and progesterone receptor (PgR) status were not reported. dExcluding patients with ductal carcinoma in situ (DCIS). eWeighted mean. fFor recurrence only; follow-up for mortality was 55.2 months. gPersonal communication. hExcluding DCIS, stages III and IV, and ER-positive patients. DFI, disease-free interval; HT, hormone therapy; NA, not able to calculate; NR, not reported.
Summary of observational studies
Of the eight observational studies, six were clinical experiences [22-27]. The eight studies involved a total of 3710 patients with a mean age of 59.7 years, a mean DFI of 49.2 months, a duration of HT use of 28 months, and a mean follow-up period after HT initiation of 57.1 months (Table 1). A combined total of 552 recurrences (109 among HT users) and 460 deaths (51 among HT users) occurred in these trials. The pooled RR for the observational studies was 0.64 (95% CI 0.50–0.82).
All studies
Most studies included both combination HT and unopposed estrogens without stratifying risk estimates according to preparation. Three of the observational studies [22,24,25] reported obtaining informed consent but only from women who used HT. Three studies [20,24,26] reported similar restaging for treatment and control groups at the beginning of the observation period, although one of these [26] did not report whether those found to have occult metastasis were excluded. Not all studies reported the DFI for the control groups, but several reported matching control individuals according to DFI [22,27]. Prognostic factors for HT users and nonusers differed in most studies (Table 1). On average, HT users were more than 3 years younger than nonusers and were more likely to be node negative. The average duration of HT use was 26.6 months, with an average duration of follow up after initiation of HT of 53 months. The mean DFI was 36.9 months for HT users and 55.6 for nonusers.
Among the 1,191 HT users in nine studies reporting recurrences, 137 (11.7%) experienced a recurrence of their breast cancer during follow up. Among the 2,477 nonusers in these studies, 451 (18.2%) had a recurrence. The average annual recurrence rate was 3.3% (range 0.6–7.1%), with substantially higher rates in the randomized trials. Combining all studies yielded a RR for recurrence of 0.84 (95% CI 0.54–1.3; Fig. 1), with statistically significant heterogeneity (Q = 25.3).
Figure 1.
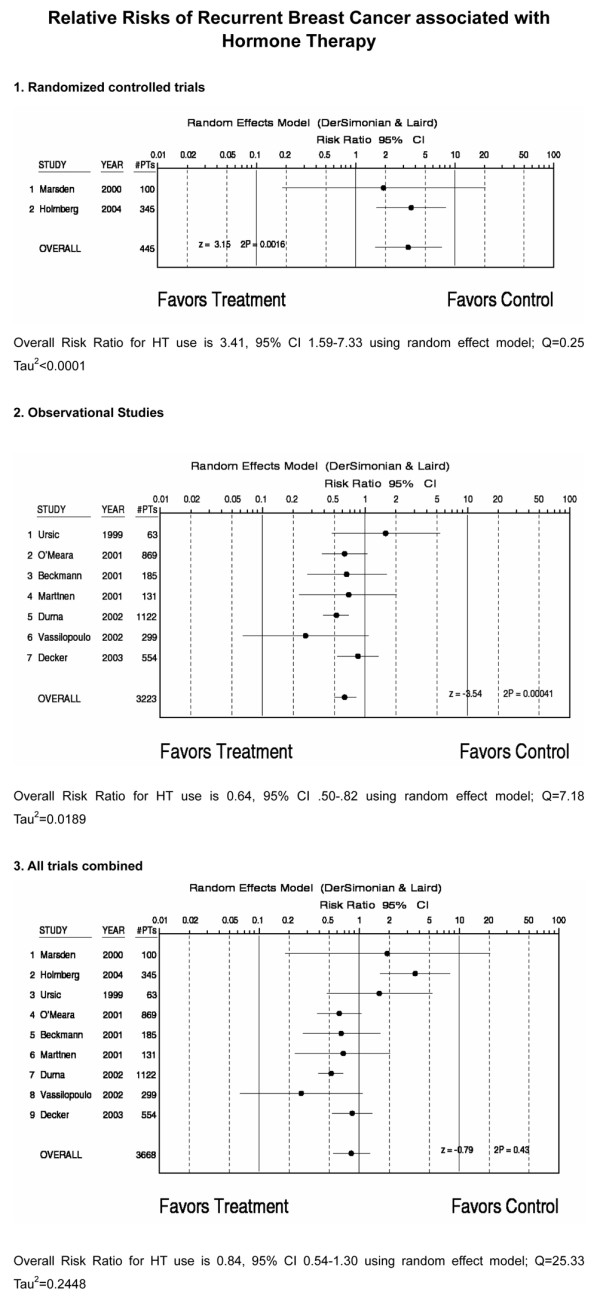
Relative risks for recurrent breast cancer associated with hormone therapy (HT). Each black circle indicates the relative risk for recurrent breast cancer; the horizontal lines indicate the 95% confidence interval (CI). The top portion of the figure describes randomized controlled trials, the middle portion describes observational studies, and the bottom portion describes all trials combined.
