Abstract
The INCENP (inner centromere protein) is a chromosomal passenger protein that plays multiple roles in regulating mitosis and cytokinesis. The MKLP1 (mitotic kinesin-like protein) is a component of centralspindlin complex that has been implicated in assembly of midzone/midbody during mitosis and is essential for cytokinesis. In the present study, we investigated functions of INCNEP and MKLP1 and their interplay in regulating spindle midzone/midbody formation and cytokinesis in human cells. Immunofluorescence and live-cell imaging analyses have shown that, in addition to multiple chromosome segregation defects, cells that lacked INCENP by RNAi (RNA interference) exhibit abnormal spindle midzone/midbody formation, resulting in formation of binucleated/multinucleated cells. Suppression of MKLP1 expression by siRNA (small interfering RNA) did not cause any abnormality of chromosome segregation and midzone formation, but abrogated midbody formation and completion of cytokinesis. Furthermore, we show that INCENP is required for recruiting MKLP1 to the spindle midzone/midbody. Three-dimensional reconstruction imaging analysis suggests that recruitment of MKLP1 to the midzone/midbody by INCENP is a crucial step for the midbody formation and completion of cytokinesis in mammalian cells.
Keywords: centralspindlin, chromosomal passenger, cytokinesis, inner centromere protein (INCENP), midzone/midbody, mitotic kinesin-like protein (MKLP1)
Abbreviations: Cdk, cyclin-dependent kinase; DAPI, 4,6-diamidino-2-phenylindole; DMEM, Dulbecco's modified Eagle's medium; dsRNA, double-stranded RNA; ECFP, enhanced cyan fluorescent protein; EYFP, enhanced yellow fluorescent protein; FBS, foetal bovine serum; INCENP, inner centromere protein; MgcRacGAP, male-germ-cell Rac GTPase-activating protein; MKLP1, mitotic kinesin-like protein; CeMKLP1, C. elegans homologue of MKLP1; MT, microtubule; Ni-NTA, Ni2+-nitrilotriacetate; RNAi, RNA interference; siRNA, small interfering RNA; esiRNA, endoribonuclease RNase III-prepared siRNA
INTRODUCTION
Successful cell division requires the temporal–spatial co-ordination of nuclear division (mitosis) and cytoplasmic division (cytokinesis) to ensure that each daughter cell receives a full set of chromosomes together with a proper complement of cytoplasm and organelles. Errors during mitosis and cytokinesis can lead to numerous deleterious events, including chromosome instability, which can have severe consequences for an organism such as cell death, birth and developmental defects, and cancer. In the metaphase to anaphase transition, antiparallel non-kinetochore interdigitating MTs (microtubules) between separating chromosomes bundle together to form a unique spindle structure, the spindle midzone. The spindle midzone plays an important role in determining the position of the cleavage furrow in animal cells [1]. As the cleavage furrow ingresses, it constricts components of the midzone into a focused structure called the midbody. The mechanism by which the midzone/midbody is assembled remains unclear. However, recent genetic and biochemical studies from cultured mammalian cells, Caenorhabditis elegans and Drosophila have begun to reveal factors that are involved in the process. These factors include chromosomal passenger proteins, the kinesin-like motors and the associated proteins, kinases, phosphatase and the spindle midzone bundling protein PRC1 [2–10].
Chromosomal passenger proteins, which include INCENP (inner centromere protein), Aurora-B kinase, Survivin, Borealin and TD60, are a group of proteins that localize initially to chromosomes and centromeres, transfer to the spindle midzone in early anaphase and then concentrate at the midbody during cytokinesis [3,11–18]. INCENP, Aurora-B kinase, Survivin and Borealin interact with each other to form complex(es) in vivo [17–20]. As their subcellular localization implicated, chromosomal passenger proteins have been shown to be involved in chromosome condensation, congression, segregation, the spindle dynamics and cytokinesis in various eukaryotic organisms [3,13,18,21]. For instance, abrogation of function of Aurora-B, INCENP or Survivin by RNAi (RNA interference), by dominant-negative and deactivating temperature-sensitive mutants or by specific inhibitors leads to a wide range of defects in mitosis and cytokinesis in animal cells [3,22–28]. Consistent with the findings, knockout experiments with mice revealed that INCENP and Survivin are essential for cytokinesis [29,30].
The exact functional role of chromosome passenger proteins in cytokinesis remains elusive, although they might recruit or target their downstream targets to regulate midzone/midbody formation and/or cleavage furrowing during cytokinesis [31,32]. In C. elegans, Aurora-B has been implicated in anaphase spindle midzone/midbody organization by recruiting MKLP1 (mitotic kinesin-like protein) which bundles and stabilizes the spindle midzone/midbody interdigitating MTs. Aurora-B binds to ZEN-4/CeMKLP1 (a C. elegans homologue of MKLP1), and both Aurora-B and INCENP are required to recruit ZEN-4/CeMKLP1 to the spindle midzone [24]. However, results in Drosophila are controversial. Giet and Glover [25] showed that Aurora-B and INCENP were required for recruiting the Drosophila MKLP1 homologue, Pavarotti, to the midzone, whereas Adams et al. [33] indicated that the midzone association of Pavarotti was not dependent on Aurora-B or INCENP.
The midzone-associated kinesin motors and their binding proteins, such as centralspindlin MKLP1 and MgcRacGAP (male-germ-cell Rac GTPase-activating protein), have been shown to play important roles in cytokinesis in animal cells [4,32,34–36]. Although initial immunodepletion experiments implicated MKLP1 in mitotic progression [37], recent studies indicated that MKLP1 family members function specifically in cytokinesis. Mishima et al. [4] show that MKLP1 interacts specifically with MgcRacGAP in vivo to form a heterotetrameric complex. This complex, but not the individual component, promotes antiparallel MT bundling in vitro. MKLP1 is a downstream target of Cdc2/cyclin B1 and Cdk (cyclin-dependent kinase) phosphorylation of MKLP1 has been implicated to control the timing of midzone formation [8]. These results suggest that MKLP1 is an essential factor for midzone formation. However, other studies reveal different views [38,39]. Immunofluorescence analysis and time-lapse imaging reveal that depletion of MKLP1 by microinjection of anti-MKLP1 antibodies or MKLP1 siRNA (small interfering RNA) in mammalian cells does not perturb midzone formation. Instead, it inhibits midbody formation and completion of cytokinesis. These results indicate that MKLP1 functions mainly in the late stage of cytokinesis.