Discussion
Estimates from observational studies of HT among breast cancer survivors suggest that HT prevents breast cancer recurrence, whereas estimates from randomized trials suggest the opposite. Because of statistically significant heterogeneity, these estimates should not be combined. Although all of the trials included in our analyses contained methodological weaknesses, the nonrandomized studies had design features that could introduce selection, reporting, and/or publication biases. The selection of healthier women to begin HT, the benefit of restaging before initiation of HT, the short duration of HT exposure and follow up, the potential effects of HT on mammograms that could obscure the diagnosis of recurrent or new breast cancers, and publication bias favoring publication and/or completion of studies reporting a protective effect of HT could explain the apparent protective effect of short-term HT on recurrence among breast cancer survivors in these studies.
Systematic serial restaging with blood tests and imaging during follow up is no longer generally recommended. However, their use detects breast cancer recurrence earlier. Balanced restaging was defined in only two out of seven observational studies. If breast cancer survivors contemplating HT use were more likely to have restaging, then the imbalance could account for the apparent protective effect of HT in observational studies. Although the description of prognostic factors was rarely complete, HT users in observational studies were younger and had more favorable prognostic profiles than did control individuals. This process also selected women with severe vasomotor symptoms, who have lower estradiol and testosterone levels; higher levels of these hormones have been associated with increased breast cancer risk. As a result, it is possible that women who were more likely to be offered HT [20] had lower recurrence risks. It is important to note that the majority of observational studies included in these analyses were not designed as observational studies from the start but rather as clinical experiences. Had these observational studies been more rigorously designed, using modern epidemiological techniques, many of these biases could have been minimized.
The adverse effect of combined HT on mammographic breast cancer detection [5] might have affected recurrence detection. Both recurrent and new breast cancers, which account for 10–20% of cancer events in women with prior lumpectomy, could have falsely appeared lower in HT users because of HT-related interference with mammographic diagnosis. However, this factor is probably not large, given the sharp increase in risk observed even after short-term HT use in randomized trials [36] and that the increase in risk pertained to distant as well as local recurrences.
The randomized trial reported by Holmberg and colleagues [9] overcomes many of the shortcomings of observational studies and provides the best available data on the impact of HT in breast cancer survivors. Although their unblinded design and lack of a placebo group could result in selective attrition, follow-up rates were comparable among HT users and nonusers. These investigators also reported summary interim analyses of a similar randomized trial, the Stockholm trial, with a relative hazard ratio of 0.82 (95% CI 0.35–1.9). This trial was not included in this analysis because its findings have not yet been reported in full; the reasons for its discrepant findings are unclear at this time.
Conclusion
Observational studies of HT use in breast cancer survivors have design limitations that cannot be controlled for using standard statistical methods and hence should be considered essentially uninformative with respect to the safety of HT use in breast cancer survivors. Only randomized clinical trials are likely to provide reliable estimates of the effect of HT use in this setting.
Abbreviations
CI = confidence interval; DFI = disease-free interval; HT = hormone therapy; RR = relative risk.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
NC conceived the study (with RC), designed the study, reviewed the source studies, abstracted data, drafted the paper, and supervised the statistical analyses. JK participated in the design of the study and reviewed the source studies, abstracted data, carried out the meta-analysis, and helped to draft the manuscript. RC conceived of the study (with NC), designed the analysis, participated in its coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was supported, in part, by the Agency for Healthcare Quality (RO1 HS01332901), American Cancer Society Breast Cancer Prevention Forum, and the Robert Wood Johnson Foundation Generalist Physician Faculty Scholars Award (#033958).
This work was presented at the 24th Annual Meeting of the Society for Medical Decision Making in Baltimore (MD, USA) in October 2002.
See related commentary by Colditz: http://breast-cancer-research.com/content/7/4/168
Contributor Information
Nananda F Col, Email: ncol@lifespan.org.
Jung A Kim, Email: joyhippo@hanyang.ac.kr.
Rowan T Chlebowski, Email: rchlebow@whi.org.