To elucidate further the functions of chromosomal passenger and centralspindlin proteins, and their interplay in spindle midzone/midbody formation and cytokinesis, we examined the effects of inhibition of INCENP or MKLP1 expression by siRNA in HeLa cells using immunofluorescence analysis, three-dimensional reconstruction imaging and time-lapse microscopy. Our results indicate that, although both INCENP and MKLP1 are midzone/midbody-associated proteins and are essential for cytokinesis, they regulate different stages of the processes. INCENP is crucial for spindle midzone/midbody assembly, whereas MKLP1 mainly plays an essential role for midbody formation. INCENP is required for recruiting MKLP1 to the spindle midzone/midbody, a crucial step for midbody formation and completion of cytokinesis.
MATERIALS AND METHODS
Plasmids and antibodies
The full-length human INCENP cDNA was assembled from two human EST (expressed sequence tag) clones (IMAGE 5563252 and 1624715). The cDNA was fully sequenced and was then subcloned into mammalian expression vector pEGFP-C1. To produce His-tagged INCENP-C-terminus fusion protein, an INCENP cDNA fragment corresponding to nucleotides 2385–2736 was amplified by PCR and subcloned into the pHis8 vector [40]. After expression in Escherichia coli BL21 (DE) pLysS strain, His–INCENP-C-terminus was purified by Ni-NTA (Ni2+-nitrilotriacetate)–agarose chromatography. pEYFP-tubulin and pECFP-H2B plasmids were generated as described previously [41,42].
To generate anti-INCENP antibodies, rabbits (471/472) were immunized with purified His-tagged INCENP-C-terminus fusion protein. Anti-INCENP antibodies were affinity-purified by incubating serum with His-tagged INCENP-C-terminus–Ni-NTA–agarose beads. Anti-Aurora-B (AIM1) monoclonal and anti-MKLP1 rabbit polyclonal antibodies were purchased from Transduction Laboratories and Santa Cruz Biotechnology respectively. Alexa Fluor® 488-conjugated anti-INCENP antibodies were generated following the instructions for amine-reactive probes (Molecular Probes) and purified by Bio-Spin 30 columns (Bio-Rad). All secondary antibodies used were purchased from SouthernBiotech.
siRNAs
siRNAs were synthesized by Dharmacon Research. RNA oligonucleotide sequences used for targeting INCENP and MKLP1 were (AA)GGACUUGGUGUGGCUUGAG (INCENP) and (AA)GAGUGUUGCAUAGAAGUGA (MKLP1) respectively. Scrambled siRNA, used as control siRNA, was purchased from Dharmacon Research. INCENP esiRNA (endoribonuclease RNase III-prepared siRNA) or MKLP1 esiRNA was generated using the E. coli RNase III method [43], with some modifications. In brief, ∼400 bp 3′ untranslated region DNA fragments of human INCENP and MKLP1 were amplified by PCR from sheared genomic DNA of normal human foreskin fibroblasts using sequence-specific 5′ and 3′ primers containing a T7 promoter sequence at the 5′ end. The INCENP primers used for PCR were 5′-GCGTAATACGACTCACTATAGGCTTCTTGGCATGCCATTGTGG-3′ and 5′-GCGTAATACGACTCACTATAGGCTGCACCTGGTGATCCTGAGG-3′. The MKLP1 primers used for PCR were 5′-GCGTAATACGACTCACTATAGGCTCGAAAGCCATGCCAGAAGC-3′ and 5′-GCGTAATACGACTCACTATAGGAGACCAGGGCTGGAGAAGTCA-3′. As a control, a fragment of the luciferase coding region was amplified from a luciferase cDNA sequence-specific 5′ and 3′ primers (5′-GCGTAATACGACTCACTATAGGACATCTCATCTACCTCCCGGT-3′ and 5′-GCGTAATACGACTCACTATAGGTGCGCCCCCAGAAGCAATTTC-3′). The PCR products were subjected to in vitro transcription to produce dsRNAs (double-stranded RNAs) using an in vitro T7 transcription kit (Ambion). dsRNAs were then treated with DNase I, precipitated with 7.5 M LiCl, washed with 70% (v/v) ethanol, dried and resuspended in water at 1 μg/μl. dsRNAs (50 μg) were digested with 2.5 μg of GST (glutathione S-transferase)–RNase III at 37 °C for 3 h. The reactions were stopped by the addition of 20 mM EDTA, and products were purified through QIAquick spin columns (Qiagen). esiRNAs were precipitated by ethanol and dissolved in water. The concentrations of esiRNAs were determined by measuring absorbance at 260 nm. The quality of esiRNAs was also evaluated by electrophoresis on 4% low-melting agarose gels.
Cell culture and transfection
HeLa cells were cultured in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% FBS (foetal bovine serum). Transfection of HeLa cells with siRNA duplex oligonucleotides was performed using Oligofectamine (Invitrogen) as described previously [44]. In brief, 1×105 cells were grown on 35-mm-diameter dishes overnight and were then transfected with 120 nM siRNA using Oligofectamine in serum-free DMEM. At 4 h after transfection, equal volumes of DMEM containing 20% FBS were added into the dishes. At 24–72 h after transfection, cells were harvested or fixed for immunoblotting or immunostaining analysis.
Immunoprecipitation, immunoblotting and immunofluorescence analysis
For immunoprecipitation and immunoblotting, HeLa cells were cultured with 100 ng/ml nocodazole overnight. Cells were then collected and lysed in lysis buffer (50 mM Tris/HCl, pH 8.0, 0.4 M NaCl, 1% Nonidet P40, 0.5% deoxycholate, 1 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml pepstatin and 1 μg/ml aprotinin) on ice for 30 min. Cell lysates were incubated at 4 °C with affinity-purified anti-INCENP antibodies or pre-immune antisera for 2 h and then with Protein A–Sepharose beads for 2 h. Samples were spun down at 10000 g for 2 min at 4 °C and washed twice with lysis buffer. Bead-bound proteins were subjected to SDS/PAGE (7.5% polyacrylamide), transferred on to PVDF membranes and then immunoblotted with anti-INCENP antibodies. For immunofluorescence analysis, cells grown on glass coverslips were fixed in PBS containing 3% (w/v) formaldehyde and 2% (w/v) sucrose at room temperature (25 °C) for 5 min. After permeabilization and block in PBS containing 0.4% (v/v) Triton X-100 and 10% (v/v) goat serum, cells were incubated with primary antibodies (anti-INCENP, 1:500 dilution; anti-MKLP1, 1:100 dilution) for 2 h at room temperature. After washing, cells were co-stained with secondary antibodies (1:200 dilution) and DAPI (4,6-diamidino-2-phenylindole) (0.5 μg/ml) for 1 h at room temperature. After five washes, cells were mounted with FluoroGuard (Bio-Rad) and photographed using a Leica DM IRE2 fluorescence microscope.
Time-lapse microscopy
HeLa cells were grown on 35-mm-diameter glass-bottom microwell dishes (MatTek) overnight and transfected with 0.5 μg of pEYFP-tubulin and 0.5 μg of pECFP-H2B plasmids, together with 100 nM siRNA using Oligofectamine. At 24 h after transfection, cells were cultured overnight in CO2-independent medium (Gibco) containing 10% (v/v) FBS. Then, dishes were covered with mineral oil (Sigma) and transferred to a heated stage (37 °C) on a Zeiss Axiovert 100M microscope. Phase-contrast and fluorescence images of live cells were collected at 2 min intervals, for 9–10 h and processed using the Slidebook 3.0 software (Intelligent Imaging Innovations).
Three-dimensional imaging reconstitution
Serial thin sections (0.3 μm) of immunofluorescent stained cells were scanned under a 63× oil-immersion objective using an inverted Leica DMIRE2 microscope. Images were imported into the library of Volocity (Improvision) and rendered to three-dimensional volume. The three-dimensional images were generated using Volocity software and exported as QuickTime movies.
RESULTS
Successful repression of the midzone-associated proteins, INCENP and MKLP1, in HeLa cells using siRNA
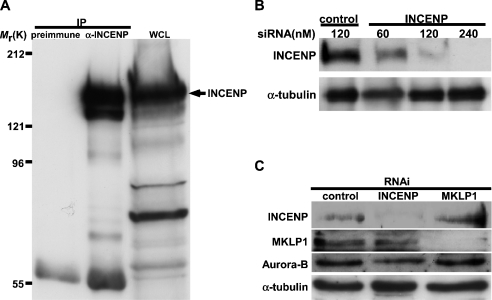
To study human INCENP function, we first generated rabbit polyclonal anti-INCENP antibodies against the bacterially expressed His-tagged C-terminus of human INCENP (see the Materials and methods section). To test anti-INCENP antibody specificity, HeLa cell lysates were immunoprecipitated with affinity-purified anti-INCENP antibodies or pre-immune antisera. The immunoprecipitates were subjected to SDS/PAGE (7.5% polyacrylamide) and then blotted with anti-INCENP antibodies. Multiple bands around 125–135 kDa in the immunoblots were detected from the immunoprecipitates with anti-INCENP antibodies, but not from the immunoprecipitates with control pre-immune antisera (Figure 1A). Similar results were obtained from other human cell lines, U2OS, HEK-293T (human embryonic kidney) and HCT116 (results not shown). Since the predicted molecular mass of human INCENP is ∼135 kDa [45], the results indicated that anti-INCENP antibodies specifically recognized the endogenous INCENP. Multiple bands of INCENP detected in the immunoblots might represent the post-translational modification isoforms (e.g. phosphorylation) of INCENP as reported previously [20].
Figure 1. Specificity of anti-(human INCENP) antibodies (α-INCENP) and depletion of INCENP or MKLP1 expression by corresponding siRNA in HeLa cells.
(A) Nocodazole-treated HeLa cells were lysed in lysis buffer, and cell lysates were immunoprecipitated with pre-immune antisera or anti-INCENP antibodies. Immunoprecipitates and one-tenth of whole-cell lysates (WCL) used in immunoprecipitations were subjected to SDS/PAGE (7.5% polyacrylamide), transferred on to a PVDF membrane and immunoblotted with anti-INCENP antibodies. Mr sizes are shown (×1000). (B) HeLa cells grown on six-well plates were transfected with control siRNA (120 nM) or INCENP siRNA (60–240 nM). At 3 days after transfection, cells were lysed in lysis buffer. Cell lysates were subjected to SDS/PAGE (7.5% polyacrylamide), transferred on to a PVDF membrane and then immunoblotted with anti-INCENP or anti-α-tubulin antibodies. (C) HeLa cells were transfected with 120 nM control siRNA, INCENP siRNA or MKLP1 siRNA. At 3 days after transfection, cells were lysed in lysis buffer. Cell lysates were subjected to SDS/PAGE (7.5% polyacrylamide), transferred on to a PVDF membrane and then immunoblotted with antibodies as indicated.
To investigate the roles of INCENP or MKLP1 in spindle midzone/midbody formation and cytokinesis, we ablated INCENP or MKLP1 expression in HeLa cells using siRNA produced by chemical synthesis or esiRNA generated by the E. coli RNase III method (see the Materials and methods section). Immunoblotting analyses indicated that transfection of INCENP- or MKLP1-specific siRNA or esiRNA, but not control siRNA or esiRNA, effectively ablated expression levels of INCENP or MKLP1 in HeLa cells (Figures 1B and 1C, and Supplementary Figure S1 at http://www.BiochemJ.org/bj/389/bj3890373add.htm). Inhibition of INCENP expression did not cause a substantial reduction of MKLP1 expression and vice versa (Figure 1C). There was no reduction of Aurora-B or α-tubulin expression in INCENP, MKLP1 and control siRNA-transfected cells. Thus the results indicated that the expression of INCENP or MKLP1 could be specifically ablated by INCENP or MKLP1 siRNA or esiRNA in HeLa cells.
Ablation of INCENP expression by siRNA causes poorly organized spindle midzone and abortive midbody in cytokinesis
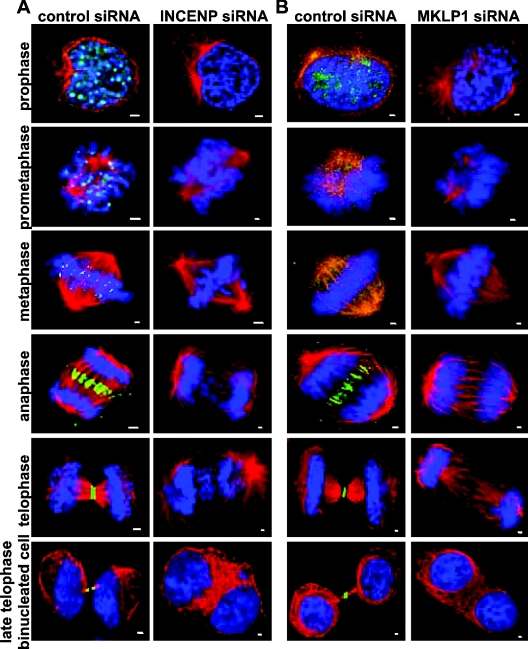
To examine morphological changes in INCENP siRNA-treated cells during mitosis/cytokinesis, we stained INCENP siRNA- or control siRNA-treated cells with anti-INCENP to monitor the expression and subcellular localization of INCENP. In parallel, cells were also co-stained with DAPI and anti-α-tubulin antibodies to verify the position of chromosomes and the mitotic spindle. Consistent with previous reports [22,46], INCENP displayed highly dynamic subcellular localization during the cell cycle in control cells. INCENP was initially detected on chromatin in interphase (late G2 [22]), and translocated to the centromeres in early mitosis (prophase, prometaphase and metaphase), localized to the spindle midzone and cleavage furrow in anaphase, and then concentrated at the border of the spindle midbody during cytokinesis (Figure 2A).