References
- Carpenter J, Johnson D, Wagner L, Andrykowski M. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Nurs Forum. 2002;29:E16–E25. doi: 10.1188/02.ONF.E16-E25. [DOI] [PubMed] [Google Scholar]
- MacLennan A, Lester S, Moore V. Oral estrogen replacement therapy versus placebo for hot flushes: a systematic review. Climacteric. 2001;4:58–74. [PubMed] [Google Scholar]
- Pritchard K, Khan H, Levine M, Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer Clinical practice guidelines for the care and treatment of breast cancer: The role of hormone replacement therapy in women with a previous diagnosis of breast cancer. CMAJ. 2002;166:1017–1022. [PMC free article] [PubMed] [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 without breast cancer. Lancet. 1997;350:1047–1059. doi: 10.1016/S0140-6736(97)08233-0. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- Alving B. NIH asks participants in Women's Health Initiative estrogen-alone study to stop study pills, begin follow-up phase. NIH News. 2004. http://www.nhlbi.nih.gov/new/press/04-03-02.htm (last accessed 19 April 2005). [DOI] [PubMed]
- Col NF, Hirota LK, Orr RK, Erban JK, Wong JB, Lau J. Hormone replacement therapy after breast cancer: a systematic review and quantitative assessment of risk. J Clin Oncol. 2001;19:2357–2363. doi: 10.1200/JCO.2001.19.8.2357. [DOI] [PubMed] [Google Scholar]
- Chlebowski R, Col N. Menopausal hormone therapy after breast cancer. Lancet. 2004;363:410–411. doi: 10.1016/S0140-6736(04)15519-0. [DOI] [PubMed] [Google Scholar]
- Holmberg L, Anderson H, for the HABITS steering and data monitoring committees HABITS (hormonal replacement therapy after breast cancer – is it safe?), a randomised comparison: trial stopped. Lancet. 2004;363:453–455. doi: 10.1016/S0140-6736(04)15493-7. [DOI] [PubMed] [Google Scholar]
- Eden JA, Wren BG. Hormone replacement therapy after breast cancer: a review. Cancer Treat Rev. 1996;22:335–343. doi: 10.1016/S0305-7372(96)90006-7. [DOI] [PubMed] [Google Scholar]
- Eden JA, Bush T, Nand S, Wren BG. A case–control study of combined continuous estrogen-progestin replacement therapy among women with a personal history of breast cancer. Menopause. 1995;2:67–72. [Google Scholar]
- Dew J, Eden J, Beller E, Magarey C, Schwartz P, Crea P, Wren B. A cohort study of hormone replacement therapy given to women previously treated for breast cancer. Climacteric. 1998;1:137–142. doi: 10.3109/13697139809085529. [DOI] [PubMed] [Google Scholar]
- Lau J. Metaanalyst. Boston, MA, USA; [Google Scholar]
- Vassilopoulou-Sellin R, Asmar L, Hortobagyi GN, Klein MJ, McNeese M, Singletary SE, Theriault RL. Estrogen replacement therapy after localized breast cancer: clinical outcome of 319 women followed prospectively. J Clin Oncol. 1999;17:1482–1487. doi: 10.1200/JCO.1999.17.5.1482. [DOI] [PubMed] [Google Scholar]
- Beckmann MW, Jap D, Djahansouzi S, Nestle-Kramling C, Kuschel B, Dall P, Brumm C, Bender HG. Hormone replacement therapy after treatment of breast cancer: effects on postmenopausal symptoms, bone mineral density and recurrence rates. Oncology. 2001;60:199–206. doi: 10.1159/000055319. [DOI] [PubMed] [Google Scholar]
- Durna EM, Crowe SM, Leader LR, Eden JA. Quality of life of breast cancer survivors: the impact of hormonal replacement therapy. Climacteric. 2002;5:266–276. [PubMed] [Google Scholar]
- Natrajan P, Soumakis K, Gambrell R., Jr Estrogen replacement therapy in women with previous breast cancer. Am J Obstet Gynecol. 1999;181:288–295. doi: 10.1016/s0002-9378(99)70550-8. [DOI] [PubMed] [Google Scholar]
- Natrajan P, Gambrell R. Estrogen replacement therapy in patients with early breast cancer. Am J Obstet Gynecol. 2002;187:289–294. doi: 10.1067/mob.2002.125999. [DOI] [PubMed] [Google Scholar]
- Dew J, Wren B, Eden J. A cohort of topical vaginal estrogen therapy in women previously treated for breast cancer. Climacteric. 2003;6:45–52. [PubMed] [Google Scholar]