Figure 2. Immunofluorescence analyses of HeLa cells treated with INCENP or MKLP1 siRNA during mitosis and cytokinesis.
HeLa cells grown on glass coverslips were transfected with control, INCENP or MKLP1 siRNA as described in Figure 1(C). At 2 days after transfection, cells were fixed and stained with anti-INCENP antibodies (green, A), anti-MKLP1 antibodies (green, B), anti-α-tubulin antibodies (red) and DAPI (DNA, blue). Cells at different stages of mitosis and cytokinesis were viewed under a fluorescent microscope, and images were taken using a digital CCD (charge-coupled device) camera. Scale bars, 1 μm.
In contrast, INCENP was not detected in the majority of cells treated with INCENP siRNA. The results were consistent with immunoblotting analysis, indicating that the expression of INCENP was effectively ablated by INCENP siRNA. Cells expressing undetectable levels of endogenous INCENP displayed severe mitotic and cytokinetic defects when compared with control cells (Figure 2A). In prophase and prometaphase, INCENP-depleted cells exhibited poorly condensed dumpy chromosomes. Although assembly of the bipolar mitotic spindle was observed in INCENP-depleted cells, maloriented chromosomes were frequently detected. In metaphase, chromosomes did not align on the metaphase plate completely. In anaphase and telophase, as some sister chromosomes separated and moved to the opposite poles of the spindle, others were lagging behind. The MT arrays of the spindle midzone were somewhat disorganized, and the formation of the midbody was severely inhibited. INCENP-depleted cells initiated cytokinesis, but they ultimately failed in the completion of cytokinesis and became binucleated/multinucleated cells. Similar results were also observed in cells transfected with INCENP esiRNA (Supplementary Figure S1 at http://www.BiochemJ.org/bj/389/bj3890373add.htm). Together, the results indicated that INCENP play multiple roles in regulation of chromosome segregation, spindle midzone/midbody formation and completion of cytokinesis.
Suppression of MKLP1 expression inhibits midbody formation and completion of cytokinesis
We next examined the effects of inhibition of MKLP1 expression on the progression of mitosis and cytokinesis. Immunofluorescence analysis was performed in control cells and cells treated with MKLP1 siRNA using anti-MKLP1 antibodies to monitor the expression and localization of MKLP1. In parallel, cells were also co-stained for DNA and α-tubulin to verify the position of chromosomes and the mitotic spindle. Consistent with previous reports [37], MKLP1 protein localized to the spindle poles and spindle in early mitosis, distributed to the spindle midzone in anaphase and then concentrated to the central portion of the midbody in telophase in control cells (Figure 2B). There were no obvious abnormalities on chromosome congression and chromosome segregation, with the spindle and spindle midzone formation in prometaphase, metaphase and anaphase cells expressing undetectable levels of endogenous MKLP1 (Figure 2B). The results indicated that cells that lacked MKLP1 progressed normally from prophase to early anaphase. However, despite assembly of the normal spindle midzone in anaphase, the proper formation of the midbody was severely inhibited in telophase cells that lacked MKLP1 (Figure 2B). Although the initiation of cytokinesis occurred in these cells, cytokinesis could not complete, resulting in the formation of binucleated/multinucleated cells. Similar results were also observed in cells transfected with MKLP1 esiRNA (Supplementary Figure S1 at http://www.BiochemJ.org/bj/389/bj3890373add.htm). These results indicated that MKLP1 is essential for midbody formation and completion of cytokinesis in HeLa cells.
Temporal involvement of INCENP and MKLP1 in midzone/midbody formation and cytokinesis
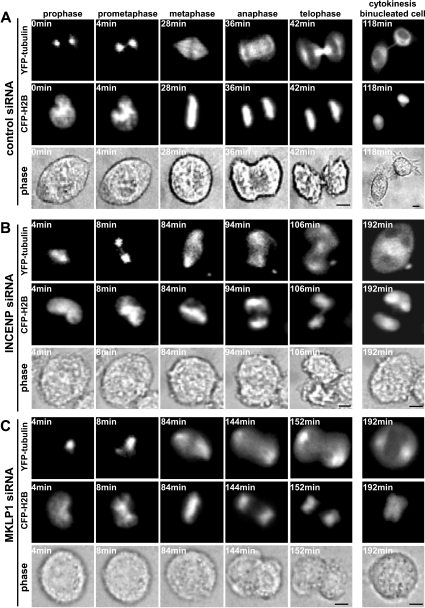
Chromosome segregation and spindle assembly and disassembly during mitosis/cytokinesis are highly dynamic processes. Recently, we developed an experimental system to study these dynamic processes in mammalian cells. In the system, mammalian expression plasmids expressing human α-tubulin fused with an EYFP (enhanced yellow fluorescent protein) and human histone H2B fused with ECFP (enhanced cyan fluorescent protein) were generated and used to mark the mitotic spindle and chromosomes respectively. Mammalian cells were simultaneously transfected with EYFP–tubulin and ECFP–H2B together with plasmids (or siRNAs) of interest, and live-cell images of transfected cells were obtained by time-lapse microscopy. As shown in Figure 3(A), HeLa cells transfected with EYFP–tubulin and ECFP–H2B together with control siRNA showed that EYFP–tubulin and ECFP–H2B localized in cytoplasmic cytoskeleton arrays and nuclei in interphase (results not shown) and then localized on mitotic spindles and chromosomes during mitosis respectively. Like non-transfected cells, these cells progressed through mitosis and cytokinesis normally and finished the processes (from prophase to completion of separation of two daughter cells) within 2–3 h (Figure 3A, and Supplementary Video S1 at http://www.BiochemJ.org/bj/389/bj3890373add.htm).
Figure 3. Time-lapse imaging analysis of HeLa cells treated with control, INCENP or MKLP1 siRNA during mitosis and cytokinesis.
HeLa cells grown on glass-bottom microwell dishes were transfected with EYFP–tubulin and ECFP–H2B plasmids together with control siRNA (A), INCENP siRNA (B) or MKLP1 siRNA (C). At 2 days after transfection, cells were placed on a heated stage (37 °C), and time-lapse images were collected under a Zeiss Axiovert 100M inverted fluorescent microscope at 2 min intervals using an automatic digital CCD (charge-coupled device) camera for 9–10 h. The movies were edited using Slidebook 3.0 and Macromedia Director MX software. Representative images of the movies are shown. The top panels show the images of EYFP–tubulin. The middle panels show the images of ECFP–H2B. The bottom panels show the images of phase contrast. Scale bars, 5 μm.
We examined cells transfected with EYFP–tubulin and ECFP–H2B together with INCENP siRNA through mitosis and cytokinesis using time-lapse microscopy. In contrast with control cells, cells transfected with EYFP-tubulin and ECFP-H2B together with INCENP siRNA clearly showed multiple mitotic/cytokinetic defects (Figure 3B, and Supplementary Video S2 at http://www.BiochemJ.org/bj/389/bj3890373add.htm). Although no obvious abnormalities of bipolar mitotic spindle formation were observed in INCENP siRNA-treated cells, the transitions of prometaphase to metaphase and metaphase to anaphase were severely delayed, and abnormal chromosome congression and alignment were detected. As INCENP-depleted cells eventually progressed to anaphase, and the sister chromosomes began to separate, the anaphase features in these cells appeared highly aberrant. Some of the sister chromosomes pulled to the opposite poles of the spindle and others lagged behind. The midzone interdigitating microtubule bundles were poorly established between separating chromosomes. The spindle pole forces used for segregating sister chromosomes evidently did not retain strength. As a result, the separating sister chromosomes moved backwards and resided in close proximity. While formation and ingression of the cleavage furrow occurred in INCENP siRNA-treated cells, the furrowing remained incomplete, and, ultimately, cytokinesis failed. These results, together with the immunofluorescence analysis described above, demonstrated clearly that INCENP is required not only for chromosome congression and segregation, but also for spindle midzone/midbody formation and completion of cytokinesis.
We then examined the progression of mitosis and cytokinesis in MKLP1 siRNA-treated cells using time-lapse microscopy. Similar to what we did for INCENP siRNA-treated cells, HeLa cells were simultaneously transfected with plasmids expressing EYFP–tubulin and ECFP–H2B together with MKLP1 siRNA (Figure 3C, and Supplementary Video S3 at http://www.BiochemJ.org/bj/389/bj3890373add.htm). Live-cell imaging analysis showed that, consistent with immunofluorescence analysis, there were no obvious abnormalities in chromosome condensation, congression and segregation or bipolar spindle formation and dynamics in prophase, prometaphase, metaphase and early anaphase in MKLP1 siRNA-treated cells. However, the features of the mitotic spindle in late anaphase and telophase appeared highly aberrant in these cells. The proper formation of midbody was severely impaired, and the separating sister chromosomes moved backwards and came into close proximity. Although formation and ingression of the cleavage furrow was observed in MKLP1 siRNA-treated cells, the furrowing aborted quickly. As chromosomes were decondensed, MKLP1 siRNA-treated cells became binucleated. These results indicate that MKLP1 plays an essential role for the midbody formation and completion of cytokinesis. The inhibition of midbody formation and failure in completion of cytokinesis observed in late-telophase MKLP1 siRNA-treated cells were very similar to those observed in INCENP siRNA-treated cells, suggesting that both proteins are involved in midbody formation.
INCENP is required for recruiting MKLP1 to the spindle midzone/midbody and the completion of cytokinesis
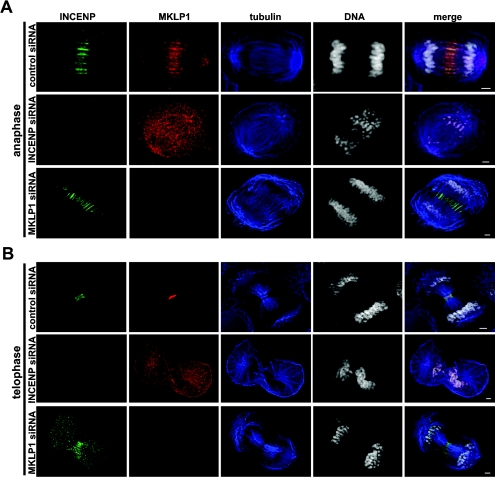
To determine the interplay between INCENP and MKLP1 in midzone/midbody formation and cytokinesis, HeLa cells transfected with control, INCENP or MKLP1 siRNA were stained with anti-MKLP1, Alexa Fluor® 488-conjugated anti-INCENP, anti-α-tubulin antibodies and DAPI. The subcellular localizations of INCENP and MKLP1 in these cells were examined under a fluorescent microscope. As shown in Figure 4, INCENP and MKLP1 co-localized to the spindle midzone in anaphase in control cells. In late-telophase cells, INCENP localized to the borders of the midbody, whereas MKLP1 concentrated on the centre of the midbody in these cells.
Figure 4. Association of MKLP1 with the mitotic spindle midzone/midbody is dependent on INCENP.
HeLa cells grown on coverslips were transfected with control, INCENP or MKLP1 siRNA. At 48 h after transfection, cells were fixed and stained with anti-INCENP antibodies (green), anti-MKLP1 antibodies (red), anti-α-tubulin antibodies (blue) and DAPI (DNA, white). Images were obtained under a fluorescent microscope using a digital CCD (charge-couple device) camera. Shown are anaphase (A) and telophase (B) cells. Scale bars, 1 μm.
The subcellular localization of MKLP1 was not affected in early mitosis in INCENP siRNA-treated cells. As in control cells, MKLP1 localized to the spindle (results not shown). However, the subcellular localization of MKLP1 was clearly affected in late mitosis and cytokinesis in cells that lacked INCENP. Unlike in control cells, where MKLP1 co-localized with INCENP in the spindle midzone and midbody, MKLP1 was found along with the entire mitotic spindle in anaphase cells and in a more diffused pattern in telophase cells that lacked INCENP (Figure 4). These results indicated that the association of MKLP1 with the spindle midzone and midbody during late mitosis and cytokinesis is INCENP-dependent. We examined whether MKLP1 interacts with INCENP in vivo. MKLP1 did not co-immunoprecipitate with INCENP in G2/M phase HeLa cell lysates (results not shown). The results indicated that MKLP1 does not interact with INCENP.
We then examined the subcellular localization of INCENP in MKLP1 siRNA-treated cells. The subcellular localizations of INCENP with centromeres in early mitosis and with the spindle midzone in anaphase were not affected in cells that lacked MKLP1 when compared with control cells (Figure 4A, and results not shown). However, in telophase cells that lacked MKLP1, because formation of the midbody was inhibited, the midbody localization of INCENP was greatly affected (Figure 4B). When compared with the control cells, the staining pattern of INCENP was disorganized and disarrayed in these cells, although INCENP was still co-localized with the spindle MTs in between separating chromosomes (Figure 4B). These results indicated that INCENP is not dependent on MKLP1 for its association with the spindle midzone. However, both INCENP and MKLP1 are required for the formation of functional midbody.