- Marsden J, Whitehead M, A'Hern R, Baum M, Sacks N. Are randomized trials of hormone replacement therapy in symptomatic women with breast cancer feasible? Fertil Steril. 2000;73:292–299. doi: 10.1016/S0015-0282(99)00510-5. [DOI] [PubMed] [Google Scholar]
- Vassilopoulou-Sellin R, Cohen DS, Hortobagyi GN, Klein MJ, McNeese M, Singletary SE, Smith TL, Theriault RL. Estrogen replacement therapy for menopausal women with a history of breast carcinoma: results of a 5-year, prospective study. Cancer. 2002;95:1817–1826. doi: 10.1002/cncr.10913. [DOI] [PubMed] [Google Scholar]
- DiSaia P, Brewster W, Ziogas A, Anton-Culver H. Breast cancer survival and hormone replacement therapy: a cohort analysis. Am J Clin Oncol. 2000;23:541–545. doi: 10.1097/00000421-200012000-00001. [DOI] [PubMed] [Google Scholar]
- Durna E, Wren B, Heller G, Leader L, Sjoblom P, Eden J. Hormone replacement therapy after a diagnosis of breast cancer: cancer recurrence and mortality. Med J Aust. 2002;177:347–351. doi: 10.5694/j.1326-5377.2002.tb04835.x. [DOI] [PubMed] [Google Scholar]
- Beckmann MW, Jap D, Djahansouzi S, Nestle-Kramling C, Kuschel B, Dall P, Brumm C, Bender HG. Hormone replacement therapy after treatment of breast cancer: effects on postmenopausal symptoms, bone mineral density and recurrence rates. Oncology. 2001;60:199–206. doi: 10.1159/000055319. [DOI] [PubMed] [Google Scholar]
- Decker DA, Pettinga JE, VanderVelde N, Huang RR, Kestin L, Burdakin JH. Estrogen replacement therapy in breast cancer survivors: a matched-controlled series. Menopause. 2003;10:277–285. doi: 10.1097/01.GME.0000061806.76067.E9. [DOI] [PubMed] [Google Scholar]
- Marttunnen M, Hietanen P, Pyrhonen S, Tiitinen A, Ylikorkala O. A prospective study on women with a history of breast cancer and with or without estrogen replacement therapy. Maturitas. 2001;39:217–225. doi: 10.1016/S0378-5122(01)00211-0. [DOI] [PubMed] [Google Scholar]
- Ursic-Vrscaj M, Bebar S. A case-control study of hormone replacement therapy after primary surgical breast cancer treatment. Eur J Surg Oncol. 1999;25:146–151. doi: 10.1053/ejso.1998.0617. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Davidson NE, Schapira DV, Grunfeld E, Muss HB, Vogel VG, III, Somerfield MR. American Society of Clinical Oncology 1998 update of recommended breast cancer surveillance guidelines. J Clin Oncol. 1999;17:1080–1082. doi: 10.1200/JCO.1999.17.3.1080. [DOI] [PubMed] [Google Scholar]
- Temple LK, Wang EE, McLeod RS. Preventive health care, 1999 update: 3. Follow-up after breast cancer. Canadian Task Force on Preventive Health Care. CMAJ. 1999;161:1001–1008. [PMC free article] [PubMed] [Google Scholar]
- American Society of Clinical Oncology Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. J Clin Oncol. 1996;14:2843 –2877. doi: 10.1200/JCO.1996.14.10.2843. [DOI] [PubMed] [Google Scholar]
- Guthrie J, Dennerstein L, Hopper J, Burger H. Hot flushes, menstrual status, and hormone levels in a population-based sample of midlife women. Obstet Gynecol. 1996;88:437–442. doi: 10.1016/0029-7844(96)00196-2. [DOI] [PubMed] [Google Scholar]
- Overlie I, Moen M, Holte A, Finset A. Androgens and estrogens in relation to hot flashes during the menopausal transition. Maturitas. 2002;41:69–77. doi: 10.1016/S0378-5122(01)00256-0. [DOI] [PubMed] [Google Scholar]
- Cauley JA, Lucas FL, Kuller LH, Stone K, Browner W, Cummings SR. Elevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancer. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1999;130:270–277. doi: 10.7326/0003-4819-130-4_part_1-199902160-00004. [DOI] [PubMed] [Google Scholar]
- Endogenous Hormones and Breast Cancer Collaborative Group Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- O'Meara ES, Rossing MA, Daling JR, Elmore JG, Barlow WE, Weiss NS. Hormone replacement therapy after a diagnosis of breast cancer in relation to recurrence and mortality. J Natl Cancer Inst. 2001;93:754–762. doi: 10.1093/jnci/93.10.754. [DOI] [PubMed] [Google Scholar]