To explore further the roles of INCENP and MKLP1 in the midbody formation, we examined the three-dimensional structures of the midbody or mitotic spindle in INCENP-depleted or MKLP1-depleted telophase cells (see the Materials and methods section). The three-dimensional reconstruction imaging analysis revealed remarkable details of the midbody or spindle structures in these cells (see Supplementary Videos S4, S5 and S6 at http://www.BiochemJ.org/bj/389/bj3890373add.htm). In control cells, the bipolar telophase spindle MTs bundled to form a unique, geometrical and well-organized midbody structure between the two sets of separating chromosomes. MKLP1 localized to the centre and INCENP localized to the borders of the midbody, as the reconstructed image was rotated around the spindle equator orthogonally (see Supplementary Video S4 at http://www.BiochemJ.org/bj/389/bj3890373add.htm). In contrast, telophase cells that lacked INCENP or MKLP displayed striking aberrant spindle morphologies. In telophase cells that lacked MKLP1, the bipolar spindle MTs were poorly organized and disarrayed. Although INCENP was still co-localized with MTs in the middle of the spindle, no well-organized midbody structure with disconnected interdigitating MTs between two half spindles was detected (see Supplementary Video S6 at http://www.BiochemJ.org/bj/389/bj3890373add.htm). In telophase cells that lacked INCENP, the midbody structure was completely impaired. Dispersed MTs were observed in between two sets of incompletely separating chromosomes, and diffuse staining patterns of MKLP1 were detected in these cells (see Supplementary Video S5 at http://www.BiochemJ.org/bj/389/bj3890373add.htm). Taken together, these studies suggested that recruitment of MKLP1 by INCENP to the midzone/midbody is a crucial step for the midbody formation.
DISCUSSION
In the present study, we examined two mitotic spindle midzone-associated proteins, chromosomal passenger protein, INCENP, and centralspindlin protein, MKLP1, in regulating midzone/midbody formation and cytokinesis. Immunofluorescence analysis and time-lapse imaging, in which we monitored chromosome segregation and spindle dynamics during mitosis/cytokinesis in live cells, demonstrated that, in addition to multiple chromosome segregation defects, depletion of INCENP expression by siRNA in HeLa cells resulted in inhibition of the spindle midzone/midbody formation and failure of the completion of cytokinesis. Previous studies showed that depletion of homologues of INCENP by siRNA in C. elegans and Drosophila, overexpression of a dominant-negative mutant of chicken INCENP in HeLa cells and knockout of the INCENP gene in mice dramatically inhibited the ability of cells to achieve normal chromosome segregation during mitosis and the completion of cell separation during cytokinesis [20,22,25,30,33,47]. Our results are consistent with these findings and indicate that INCENP plays multiple essential roles in regulating chromosome segregation, spindle midzone/midbody formation and cytokinesis in human cells.
In contrast, suppression of MKLP1 expression by siRNA did not cause any abnormality of chromosome segregation and midzone formation, but abrogated midbody formation and completion of cytokinesis. Previous studies suggested that the centralspindlin complex, MKLP1–MgcRacGAP, plays a role in bundling the interdigitating MTs to form the midzone in animal cells [4]. As its name implies, both MKLP1 and MgcRacGAP localize to the midzone and are essential for cytokinesis in C. elegans, Drosophila and mammalian cells [4,39,48,49]. The MKLP1–MgcRacGAP complex binds to MTs and promotes antiparallel MT bundling in vitro [4]. MKLP1 is phosphorylated by Cdc2/cyclin B in vivo, and Cdk phosphorylation of MKLP1 negatively regulates MKLP1 motor and MT bundling activities [8]. Because inactivation of Cdc2/cyclin B activity through destruction of mitotic cyclin B is critical for metaphase to anaphase transition, it was suggested that Cdc2/cyclin B phosphorylation by MKLP1 controls the timing of midzone formation during the metaphase to anaphase transition [8]. Thus MKLP1–MgcRacGAP might be the crucial factor that regulates spindle midzone formation and cytokinesis [50]. However, our results indicated that MKLP1 is not involved in the midzone formation. Inhibition of MKLP1 expression by siRNA clearly did not perturb midzone formation (Figure 2, and Supplementary Video S3 at http://www.BiochemJ.org/bj/389/bj3890373add.htm) and did not affect the midzone association of INCENP (Figure 4B). Instead, suppression of MKLP1 expression inhibited midbody formation and completion of cytokinesis, indicating that MKLP1 is essential for these processes. Consistent with our findings, Matuliene and Kuriyama [6,38,39] have shown that an MKLP1 splicing variant, CHO-1, is required for formation of midbody matrix and completion of cytokinesis in mammalian cells. Similar late cytokinesis defects were also observed when MKLP1 homologue proteins were ablated by mutations or by siRNA in other species [9,24,25,48,51,52]. Thus the MKLP1–MgcRacGAP complex appears to be an important factor involved in constricting the midzone to the midbody that is essential for the completion of cytokinesis.
We explored the interplay between INCENP and MKLP1 in regulating the formation of midzone/midbody and completion of cytokinesis in HeLa cells. We showed that MKLP1 is not required for recruiting INCENP to the midzone, but that INCENP is essential for recruiting MKLP1 to the midzone/midbody. Three-dimensional reconstruction imaging analysis suggests that recruitment of MKLP1 to the midzone/midbody by INCENP is a crucial step for midbody formation. Thus regulation of functions of the centralspindlin complex may be a major role of chromosomal passenger complex in cytokinesis. Recently, Minoshima et al. [31] showed that human MgcRacGAP is a direct substrate of Aurora-B kinase, and Aurora-B phosphorylation of MgcRacGAP is necessary for the completion of cytokinesis. These findings, together with the results from the present study, support the notion that the MKLP1–MgcrRacGAP complex is a critical target of Aurora-B–INCENP–Survivin–Borealin complex(es) that are necessary for cytokinesis. It is noteworthy that human MKLP1 also contains two putative Aurora-B consensus phosphorylation sites. Whether MKLP1 is a direct downstream target of Aurora-B–INCENP–Survivin–Borealin complex(es) requires further studies.
Taken together, our results show that, in addition to its chromosome segregation function, human chromosomal passenger protein, INCENP, plays an essential role in regulating midzone/midbody formation and cytokinesis. In contrast, the centralspindlin protein, MKLP1, is not required for midzone formation. Instead, MKLP1 is crucial for midbody formation and completion of cytokinesis. INCENP is required for recruiting MKLP1 to the spindle midzone/midbody, a crucial step for midbody formation and completion of cytokinesis. Future work will focus on defining how the chromosomal passenger complex controls the midzone/midbody association of the centralspindlin complex and determining mechanisms by which the chromosomal passenger complex and the centralspindlin complex regulate midbody formation and completion of cytokinesis.
Multimedia adjuncts
Acknowledgments
We thank Dr Cheng-Chung Tsao for helpful discussion, Dr Nanxin Li for critical reading of the manuscript and Ningning Sai for technical support. This work was supported by grants from Edward Mallinckrodt Jr Foundation, Lisa U. Pardee Foundation and National Institutes of Health (GM67859) to W.J.
References
- 1.Glotzer M. Cleavage furrow positioning. J. Cell Biol. 2004;164:347–351. doi: 10.1083/jcb.200310112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheatley S. P., Hinchcliffe E. H., Glotzer M., Hyman A. A., Sluder G., Wang Y. L. CDK1 inactivation regulates anaphase spindle dynamics and cytokinesis in vivo. J. Cell Biol. 1997;138:385–393. doi: 10.1083/jcb.138.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams R. R., Carmena M., Earnshaw W. C. Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 2001;11:49–54. doi: 10.1016/s0962-8924(00)01880-8. [DOI] [PubMed] [Google Scholar]
- 4.Mishima M., Kaitna S., Glotzer M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev. Cell. 2002;2:41–54. doi: 10.1016/s1534-5807(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 5.Mollinari C., Kleman J. P., Jiang W., Schoehn G., Hunter T., Margolis R. L. PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. J. Cell Biol. 2002;157:1175–1186. doi: 10.1083/jcb.200111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matuliene J., Kuriyama R. Role of the midbody matrix in cytokinesis: RNAi and genetic rescue analysis of the mammalian motor protein CHO1. Mol. Biol. Cell. 2004;15:3083–3094. doi: 10.1091/mbc.E03-12-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruneberg U., Neef R., Honda R., Nigg E. A., Barr F. A. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J. Cell Biol. 2004;166:167–172. doi: 10.1083/jcb.200403084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishima M., Pavicic V., Gruneberg U., Nigg E. A., Glotzer M. Cell cycle regulation of central spindle assembly. Nature (London) 2004;430:908–913. doi: 10.1038/nature02767. [DOI] [PubMed] [Google Scholar]
- 9.Goshima G., Vale R. D. The roles of microtubule-based motor proteins in mitosis: comprehensive RNAi analysis in the Drosophila S2 cell line. J. Cell Biol. 2003;162:1003–1016. doi: 10.1083/jcb.200303022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu C., Jiang W. Cell-cycle-dependent translocation of PRC1 on the spindle by Kif4 is essential for midzone formation and cytokinesis. Proc. Natl. Acad. Sci. U.S.A. 2005;102:343–348. doi: 10.1073/pnas.0408438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackay A. M., Earnshaw W. C. The INCENPs: structural and functional analysis of a family of chromosome passenger proteins. Cold Spring Harbor Symp. Quant. Biol. 1993;58:697–706. doi: 10.1101/sqb.1993.058.01.077. [DOI] [PubMed] [Google Scholar]
- 12.Skoufias D. A., Mollinari C., Lacroix F. B., Margolis R. L. Human survivin is a kinetochore-associated passenger protein. J. Cell Biol. 2000;151:1575–1582. doi: 10.1083/jcb.151.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terada Y. Role of chromosomal passenger complex in chromosome segregation and cytokinesis. Cell Struct. Funct. 2001;26:653–657. doi: 10.1247/csf.26.653. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi T., Uhlmann F. Cell cycle: passenger acrobatics. Nature (London) 2003;426:780–781. doi: 10.1038/426780a. [DOI] [PubMed] [Google Scholar]
- 15.Mollinari C., Reynaud C., Martineau-Thuillier S., Monier S., Kieffer S., Garin J., Andreassen P. R., Boulet A., Goud B., Kleman J. P., Margolis R. L. The mammalian passenger protein TD-60 is an RCC1 family member with an essential role in prometaphase to metaphase progression. Dev. Cell. 2003;5:295–307. doi: 10.1016/s1534-5807(03)00205-3. [DOI] [PubMed] [Google Scholar]
- 16.Romano A., Guse A., Krascenicova I., Schnabel H., Schnabel R., Glotzer M. CSC-1: a subunit of the Aurora B kinase complex that binds to the survivin-like protein BIR-1 and the incenp-like protein ICP-1. J. Cell Biol. 2003;161:229–236. doi: 10.1083/jcb.200207117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gassmann R., Carvalho A., Henzing A. J., Ruchaud S., Hudson D. F., Honda R., Nigg E. A., Gerloff D. L., Earnshaw W. C. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell Biol. 2004;166:179–191. doi: 10.1083/jcb.200404001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampath S. C., Ohi R., Leismann O., Salic A., Pozniakovski A., Funabiki H. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118:187–202. doi: 10.1016/j.cell.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Wheatley S. P., Kandels-Lewis S. E., Adams R. R., Ainsztein A. M., Earnshaw W. C. INCENP binds directly to tubulin and requires dynamic microtubules to target to the cleavage furrow. Exp. Cell Res. 2001;262:122–127. doi: 10.1006/excr.2000.5088. [DOI] [PubMed] [Google Scholar]
- 20.Honda R., Korner R., Nigg E. A. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol. Biol. Cell. 2003;14:3325–3341. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vagnarelli P., Earnshaw W. C. Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma. 2004;113:211–222. doi: 10.1007/s00412-004-0307-3. [DOI] [PubMed] [Google Scholar]
- 22.Mackay A. M., Ainsztein A. M., Eckley D. M., Earnshaw W. C. A dominant mutant of inner centromere protein (INCENP), a chromosomal protein, disrupts prometaphase congression and cytokinesis. J. Cell Biol. 1998;140:991–1002. doi: 10.1083/jcb.140.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schumacher J. M., Golden A., Donovan P. J. AIR-2: an Aurora/Ipl1-related protein kinase associated with chromosomes and midbody microtubules is required for polar body extrusion and cytokinesis in Caenorhabditis elegans embryos. J. Cell Biol. 1998;143:1635–1646. doi: 10.1083/jcb.143.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Severson A. F., Hamill D. R., Carter J. C., Schumacher J., Bowerman B. The aurora-related kinase AIR-2 recruits ZEN-4/CeMKLP1 to the mitotic spindle at metaphase and is required for cytokinesis. Curr. Biol. 2000;10:1162–1171. doi: 10.1016/s0960-9822(00)00715-6. [DOI] [PubMed] [Google Scholar]
- 25.Giet R., Glover D. M. Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 2001;152:669–682. doi: 10.1083/jcb.152.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaitna S., Pasierbek P., Jantsch M., Loidl J., Glotzer M. The aurora B kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous chromosomes during meiosis. Curr. Biol. 2002;12:798–812. doi: 10.1016/s0960-9822(02)00820-5. [DOI] [PubMed] [Google Scholar]
- 27.Ditchfield C., Johnson V. L., Tighe A., Ellston R., Haworth C., Johnson T., Mortlock A., Keen N., Taylor S. S. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 2003;161:267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauf S., Cole R. W., LaTerra S., Zimmer C., Schnapp G., Walter R., Heckel A., van Meel J., Rieder C. L., Peters J. M. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saffery R., Irvine D. V., Kile B. T., Hudson D. F., Cutts S. M., Choo K. H. Cloning, expression, and promoter structure of a mammalian inner centromere protein (INCENP) Mamm. Genome. 1999;10:415–418. doi: 10.1007/s003359901014. [DOI] [PubMed] [Google Scholar]
- 30.Uren A. G., Wong L., Pakusch M., Fowler K. J., Burrows F. J., Vaux D. L., Choo K. H. Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr. Biol. 2000;10:1319–1328. doi: 10.1016/s0960-9822(00)00769-7. [DOI] [PubMed] [Google Scholar]
- 31.Minoshima Y., Kawashima T., Hirose K., Tonozuka Y., Kawajiri A., Bao Y. C., Deng X., Tatsuka M., Narumiya S., May W. S., Jr, et al. Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev. Cell. 2003;4:549–560. doi: 10.1016/s1534-5807(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 32.Maddox A. S., Oegema K. Closing the GAP: a role for a RhoA GAP in cytokinesis. Mol. Cell. 2003;11:846–848. doi: 10.1016/s1097-2765(03)00151-5. [DOI] [PubMed] [Google Scholar]
- 33.Adams R. R., Maiato H., Earnshaw W. C., Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J. Cell Biol. 2001;153:865–880. doi: 10.1083/jcb.153.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gatti M., Giansanti M. G., Bonaccorsi S. Relationships between the central spindle and the contractile ring during cytokinesis in animal cells. Microsc. Res. Tech. 2000;49:202–208. doi: 10.1002/(SICI)1097-0029(20000415)49:2<202::AID-JEMT13>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Glotzer M. Animal cell cytokinesis. Annu. Rev. Cell Dev. Biol. 2001;17:351–386. doi: 10.1146/annurev.cellbio.17.1.351. [DOI] [PubMed] [Google Scholar]
- 36.Guertin D. A., Trautmann S., McCollum D. Cytokinesis in eukaryotes. Microbiol. Mol. Biol. Rev. 2002;66:155–178. doi: 10.1128/MMBR.66.2.155-178.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nislow C., Sellitto C., Kuriyama R., McIntosh J. R. A monoclonal antibody to a mitotic microtubule-associated protein blocks mitotic progression. J. Cell Biol. 1990;111:511–522. doi: 10.1083/jcb.111.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuriyama R., Gustus C., Terada Y., Uetake Y., Matuliene J. CHO1, a mammalian kinesin-like protein, interacts with F-actin and is involved in the terminal phase of cytokinesis. J. Cell Biol. 2002;156:783–790. doi: 10.1083/jcb.200109090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matuliene J., Kuriyama R. Kinesin-like protein CHO1 is required for the formation of midbody matrix and the completion of cytokinesis in mammalian cells. Mol. Biol. Cell. 2002;13:1832–1845. doi: 10.1091/mbc.01-10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang W., Jimenez G., Wells N. J., Hope T. J., Wahl G. M., Hunter T., Fukunaga R. PRC1: a human mitotic spindle-associated CDK substrate protein required for cytokinesis. Mol. Cell. 1998;2:877–885. doi: 10.1016/s1097-2765(00)80302-0. [DOI] [PubMed] [Google Scholar]
- 41.Rusan N. M., Fagerstrom C. J., Yvon A. M., Wadsworth P. Cell cycle-dependent changes in microtubule dynamics in living cells expressing green fluorescent protein-α tubulin. Mol. Biol. Cell. 2001;12:971–980. doi: 10.1091/mbc.12.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanda T., Sullivan K. F., Wahl G. M. Histone–GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 1998;8:377–385. doi: 10.1016/s0960-9822(98)70156-3. [DOI] [PubMed] [Google Scholar]
- 43.Yang D., Buchholz F., Huang Z., Goga A., Chen C. Y., Brodsky F. M., Bishop J. M. Short RNA duplexes produced by hydrolysis with Escherichia coli RNase III mediate effective RNA interference in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 2002;99:9942–9947. doi: 10.1073/pnas.152327299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elbashir S. M., Lendeckel W., Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams R. R., Eckley D. M., Vagnarelli P., Wheatley S. P., Gerloff D. L., Mackay A. M., Svingen P. A., Kaufmann S. H., Earnshaw W. C. Human INCENP colocalizes with the Aurora-B/AIRK2 kinase on chromosomes and is overexpressed in tumour cells. Chromosoma. 2001;110:65–74. doi: 10.1007/s004120100130. [DOI] [PubMed] [Google Scholar]
- 46.Wheatley S. P., Carvalho A., Vagnarelli P., Earnshaw W. C. INCENP is required for proper targeting of Survivin to the centromeres and the anaphase spindle during mitosis. Curr. Biol. 2001;11:886–890. doi: 10.1016/s0960-9822(01)00238-x. [DOI] [PubMed] [Google Scholar]
- 47.Kaitna S., Mendoza M., Jantsch-Plunger V., Glotzer M. Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr. Biol. 2000;10:1172–1181. doi: 10.1016/s0960-9822(00)00721-1. [DOI] [PubMed] [Google Scholar]
- 48.Adams R. R., Tavares A. A., Salzberg A., Bellen H. J., Glover D. M. Pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev. 1998;12:1483–1494. doi: 10.1101/gad.12.10.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jantsch-Plunger V., Gonczy P., Romano A., Schnabel H., Hamill D., Schnabel R., Hyman A. A., Glotzer M. CYK-4: A Rho family GTPase activating protein (GAP) required for central spindle formation and cytokinesis. J. Cell Biol. 2000;149:1391–1404. doi: 10.1083/jcb.149.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCollum D. Cytokinesis: the central spindle takes center stage. Curr. Biol. 2004;14:R953–R955. doi: 10.1016/j.cub.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 51.Chen M. C., Zhou Y., Detrich H. W., 3rd Zebrafish mitotic kinesin-like protein 1 (Mklp1) functions in embryonic cytokinesis. Physiol. Genomics. 2002;8:51–66. doi: 10.1152/physiolgenomics.00042.2001. [DOI] [PubMed] [Google Scholar]
- 52.Minestrini G., Harley A. S., Glover D. M. Localization of Pavarotti-KLP in living Drosophila embryos suggests roles in reorganizing the cortical cytoskeleton during the mitotic cycle. Mol. Biol. Cell. 2003;14:4028–4038. doi: 10.1091/mbc.E03-04-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.